Abstract
Objectives: Although hypovitaminosis D appears to be highly prevalent in patients with coronavirus disease 2019 (COVID-19), its impact on their prognosis remains unclear.
Methods: In this study, serum 25-hydroxyvitamin D (Vit-D) level was measured in 200 patients hospitalized with COVID-19. The association between Vit-D and the composite endpoint of intensive care unit (ICU) admission/in-hospital death was explored using univariable and multivariable analyses. Also, serum Vit-D level in patients with COVID-19 was compared with that in age- and sex-balanced COVID-19-negative controls (i.e., 50 inpatients with sepsis).
Results: Serum Vit-D level was comparable between patients with COVID-19 and COVID-19-negative inpatients with sepsis (P = 0.397). No significant differences were found in serum Vit-D level according to COVID-19 severity at the time of hospital admission (P = 0.299). Incidence rates of the composite endpoint of ICU admission/in-hospital death did not differ significantly between patients with either Vit-D deficiency (i.e., Vit-D <20 ng/mL) or severe Vit-D deficiency (i.e., Vit-D <12 ng/mL) and those without (31% vs 35% with P = 0.649, and 34% vs 30% with P = 0.593, respectively). Vit-D level and status (i.e., Vit-D deficiency and severe Vit-D deficiency) were not prospectively associated with the risk of the composite endpoint of ICU admission/in-hospital death (P > 0.05 for all Cox regression models).
Conclusions: Regardless of the potential usefulness of Vit-D measurement to guide appropriate supplementation, Vit-D does not appear to provide helpful information for the stratification of in-hospital prognosis in patients with COVID-19.
Keywords: SARS-CoV-2, COVID-19, 25-hydroxyvitamin D, Pneumonia, Respiratory distress
Introduction
The coronavirus disease 2019 (COVID-19) continues to be a terrifying challenge for health systems worldwide, as reported daily by World Health Organization official updates [1]. In the hospital setting, therapeutic efforts to manage patients with the most common complications of this disease are not always effective [2]. Thus, approximately 20% of patients hospitalized with COVID-19 are transferred to intensive care units (ICUs) and 20% die from pulmonary and extrapulmonary complications of the disease [3]. The most unfavorable prognosis, often dictated by a lack of effective specific therapies, is recorded in older patients with greater frailty [4,5]. However, identifying circulating biomarkers that possibly suggest pathophysiological mechanisms and clinical evolution of the disease regardless of age and comorbidities, as well as direct the diagnostic and therapeutic choices, could be very helpful.
The major circulating form of vitamin D, 25-hydroxyvitamin D (Vit-D), is produced by the hepatic hydroxylation of previtamin D, which in turn is obtained in the skin from 7-dehydrocholesterol by the action of ultraviolet B rays. Subsequently, Vit-D is converted into 1,25-dihydroxyvitamin D (calcitriol), the biologically active form of vitamin D, through another hydroxylation reaction in the kidney and other tissues/cells (e.g., immune cells) [6,7]. Beyond having a key role in calcium homeostasis [8], Vit-D can exert direct antiviral effects and cooperate with type I interferon to enhance antiviral responses [9,10]. In addition, Vit-D can exert an immune-modulating action by regulating the production of inflammatory cytokines and inhibiting the proliferation of inflammatory cells [10].
By virtue of its antiviral and immunomodulating action, Vit-D could play a favorable role in the natural history of COVID-19 [11]. On the contrary, Vit-D deficiency status could be a prognostically unfavorable element in this infectious disease [11]. To date, various observational studies have assessed Vit-D levels in patients with COVID-19 and showed a unique result, namely the high prevalence of Vit-D deficiency [12,13]. Also, a recent meta-analysis of observational studies has shown that patients with Vit-D deficiency are more likely to get a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [14]. However, a similar concordance of results was not observed with respect to the association between Vit-D and COVID-19-related outcomes. In fact, the inverse associations between Vit-D level and both COVID-19 severity and prognosis that emerged in some studies [15], [16], [17] were not always confirmed in others [18], [19], [20], [21], [22].
To make the link between serum Vit-D level and prognosis even more intricate, some intervention studies have found that Vit-D supplementation could reduce the transfer of patients with COVID-19 to ICUs or their risk of death [23]. However, in other studies, Vit-D supplementation had no favorable effects on the clinical course of COVID-19 [24,25].
In this study we investigated the prevalence of Vit-D deficiency and severe Vit-D deficiency in patients with COVID-19 who were hospitalized in an internal medicine ward compared with that in COVID-19-negative patients admitted with sepsis. In addition, considering the diverging literature results, we explored the prospective association between Vit-D and risk of ICU admission/in-hospital death as a composite endpoint in patients hospitalized with COVID-19.
Methods
COVID-19 population
Patients hospitalized with COVID-19 who were admitted to the Unit of Internal Medicine of the Santa Maria della Misericordia Hospital of Perugia in Italy between December 2020 and March 2021 were consecutively enrolled. The study protocol was developed in accordance with the principles of the Helsinki Declaration, and approved by the local ethics committee. The inclusion criteria were as follows: 1) age ≥18 y; 2) positive result on real-time reverse-transcriptase-polymerase chain reaction (RT-PCR) assays testing for SARS-CoV-2 on nasal or pharyngeal swab specimens at the time of hospital admission; and 3) informed written consent. Ongoing or previous (within the last 6 mo) Vit-D supplementation was an exclusion criterion.
Control group
For the control group, 50 age- and sex-balanced COVID-19-negative patients who were admitted with sepsis to the Unit of Internal Medicine of the Santa Maria della Misericordia Hospital of Perugia in Italy between December 2020 and March 2021 were enrolled. The inclusion criteria were as follows: 1) Age ≥18 y, 2) negative result on RT-PCR assays test for SARS-CoV-2 on nasal or pharyngeal swab specimens at the time of hospital admission, 3) informed written consent, and 4) diagnosis of sepsis according to the Sepsis-3 criteria [26] at the time of hospital admission. Ongoing or previous (within the last 6 mo) Vit-D supplementation was an exclusion criterion.
Data collection
Data on demographic characteristics, coexisting medical conditions, current treatments, laboratory tests, as well as physical and instrumental examinations performed at the time of hospital admission were collected and registered in medical records. Tests for SARS-CoV-2 on nasal or pharyngeal swab specimens were performed with RT-PCR assays (Allplex 2019-nCoV Assay, Seegene, Seoul, South Korea, or Xpert Xpress SARS-CoV-2, Cepheid, Sunnyvale, CA). Arterial and venous blood samples obtained within 48 h since hospital admission were processed according to standard laboratory techniques to determine the following laboratory variables: blood gas parameters (ABL90 FLEX blood gas analyzer, Radiometer, Brønshøj, Denmark), white cell and platelet counts (Sysmex XT-2000i, Dasit, Milano, Italy), D-dimer (BCS XP Coagulation Analyzer, Siemens, Munich, Germany), C-reactive protein (CRP), blood urea nitrogen, creatinine, bilirubin, lactate dehydrogenase, fasting glucose, and albumin (AU5800 Clinical Chemistry System, Beckman Coulter, Brea, CA). Serum Vit-D was assessed on venous blood samples obtained within 48 h since hospital admission through a radioimmunoassay (Diasorin Inc. Stillwater, Minnesota). Estimated glomerular filtration rate was calculated through the Chronic Kidney Disease Epidemiology Collaboration equation. Vit-D deficiency was defined as Vit-D level <20 ng/mL and severe Vit-D deficiency as Vit-D level <12 ng/mL [27]. A radiologic diagnosis of pneumonia was based on the presence of at least one of the following radiographic signs in either chest x-ray or high-resolution computed tomography: mono- or bilateral consolidations, ground glass opacities, and crazy paving pattern. Respiratory insufficiency was defined as the presence of peripheral oxygen saturation (SpO2) ≤90% and/or arterial partial pressure of oxygen (PaO2) ≤60 mmHg and/or the need for oxygen support at the time of admission. Calculated PaO2 per fraction of inspiration oxygen ratio (PaO2/FiO2) <300 was used to define the presence of respiratory distress. The CURB-65 score was estimated for each patient by integrating five clinical/laboratory data at the time of admission (i.e., 1 = confusion [1 point]; 2 = blood urea nitrogen >19 mg/dL [1 point]; 3 = respiratory rate ≥30/min [1 point]; 4 = systolic blood pressure <90 mmHg or diastolic blood pressure ≤60 mmHg, as assessed with a sphygmomanometer [1 point]; 5 = age ≥65 y [1 point]) [28,29]. The Charlson comorbidity index was calculated for each patient by integrating information on coexisting medical conditions [30]. Data on clinical course (i.e., in-hospital medical treatments and need of noninvasive ventilation [NIV]) and in-hospital outcomes (i.e., composite endpoint of ICU admission/in-hospital death or hospital discharge) were collected and registered in medical records.
Statistical analysis
The study sample size was calculated based on the results of a previous study [31] by assuming a type I error of 0.05, type II error of 0.2, ratio of unexposed (Vit-D ≥20 ng/mL) to exposed (Vit-D <20 ng/mL) group of 0.56, probability of event (i.e., ICU admission/in-hospital death) in the unexposed group of 0.02, and probability of event in the exposed group of 0.13. The estimated sample size was 206 patients, but we performed our analysis in 200 patients as an interim analysis. The SPSS statistical package, version 24.0 (SPSS Inc, Chicago, IL) was used for all statistical analyses. The Shapiro test was used to verify the normality of the study variables. Categorical variables were expressed as percentages and continuous variables as mean ± standard deviation or median and interquartile ranges. The independent samples t test, Mann–Whitney U-test, and χ2 test were used for two-group comparisons. The Kruskal–Wallis test was used for multiple-group comparisons of nonparametric variables. The χ2 test was used to compare multiple independent categorical variables. Correlation analyses between the study variables were performed using the Pearson's and Spearman's coefficients of correlation.
Time-to-event analyses were performed to assess the association between Vit-D and the composite endpoint of ICU admission/in-hospital death (primary endpoint), as well as the association between Vit-D and in-hospital death as a single endpoint (secondary endpoint).
For five patients, who did not meet the aforementioned endpoints and were still hospitalized at the time of the analysis, the event date was censored on April 3, 2021. The association between Vit-D, either as a continuous or categorical variable (i.e., serum Vit-D level, Vit-D deficiency, and severe Vit-D deficiency), and either the composite endpoint of ICU admission/in-hospital death or in-hospital death as a single endpoint was evaluated through Cox proportional hazard models by adjusting for potential confounders. Statistical significance was assumed if a null hypothesis could be rejected at P < 0.05.
Results
Characteristics of patients with COVID-19
The main characteristics of 200 patients with COVID-19 categorized according to the presence or absence of Vit-D deficiency (i.e., Vit-D <20 ng/mL vs Vit-D ≥20 ng/mL) are shown in Table 1 . The prevalent symptoms reported at the time of hospital admission were fever, dyspnea, and cough (65%, 64%, and 41% of patients, respectively). According to the National Institutes of Health classification of COVID-19 severity [32], 22 (11%), 26 (13%), and 152 (76%) patients had mild (i.e., signs and symptoms of COVID-19 without shortness of breath, dyspnea, or abnormal chest imaging), moderate (i.e., lower respiratory disease during clinical assessment or imaging and SpO2 ≥94% on room air at sea level) and severe COVID-19 (i.e., SpO2 <94% on room air at sea level, PaO2/FiO2 <300 mmHg, respiratory frequency >30 breaths/min, or lung infiltrates >50%), respectively.
Table 1.
Characteristics of patients with COVID-19 categorized according to the presence or absence of Vit-D deficiency (Vit-D <20 ng/mL vs Vit-D ≥20 ng/mL).
| Total N = 200 | Vit-D <20 ng/mL n = 160 | Vit-D ≥20 ng/mL n = 40 | P-value* | |
|---|---|---|---|---|
| Age, y | 74 (15) | 72 (16) | 78 (13) | 0.031 |
| Male sex, % | 55 | 56 | 47 | 0.320 |
| Body mass index, kg/m2 | 26.2 (4.1) | 26.4 (4.1) | 25.0 (4.1) | 0.063 |
| Current smoking status, % | 17 | 18 | 13 | 0.482 |
| Hypertension, % | 61 | 61 | 62 | 0.884 |
| Type 2 diabetes, % | 20 | 21 | 20 | 0.930 |
| Previous cardiovascular event, % | 18 | 19 | 17 | 0.856 |
| Active cancer, % | 8 | 9 | 5 | 0.375 |
| Previous venous thromboembolism, % | 5 | 5 | 5 | 1.000 |
| Atrial fibrillation, % | 19 | 19 | 17 | 0.787 |
| Chronic obstructive pulmonary disease, % | 14 | 14 | 15 | 0.839 |
| Obesity, % | 22 | 31 | 22 | 0.301 |
| Charlson comorbidity index | 4 (3–6) | 4 (3–6) | 4 (3–7) | 0.524 |
| Aangiotensin converting enzyme inhibitors, % | 26 | 24 | 32 | 0.295 |
| Angiotensin receptor blockers, % | 14 | 14 | 17 | 0.547 |
| Beta-blockers, % | 31 | 29 | 40 | 0.196 |
| Calcium channel blockers, % | 22 | 22 | 22 | 0.932 |
| Diuretics, % | 35 | 34 | 40 | 0.506 |
| Statins, % | 16 | 16 | 17 | 0.848 |
| Direct oral anticoagulants, % | 15 | 14 | 17 | 0.621 |
| Vitamin-K antagonists, % | 3 | 4 | 0 | 0.214 |
| Low molecular weight heparin, % | 21 | 19 | 27 | 0.259 |
| Antiplatelets, % | 23 | 24 | 20 | 0.614 |
| Insulin, % | 13 | 14 | 10 | 0.520 |
| Oral hypoglycemic agents, % | 11 | 11 | 12 | 0.825 |
| Systolic blood pressure, mmHg | 131 (20) | 132 (20) | 130 (20) | 0.573 |
| Diastolic blood pressure, mmHg | 77 (12) | 78 (12) | 75 (10) | 0.155 |
| Leukocytes, X 103/μL | 8.0 (5.7–11.0) | 7.9 (5.7–10.8) | 8.9 (5.9–11.3) | 0.480 |
| Platelets, X 103/μL | 220 (160–276) | 220 (161–279) | 224 (152–271) | 0.546 |
| D-dimer, ng/mL | 895 (530–1762) | 903 (493–1943) | 875 (604–1455) | 0.913 |
| C-reactive protein, mg/dL | 6.9 (3.6–12.1) | 6.4 (3.6–11.2) | 10.0 (3.3–14.6) | 0.148 |
| Fasting glucose, mg/dL | 121 (102–155) | 120 (101–156) | 133 (110–153) | 0.306 |
| Estimated glomerular filtration rate, mL/min | 69 (27) | 71 (27) | 62 (27) | 0.079 |
| Lactate dehydrogenase, UI/L | 307 (236–432) | 300 (235–406) | 337 (253–469) | 0.213 |
| Albumin, g/dL | 3.4 (3–3.7) | 3.4 (3.0–3.7) | 3.5 (3.0–3.7) | 0.625 |
| Arterial partial pressure of oxygen/ fraction of inspiration oxygen | 248 (147–294) | 247 (161–290) | 254 (130–304) | 0.715 |
| CURB-65 score | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.296 |
| Vit-D, ng/mL | 11 (7–18) | 10 (6–14) | 28 (23–34) | < 0.001 |
Vit-D, 25-hydroxyvitamin D. Values are expressed as mean (standard deviation), median (25–75th percentile) or percentage.
P-value for comparison between patients with COVID-19 with and without Vit-D deficiency.
Clinical course and in-hospital outcomes of patients with COVID-19
The clinical management of patients with COVID-19 was conducted according to available scientific evidence and recommendations at the time of enrollment. Corticosteroid treatment (dexamethasone 6 mg daily) was administered to 180 patients with respiratory insufficiency (90%). Antiviral therapy with remdesivir was prescribed to 48 patients who fulfilled the prescription criteria of the Italian drug agency (24%). Anticoagulant therapy was introduced in 188 patients (94%). Upon admission, radiographic signs of pneumonia were documented in 170 patients (85%). During the hospital stay, 76 patients needed NIV (38%), 24 patients were admitted to ICU (12%), 40 patients (20%) died, and 64 patients (32%) met the composite endpoint of ICU admission/in-hospital death. The median time from hospitalization to ICU admission was 2 d (range, 1–5 d), and the median time from hospitalization to death was 10 d (range, 6–16 d).
Covariates of Vit-D
Age was significantly lower in patients with Vit-D deficiency compared with those without (Table 1). A significant crude correlation was found between Vit-D and albumin (r = 0.149; P = 0.037). No significant correlations were found between Vit-D and any of the other continuous study variables. No significant correlation emerged between Vit-D and CRP (Supplementary Fig. 1).
Vit-D level did not differ significantly according to COVID-19 severity (P = 0.299) nor according to the presence of radiographic signs of pneumonia (P = 0.532), respiratory insufficiency (P = 0.342), or respiratory distress (P = 0.383) at the time of hospital admission. No significant difference was found between the serum Vit-D level of patients who needed NIV during their hospital stay and those who did not (P = 0.303).
The rates of radiographic signs of pneumonia, respiratory insufficiency, respiratory distress, and the need of NIV did not differ significantly according to Vit-D deficiency or severe Vit-D deficiency (P = 0.932, 0.754, 0.334, and 0.611, respectively, for Vit-D deficiency, and P = 0.857, 0.700, 0.502, and 0.162, respectively, for severe Vit-D deficiency).
Vit-D and in-hospital prognosis of patients with COVID-19
Baseline characteristics of patients with COVID-19 according to the composite endpoint of ICU admission/in-hospital death are reported in Table 2 , and the baseline characteristics of patients with COVID-19 categorized according to the single endpoint of in-hospital death are reported in Supplementary Table 1. The detection of radiographic signs of pneumonia, respiratory insufficiency, and respiratory distress at the time of hospital admission, as well as the need of NIV during the hospital stay were more prevalent in patients who met the composite endpoint of ICU admission/in-hospital death compared with those who did not (P = 0.013, < 0.001, < 0.001, and < 0.001, respectively). Conversely, the detection of radiographic signs of pneumonia, respiratory insufficiency, and respiratory distress at the time of hospital admission, as well as the need of NIV during the hospital stay were comparable between survivors and nonsurvivors (P = 0.055, 0.635, 0.504, and 0.827, respectively).
Table 2.
Characteristics of patients with COVID-19 categorized according to the composite endpoint of ICU admission/in-hospital death
| Non-ICU admitted/discharged alive n = 136 | ICU admitted/nonsurvivors n = 64 | P-value | |
|---|---|---|---|
| Age, y | 72 (16) | 77 (13) | 0.025 |
| Male sex, % | 52 | 59 | 0.342 |
| Body mass index, kg/m2 | 26.4 (4.2) | 25.6 (4.0) | 0.223 |
| Current smoking status, % | 18 | 17 | 0.908 |
| Hypertension, % | 61 | 62 | 0.842 |
| Type 2 diabetes, % | 15 | 31 | 0.010 |
| Previous cardiovascular event, % | 12 | 19 | 0.314 |
| Active cancer, % | 8 | 9 | 0.761 |
| Previous venous thromboembolism, % | 5 | 5 | 0.889 |
| Atrial fibrillation, % | 18 | 20 | 0.746 |
| Chronic obstructive pulmonary disease, % | 13 | 16 | 0.650 |
| Obesity, % | 31 | 25 | 0.428 |
| Charlson comorbidity index | 4 (2–6) | 5 (4–6) | 0.010 |
| Aangiotensin converting enzyme inhibitors, % | 26 | 26 | 0.901 |
| Angiotensin receptor blockers, % | 12 | 19 | 0.242 |
| Beta-blockers, % | 28 | 39 | 0.114 |
| Calcium channel blockers, % | 19 | 28 | 0.151 |
| Diuretics, % | 32 | 42 | 0.175 |
| Statins, % | 16 | 16 | 0.979 |
| Direct oral anticoagulants, % | 15 | 16 | 0.865 |
| Vitamin-K antagonists, % | 3 | 3 | 0.943 |
| Low molecular weight heparin, % | 19 | 25 | 0.341 |
| Antiplatelets, % | 18 | 33 | 0.024 |
| Insulin, % | 10 | 19 | 0.088 |
| Oral hypoglycemic agents, % | 9 | 17 | 0.084 |
| Systolic blood pressure, mmHg | 132 (20) | 130 (20) | 0.437 |
| Diastolic blood pressure, mmHg | 78 (11) | 75 (11) | 0.085 |
| Leukocytes, X 103/μL | 7.9 (5.4–10.7) | 8.4 (5.9–11.9) | 0.268 |
| Platelets, X 103/μL | 225 (162–283) | 204 (157–269) | 0.252 |
| D-dimer, ng/mL | 833 (551–1748) | 944 (482–1768) | 0.967 |
| C-reactive protein, mg/dL | 6.2 (3.2–11) | 7.4 (4.6–14.8) | 0.028 |
| Fasting glucose, mg/dL | 120 (103–152) | 127 (101–160) | 0.703 |
| Estimated glomerular filtration rate, mL/min | 74 (26) | 59 (26) | < 0.001 |
| Lactate dehydrogenase, UI/L | 3.4 (3.1–3.7) | 3.3 (3–3.6) | 0.113 |
| Albumin, g/dL | 279 (230–399) | 366 (296–467) | 0.003 |
| Arterial partial pressure of oxygen/fraction of inspiration oxygen | 260 (198–303) | 152 (133–271) | < 0.001 |
| CURB-65 score | 2 (1–2) | 2 (1–3) | < 0.001 |
| Vit-D, ng/mL | 11 (8–18) | 11 (6–18) | 0.839 |
ICU, intensive care unit; Vit-D, 25-hydroxyvitamin D.
Values are expressed as mean (standard deviation), median (25–75th percentile) or percentage.
No significant difference was found in serum Vit-D level between patients who were admitted to ICU or who died compared with those who were not admitted to ICU/were discharged alive (Table 2) nor between patients who died and those who did not (Supplementary Table 1). No significant differences were found in the prevalence of Vit-D deficiency or severe Vit-D deficiency between patients who met the composite outcome of ICU admission/in-hospital death and those who did not (P = 0.649 and 0.593, respectively) nor between survivors and nonsurvivors (P = 1.000 and 0.357, respectively). Incidence rates of the composite endpoint of ICU admission/in-hospital death did not differ significantly between patients with either Vit-D deficiency (Vit-D <20 ng/mL) or severe Vit-D deficiency (Vit-D <12 ng/mL) and those without (31% vs 35% and P = 0.649 for Vit-D deficiency, 34% vs 30% and P = 0.593 for severe Vit-D deficiency, respectively). Rates of in-hospital death did not differ significantly across serum Vit-D quintiles (Vit-D <6.1 ng/mL in 1st quintile, 6.1 ng/mL ≥ Vit-D <9.7 ng/mL in 2nd quintile, 9.7 ng/mL ≥ Vit-D <13.9 ng/mL in 3rd quintile, 13.9 ng/mL ≥ Vit-D <20 ng/mL in 4th quintile, Vit-D ≥20 ng/mL in 5th quintile; P = 0.769; Supplementary Fig. 2).
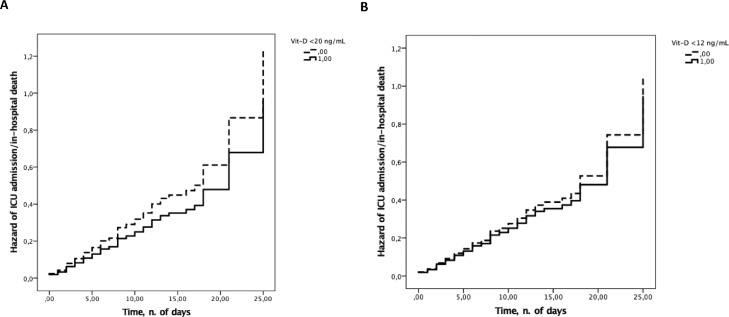
Three Cox proportional hazard models were plotted (Models 1, 2, and 3), including the time to ICU admission/in-hospital death as the time variable, ICU-admission/in-hospital death as the status variable, and the following independent variables: Model 1 has Vit-D (serum Vit-D level in Model 1a, Vit-D deficiency in Model 1b, and severe Vit-D deficiency in Model 1c), age, and male sex; Model 2 has Vit-D (serum Vit-D level in Model 2a, Vit-D deficiency in Model 2b, severe Vit-D deficiency in Model 2c), age, male sex, and coexisting medical conditions/current treatments varying significantly according to the in-hospital prognosis (i.e., type 2 diabetes, antiplatelet therapy, and Charlson comorbidity index); and Model 3 has Vit-D (serum Vit-D level in Model 3a, Vit-D deficiency in Model 3b, severe Vit-D deficiency in Model 3c), age, male sex, and clinical/laboratory parameters varying significantly according to the in-hospital prognosis (i.e., CURB-65 score, PaO2/FiO2, CRP, lactate dehydrogenase, and estimated glomerular filtration rate). In none of the three models, Vit-D was significantly associated with ICU admission/in-hospital death (Table 3 ). Independent predictors of ICU admission/in-hospital death were type 2 diabetes in Model 2 (P = 0.015, 0.016, and 0.015 in Models 2a, 2b, and 2c, respectively) and PaO2/FiO2 in Model 3 (P = 0.007, 0.006, and 0.006 in Models 3a, 3b, and 3c, respectively). Cox regression plots of the age- and sex-adjusted cumulative hazard of the composite outcome of ICU admission/in-hospital death according to Vit-D deficiency and severe Vit-D deficiency are reported in Figure 1 . No significant prospective association emerged between Vit-D (serum Vit-D level, Vit-D deficiency, severe Vit-D deficiency) and in-hospital death as single endpoint (Supplemental Table 2).
Table 3.
Risk of intensive care unit admission/in-hospital death according to Vit-D in patients with COVID-19
| Model 1* | 1a | HR | 95% CI | P-value |
| Vit-D | 1.013 | 0.991–1.035 | 0.240 | |
| 1b | HR | 95% CI | P-value | |
| Vit-D deficiency† | 0.783 | 0.427–1.436 | 0.430 | |
| 1c | HR | 95% CI | P-value | |
| Severe Vit-D deficiency‡ | 0.912 | 0.551–1.510 | 0.720 | |
| Model 2§ | 2a | HR | 95% CI | P-value |
| Vit-D | 1.015 | 0.992–1.037 | 0.202 | |
| 2b | HR | 95% CI | P-value | |
| Vit-D deficiency† | 0.778 | 0.420–1.441 | 0.424 | |
| 2c | HR | 95% CI | P-value | |
| Severe Vit-D deficiency‡ | 0.881 | 0.533–1.457 | 0.622 | |
| Model 3¶ | 3a | HR | 95% CI | P-value |
| Vit-D | 1.015 | 0.991–1.039 | 0.231 | |
| 3b | HR | 95% CI | P-value | |
| Vit-D deficiency† | 0.891 | 0.462–1.718 | 0.731 | |
| 3c | HR | 95% CI | P-value | |
| Severe Vit-D deficiency‡ | 0.841 | 0.490–1.441 | 0.528 |
CI, confidence interval; HR, hazard ratio.
Adjusted for age and male sex.
Vit-D <20 ng/mL.
Vit-D <12 ng/mL.
Adjusted for age, male sex, type 2 diabetes, antiplatelet therapy, and Charlson comorbidity index.
Adjusted for age, male sex, CURB-65 score, arterial partial pressure of oxygen/fraction of inspiration oxygen, C-reactive protein, lactate dehydrogenase, and estimated glomerular filtration rate.
Fig. 1.
Age- and sex-adjusted hazard of intensive care unit admission/in-hospital death according to (A) 25-hydroxyvitamin D deficiency and (B) severe 25-hydroxyvitamin D deficiency.
Comparison of Vit-D between patients with COVID-19 and COVID-19-negative controls
The main characteristics of age- and sex-balanced COVID-19 negative controls are reported in Supplementary Table 3. Serum Vit-D level was comparable between patients with COVID-19 and COVID-19-negative inpatients with sepsis (P = 0.397). Prevalence of Vit-D deficiency was 80% and 78% in patients with COVID-19 and COVID-19-negative inpatients with sepsis, respectively (P > 0.05 for comparison between the two groups). Prevalence of severe Vit-D deficiency was 53% and 50% in patients with COVID-19 and COVID-19-negative inpatients with sepsis, respectively (P > 0.05 for comparison between the two groups).
Discussion
In this prospective study of patients hospitalized for COVID-19, two main results emerged. First, patients with COVID-19 had comparable Vit-D levels to those of age- and sex-balanced COVID-19-negative inpatients with sepsis. Second, serum Vit-D level was not cross-sectionally associated with any of the clinical parameters of COVID-19 severity nor prospectively associated with the in-hospital prognosis of patients with COVID-19.
Prevalence of Vit-D deficiency in patients hospitalized with COVID-19
In line with the literature data [33,34], a high prevalence of Vit-D deficiency and severe Vit-D deficiency emerged in this cohort of patients hospitalized with COVID-19, with 80% and 53% of enrolled patients having shown these two conditions, respectively. However, the prevalence of Vit-D deficiency and severe Vit-D deficiency was not dissimilar to that observed in COVID-19-negative inpatients with sepsis. This finding suggests a possible pathophysiological link between Vit-D and infections. In this regard, two different albeit nonmutually exclusive speculations are plausible, with the first relating to a possible direct causality and the second to a possible reverse causation between Vit-D and infections.
With regard to the first hypothesis (i.e., direct causality), the state of Vit-D deficiency, possibly preexisting to the contact with pathogens, could affect an increased probability of getting both viral and bacterial infections. Indeed, evidence shows that Vit-D deficiency can promote different viral infections [35], including COVID-19 [12]. In addition, a significant association between hypovitaminosis D and increased susceptibility to sepsis has been reported [36].
However, although Vit-D plays an undoubted role in modulating the immune response to infections [10], the literature on this topic currently remains very controversial [37]. On the other hand, reverse causation also could explain the association between low serum Vit-D level and COVID-19. In this regard, a combination of factors characterizing the population affected by COVID-19 (e.g., preferential involvement of older age groups, state of profound debilitation and malnutrition related to the course of the disease, reduced sun exposure due to default isolation preceding and after hospitalization) [4,5,38,39] may contribute to the reduction in serum level of Vit-D. Partly supporting this interpretation, a nonspecific marker of nutritional status as reduced albumin, which is particularly prevalent among patients with COVID-19 [40], was directly correlated with Vit-D level in this study.
Nonetheless, regardless of the direction of the association between COVID-19 and hypovitaminosis D, this latter condition has been proposed to alter the homeostasis of numerous biologic processes, thereby possibly promoting the progression of the disease independently from its influence on immune response.
Vit-D and COVID-19 clinical course
In this study, we did not find any association between Vit-D and any clinical parameter of COVID-19 severity at the time of hospital admission or during hospitalization, including the presence of radiologic signs of pneumonia, respiratory insufficiency, respiratory distress, and the need for NIV. Also, serum Vit-D level, Vit-D deficiency, and severe Vit-D deficiency were not prospectively associated with the composite outcome of ICU admission/in-hospital death nor with in-hospital death as a single endpoint (Table 3; Supplementary Table 2). Conversely, as already reported in previous studies [41,42], PaO2/FiO2 and type 2 diabetes were also significant and independent predictors of a poor prognosis in this cohort of patients hospitalized with COVID-19. Our finding of the lack of an association between serum Vit-D level and severity of COVID-19 fits into the context of a series of conflicting observational studies with either a retrospective or prospective design exploring the association between Vit-D and COVID-19 severity/outcomes. Indeed, some retrospective studies in patients hospitalized for COVID-19 have found a significant association between low Vit-D level and either disease severity or in-hospital outcomes [43], [44], [45], but others have not confirmed this relationship [33,46]. Similarly, discrepant results have been highlighted in some prospective studies examining the association between low Vit-D level and either COVID-19 severity or prognosis [18,19,22,31,47]. Also, further fueling inconsistencies in the literature on Vit-D, in contrast with previous studies suggesting a possible J-shaped relationship between serum Vit-D and either cardiovascular mortality or all-cause mortality [48,49], we did not find any significant difference in rates of in-hospital death across Vit-D quintiles (Supplementary Fig. 2).
The reasons why the results of this study may diverge or agree with those from other studies may be the most varied. First, the prospective analysis of the association between Vit-D and COVID-19 prognosis, as performed in this study, provides more reliable results than those derived from retrospective studies due to the intrinsic limitations of the latter ones (e.g., impossibility of assessing the temporal relationship between the study variables, and potential confounding effect due to unmeasured variables). In addition, compared with other prospective studies that have documented a negative impact of Vit-D deficiency on the prognosis of patients with COVID-19, the population of this study was older [18,19,22,33,47], which might have masked a possible weak association between low Vit-D level and in-hospital prognosis. Furthermore, in this study, the period of observation lasted until the occurrence of ICU admission/in-hospital death or until the conclusion of hospitalization (i.e., on average 13 d for patients who were discharged alive). Therefore, a relatively short-term follow up might have compromised the possibility of detecting a long-term impact of Vit-D deficiency on COVID-19 prognosis. Based on these observations, our findings cannot be generalized to younger populations of patients with COVID-19 nor they can be extended to the relationship between Vit-D and long-term prognosis of patients with COVID-19.
However, discrepancies between previous studies and our study may, at least in part, be attributed to different methods of measuring circulating levels of Vit-D. To this regard, measurement of total serum Vit-D may have the limitation of underestimating possible interindividual differences in the proportion of free Vit-D (bioactive form) and Vit-D bound to the Vit-D binding protein (DBP). Indeed, a well-known high variability exists in DBP affinity for Vit-D, which can be affected by several DBP genetic variants and pathophysiological conditions [50]. In addition, DBP circulating levels can be significantly influenced by the activation of systemic inflammation [50]. Overall, the disagreement of the results from observational studies relating to the relationship between Vit-D level and COVID-19 prognosis mirrors discrepancies that emerged in the few intervention studies exploring the effects of Vit-D supplementation in patients with COVID-19 [23], [24], [25].
Limitations
Some limitations of this study deserve attention. First, the population examined in this study was numerically limited, confined to a relatively advanced age group, and followed for a relatively short period of hospitalization. Therefore, generalizability of the observed results to other clinical settings must be considered with extreme caution. Second, the relatively small sample size that was included in this interim analysis may undermine the reliability of the observed results. Third, the assay we used to measure serum Vit-D has the intrinsic limitation of accounting only for total Vit-D, which is mostly bound to DBP. Accordingly, some factors influencing circulating DBP levels, including systemic inflammation [50], may have confounded Vit-D measurements. Such a limitation might be overcome by demonstrating the replicability of the study results when also incorporating free Vit-D measures or, alternatively, pre-COVID-19 Vit-D measures from the same population. However, in the setting of our study, neither the option of measuring free Vit-D nor that of recording pre-COVID-19 Vit-D measures from the same population was available. Finally, the control population (i.e., COVID-19-negative inpatients with sepsis), albeit balanced in terms of age and sex with that of patient with COVID-19, was numerically too small to allow for an adequate adjustment of between-group differences in Vit-D for a consistent number of confounding factors. However, comparable results have been previously reported for the prevalence of hypovitaminosis D in populations similar to our control group [51,52].
Conclusions
In patients with COVID-19 hospitalized in an internal medicine ward, the prevalence of Vit-D deficiency is extremely high but not dissimilar to that seen in COVID-19-negative patients hospitalized for sepsis. Low levels of Vit-D neither highlight more severe COVID-19 nor predict an unfavorable in-hospital prognosis. Although the long-term prognostic value of Vit-D and the clinical impact of Vit-D supplementation in COVID-19 remain uncertain, our data do not support the utility of Vit-D measurement for the prognostic stratification of patients hospitalized with COVID-19.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.nut.2021.111408.
Appendix. Supplementary materials
References
- 1.World Health Organization . 2020. Official updates - Coronavirus disease.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at: Accessed April 1, 2021. [Google Scholar]
- 2.Bianconi V, Violi F, Fallarino F, Pignatelli P, Sahebkar A, Pirro M. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19? Drugs. 2020;80:1383–1396. doi: 10.1007/s40265-020-01365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a U.S. national sample of patients with COVID-19. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29058. Erratum in: JAMA Netw Open 2021;4:e2036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguye NT, Chinn J, Nahmias J, Yuen S, Kirby KA, Hohmann S, et al. Outcomes and mortality among adults hospitalized with COVID-19 at U.S. medical centers. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hägg S, Jylhävä J, Wang Y, Xu H, Metzner C, Annetorp M, et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559. doi: 10.1016/j.jamda.2020.08.014. .e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 7.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012;523:95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson PH. Vitamin D activity and metabolism in bone. Curr Osteoporos Rep. 2017;15:443–449. doi: 10.1007/s11914-017-0394-8. [DOI] [PubMed] [Google Scholar]
- 9.Untersmayr E, Kallay E. Insights in immuno-nutrition: Vitamin D as a potent immunomodulator. Nutrients. 2020;12:3554. doi: 10.3390/nu12113554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop E, Ismailova A, Dimeloe SK, Hewison M, White JH. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus. 2020;5:e10405. doi: 10.1002/jbm4.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz J, Yue S, Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. 2021;84 doi: 10.1016/j.nut.2020.111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N, Sun J, Wang X, Zhang T, Zhao M, Li H. Low vitamin D status is associated with coronavirus disease 2019 out-comes: A systematic review and meta-analysis. Int J Infect Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazemi A, Mohammadi V, Aghababaee SK, Golzarand M, Clark CCT, Babajafari S. Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: A systematic review and meta-analysis. Adv Nutr. 2021:nmab012. doi: 10.1093/advances/nmab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teshome A, Adane A, Girma B, Mekonnen ZA. The impact of vitamin D level on COVID-19 infection: Systematic review and meta-analysis. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelidi AM, Belanger MJ, Lorinsky MK, Karamanis D, Chamorro-Pareja N, Ognibene J, et al. Vitamin D status is associated with in-hospital mortality and mechanical ventilation: A cohort of COVID-19 hospitalized patients. Mayo Clin Proc. 2021;S0025–6196(21) doi: 10.1016/j.mayocp.2021.01.001. 00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennouar S, Cherif AB, Kessira A, Bennouar DE, Abdi S. Vitamin D deficiency and low serum calcium as predictors of poor prognosis in patients with severe COVID-19. J Am Coll Nutr. 2021;40:104–110. doi: 10.1080/07315724.2020.1856013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tehrani S, Khabiri N, Moradi H, Mosavat MS, Khabiri SS. Evaluation of vitamin D levels in COVID-19 patients referred to Labafinejad hospital in Tehran and its relationship with disease severity and mortality. Clin Nutr ESPEN. 2021;42:313–317. doi: 10.1016/j.clnesp.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pizzini A, Aichner M, Sahanic S, Böhm A, Egger A, Hoermann G, et al. Impact of vitamin D deficiency on COVID-19-A prospective analysis from the CovILD registry. Nutrients. 2020;12:2775. doi: 10.3390/nu12092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van den Abbeele K, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-138712. postgradmedj-2020–138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastie CE, Pell JP, Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur J Nutr. 2021;60:545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendy A, Apewokin S, Wells AA, Morrow AL. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients. medRxiv 2020;2020.06.25.20137323.
- 22.Jevalikar G, Mithal A, Singh A, Sharma R, Farooqui KJ, Mahendru S, et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci Rep. 2021;11:6258. doi: 10.1038/s41598-021-85809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannini S, Passeri G, Tripepi G, Sella S, Fusaro M, Arcidiacono G, et al. Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: A hypothesis-generating study. Nutrients. 2021;13:219. doi: 10.3390/nu13010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: A meta-analysis. QJM. 2021;114:175–181. doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, et al. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirro M, Francisci D, Bianconi V, Schiaroli E, Mannarino MR, Barsotti F, et al. NUtraceutical TReatment for hYpercholesterolemia in HIV-infected patients: The NU-TRY(HIV) randomized cross-over trial. Atherosclerosis. 2019;280:51–57. doi: 10.1016/j.atherosclerosis.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Schillaci G, Mannarino MR, Pucci G, Pirro M, Helou J, Savarese G, et al. Age-specific relationship of aortic pulse wave velocity with left ventricular geometry and function in hypertension. Hypertension. 2007;49:317–321. doi: 10.1161/01.HYP.0000255790.98391.9b. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12:2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institutes of Health. COVID-19 treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed xxx. [PubMed]
- 33.Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2021;106:e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PubMed] [Google Scholar]
- 34.Pinzon RT, Angela Pradana AW. Vitamin D deficiency among patients with COVID-19: Case series and recent literature review. Trop Med Health. 2020;48:102. doi: 10.1186/s41182-020-00277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: A systematic review and meta-analysis. BMC Anesthesiol. 2015;15:84. doi: 10.1186/s12871-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin R. Sorting out whether vitamin D deficiency raises COVID-19 risk. JAMA. 2021;325:329–330. doi: 10.1001/jama.2020.24127. [DOI] [PubMed] [Google Scholar]
- 38.Anker MS, Landmesser U, von Haehling S, Butler J, Coats AJS, Anker SD. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12:9–13. doi: 10.1002/jcsm.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaia G, Diémoz H, Maluta F, Fountoulakis I, Ceccon D, di Sarra A, et al. Does solar ultraviolet radiation play a role in COVID-19 infection and deaths? An environmental ecological study in Italy. Sci Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Violi F, Cangemi R, Romiti GF, Ceccarelli G, Oliva A, Alessandri F, et al. Is albumin predictor of mortality in COVID-19? Antioxid Redox Signal. 2020;35:139–142. doi: 10.1089/ars.2020.8142. [DOI] [PubMed] [Google Scholar]
- 41.Bellan M, Patti G, Hayden E, Azzolina D, Pirisi M, Acquaviva A, et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patients. Sci Rep. 2020;10:20731. doi: 10.1038/s41598-020-77698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. .e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo X, Liao Q, Shen Y, Li H, Cheng L. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people [corrected] J Nutr. 2021;151:98–103. doi: 10.1093/jn/nxaa332. Erratum in: J Nutr 2021;151:742–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayramoğlu E, Akkoç G, Ağbaş A, Ö Akgün, Yurdakul K, Selçuk Duru HN, et al. The association between vitamin D levels and the clinical severity and inflammation markers in pediatric COVID-19 patients: Single-center experience from a pandemic hospital. Eur J Pediatr. 2021:1–7. doi: 10.1007/s00431-021-04030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charoenngam N, Shirvani A, Reddy N, Vodopivec DM, Apovian CM, Holick MF. Association of vitamin D status with hospital morbidity and mortality in adult hospitalized patients with COVID-19. Endocr Pract. 2021 doi: 10.1016/j.eprac.2021.02.013. S1530–891X(21)00057–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walk J, Dofferhoff ASM, van den Ouweland JMW, van Daal H, Janssen R. Vitamin D – contrary to vitamin K – does not associate with clinical outcome in hospitalized COVID-19 patients. medRxiv 2020;2020.11.07.20227512.
- 47.Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10:20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durup D, Jørgensen HL, Christensen J, Tjønneland A, Olsen A, Halkjær J, et al. A reverse J-shaped association between serum 25-hydroxyvitamin D and cardiovascular disease mortality: The CopD Study. J Clin Endocrinol Metab. 2015;100:2339–2346. doi: 10.1210/jc.2014-4551. [DOI] [PubMed] [Google Scholar]
- 49.Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, Yetley EA, Looker AC, Schleicher RL, et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab. 2013;98:3001–3009. doi: 10.1210/jc.2013-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol (Lausanne) 2019;10:317. doi: 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen HB, Eshete B, Lau KH, Sai A, Villarin M, Baylink D. Serum 1,25-dihydroxyvitamin D: An outcome prognosticator in human sepsis. PLoS One. 2013;8:e64348. doi: 10.1371/journal.pone.0064348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratzinger F, Haslacher H, Stadlberger M, Schmidt RL, Obermüller M, Schmetterer KG, et al. 25(OH)D and 1,25(OH)D vitamin D fails to predict sepsis and mortality in a prospective cohort study. Sci Rep. 2017;7:40646. doi: 10.1038/srep40646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.