Dear editor,
Efficacy of corticosteroids in COVID-19 pneumonia has been reported in numerous studies.1 However, Yang et al. concluded that corticosteroids have a negative impact, suggesting that not all patients benefit from the therapy.2 Features of organizing pneumonia (OP) have been observed in radiological and histopathological studies from these patients.3, 4, 5 OP has usually a good response to corticosteroids. The possible correlation between the radiological pattern and clinical evolution has important implications. This study provides information on patient selection and clinical application of corticosteroids.
Adult patients admitted to a respiratory intermediate care unit (RICU) from November 18th, 2020 to February 18th, 2021 were prospectively enrolled. Institutional review boards authorised the study. Informed consent was waived.
A confirmed case of COVID-19 pneumonia was defined as a patient with compatible symptoms and PCR-confirmed infection. Only patients needing advanced noninvasive respiratory support (NIRS) with high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) and/or noninvasive ventilation (NIV) were included. All patients had a “do intubate” (DI) or “do not intubate” (DNI) order defined at admission. Information regarding demographics, comorbidities, blood test results and mode and usage of NIRS were recorded. The severity of respiratory failure was assessed by PaO2/FiO2 ratio before NIRS institution. Several outcomes were evaluated: length of stay, need for endotracheal intubation (ETI) and in-hospital mortality.
OP was diagnosed, based on radiology reports, in the presence of bilateral patchy consolidation areas with subpleural and/or peribronchial distribution; perilobular pattern, with thick, ill-defined linear opacities with a polygonal or arcade appearance; and/or reverse halo sign.3 Bacterial infection was excluded by clinical evaluation, procalcitonin levels, urinary antigens and blood cultures at admission. Pulmonary embolism was excluded using D-dimer levels and, if increased, CT pulmonary angiogram.
OP was treated, according to BTS 2008 guidelines6: methylprednisolone 500–1000 mg for 3–5 days, followed by 0.75–1 mg/Kg prednisone. Patients improving under 6 mg dexamethasone were switched to prednisone and methylprednisolone pulses were used as a rescue therapy if clinical deterioration and FiO2≥35%. GGO group was treated with 6 mg dexamethasone.7 Side effects were recorded.
Baseline characteristics of patients with OP and GGO were compared. Continuous and categorical variables were compared using Student's T test and Chi-squared or Fisher's exact test, respectively. The association between OP and outcomes was calculated using a logistic regression model adjusted for age, CCI, DNI order and PaO2/FiO2. A two-sided test of <0.05 was considered statistically significant.
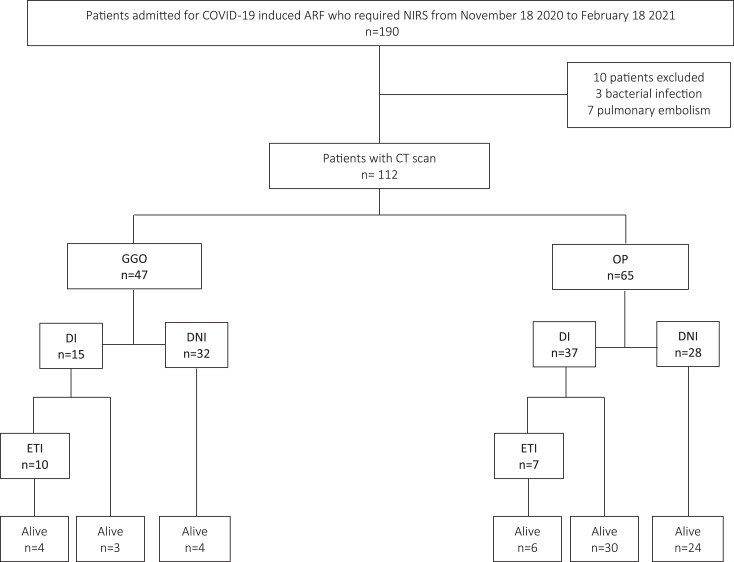
190 patients were admitted to RICU. At the time of the study, the hospital was attending 10% of the national cases of COVID-19 needing hospitalization. Due to this burden, CT scan was performed in 112. Fig. 1 illustrates patients’ allocation according to CT scan findings and outcomes. Table 1 lists patients characteristics and clinical outcomes according to cohort. Male gender was predominant and the mean age was 67.5 ± 12.1 years. OP group was significantly younger. The PaO2/FiO2 ratio was higher in OP group.
Fig. 1.
Patient allocation according to chest CT scan findings.
ARF: acute hypoxic respiratory failure; NIRS: noninvasive respiratory support; CT: computed tomography; GGO: ground glass opacities; OP: organizing pneumonia; DI: “do intubate”order; DNI: “do not intubate” order; ETI: endotracheal intubation.
Table 1.
General characteristics and clinical outcomes according to cohort.
| Demographic and clinical characteristics | ||||
|---|---|---|---|---|
| Total | Cohort |
p-value | ||
| GGO | OP | |||
| Patients | 112 | 47 | 65 | |
| Age, years | 67.5 (12.1) | 73.1 (9.0) | 63.4 (12.4) | <0.001 |
| Male | 76 (67.9) | 35 (74.5) | 41 (63.1) | 0.203 |
| CCI, points | 3.4 (1.9) | 3.8 (1.6) | 3.1 (2.1) | 0.069 |
| DNI order | 60 (53.6) | 32 (68.1) | 28 (43.1) | 0.009 |
| Length of symptoms, days | 8.5 (3.7) | 8.0 (3.5) | 8.9 (3.9) | 0.241 |
| Laboratory findings | ||||
| Leucocytes, 10⁹ cells per L | 7.9 (4.1) | 9.0 (5.1) | 7.1 (2.9) | 0.012 |
| Lymphocyte, 10⁹ cells per L | 0.9 (0.4) | 0.9 (0.4) | 1.0 (0.4) | 0.322 |
| C-reactive protein, mg/L | 152.6 (79.9) | 171.3 (82.4) | 139.0 (75.8) | 0.034 |
| Procalcitonin, ng/mL | 0.5 (0.7) | 0.6 (1.0) | 0.4 (0.5) | 0.076 |
| Ferritin>1500 ng/mL | 32 (30.8) | 15 (35.7) | 17 (27.4) | 0.037 |
| Lactate dehydrogenase, U/L | 444.1 (174.2) | 485.9 (178.5) | 413.9 (165.9) | 0.030 |
| Interleukin-6, pg/mL | 138.4 (336.1) | 228.6 (513.8) | 80.2 (96.1) | 0.025 |
| D-dimer, μg/mL | 2407.0 (8239.4) | 3032.3 (10,023.3) | 1967.3 (6762.8) | 0.509 |
| PaO2/FiO2, mmHg | 94.9 (35.1) | 86.3 (29.5) | 101.8 (37.9) | 0.024 |
| NIRS | ||||
| HFNC | 95 (67.0) | 37(78.7) | 58 (89.2) | 0.126 |
| CPAP/NIV | 37 (33.0) | 19 (40.4) | 18 (27.7) | 0.157 |
| Treatment | ||||
| Corticosteroids | 111 (99.1) | 46 (97.9) | 65 (100.0) | 0.420 |
| Remdesivir | 7 (6.3) | 3 (6.4) | 4 (6.2) | 0.961 |
| Clinical outcomes and relative probability | ||||
|---|---|---|---|---|
| Total | Cohort |
p-value | ||
| OP | OR (95% CI) | |||
| Length of stay, days | 15.4 (12.1) | 15.6 (9.6) | 1.000 (1.000–1.000) | 0.495 |
| ETI | ||||
| Crude | 17 (18.3) | 7 (10.8) | 0.08 (0.02–0.39) | 0.002 |
| Adjusted# | 0.06 (0.01–0.44) | 0.003 | ||
| Hospital mortality | 41 (36.6) | 5 (7.7) | ||
| Crude | 0.03 (0.01–0.08) | <0.001 | ||
| Adjusted# | 0.03 (0.01–0.11) | <0.001 | ||
Data are presented as n (%) or mean (SD). GGO: ground glass opacities; OP: organizing pneumonia; CCI: Charlson comorbidity index; DNI: “do not intubate”; PaO2/FiO2: arterial oxygen tension /inspiratory oxygen fraction; NIRS: noninvasive respiratory support; HFNC: high flow nasal cannula; CPAP: continuous positive airway pressure; NIV: nonivasive ventilation; ETI: endotracheal intubation.
: adjusted for age, CCI, DNI order and arterial oxygen tension/inspiratory oxygen fraction ratio.
All patients were treated with corticosteroids. Mean time to dexamethasone was 8.5 ± 3.5 days and to OP treatment was 13.0 ± 5.5 days. 75.4% of OP patients were treated with pulse methylprednisolone and the remaining with prednisone 0.75–1 mg/Kg.
There were no reports of bacterial infection in patients treated with NIRS but 10 patients who needed invasive mechanical ventilation had hospital acquired infection (3/7 in OP group vs. 7/10 in GGO group, p = 0.350). Psychosis occurred in 2 patients with OP under methylprednisolone pulses. There were no records of acute diabetic complications or gastrointestinal hemorrhage.
Length of stay was similar between groups. 25.9% needed ICU admission (24.6% in OP group vs. 31.9% in GGO group, p = 0.394). The global in-hospital mortality was 36.6%, 76.6% in GGO group vs. 8.6% in the OP group (p < 0.001). OP was associated with a significant reduction in need for ETI and in-hospital mortality after adjustment for confounders.
To our knowledge this is the first prospective study to evaluate OP outcomes. Since RECOVERY, many clinical trials have stablished the benefit of corticosteroids in COVID-19 patients.1 , 7 However, the specific mechanisms of corticosteroids in treating COVID-19 are not totally understood. The hypothesis that OP in COVID-19 is more frequent than in other viral infections may explain this steroid-responsive effect. We found OP in almost 60% of our patients.
PaO2/FiO2 ratios were extremely low and more than 50% of our patients received a “do not intubate” order, supporting the severity of acute illness and baseline frailty of our population. This might justify the global in-hospital mortality rate. Interestingly, the need for ETI and mortality rate was significantly lower in the OP group, even after adjustment for age, comorbidities, “DNI order” and severity of acute respiratory distress syndrome. Additionally, biological markers associated with worse outcomes, such as leucocyte count, C-reactive protein, lactate dehydrogenase, interleukin-6 and ferritin levels,8 were significantly lower in the OP group. These results support the theory that OP presence is by itself an independent predictor of good prognosis. In fact, literature reports that approximately 70% of OP patients will respond to corticosteroids.9
Identifying and timely treating this condition may avoid unnecessary ETI and drastically reduce mortality. An important concern is that the increasingly adopted dexamethasone protocol may be insufficient, especially in critical care patients, as OP management requires higher doses, prolonged treatment and careful tapering.6 Considering the critically ill nature of our patients, the positive impact on outcomes and the absence of significant side effects, this approach seems to be beneficial. Nevertheless, the adequate minimal dose of corticosteroids for COVID-19 OP are unknown, particularly because of the secondary nature of the disease.
One limitation of this study is the absence of histopathologic confirmation. Second, as all OP patients were treated, we cannot guarantee that the benefit found was due to OP by it-self or due to the use of high dose of corticosteroids. Finally, larger studies are necessary to confirm these results.
In conclusion, our study showed that radiological features of OP are frequent. Critically ill patients should undergo a chest CT scan in order to identify this condition as treatment with high doses of corticosteroids seems to reduce the need for ETI and hospital mortality.
Declaration of Competing Interest
None declared.
Acknowledgments
Funding
None.
Acknowledgments
Dr. Francisco Manuel Pereira Silva, Department of Radiology, Centro Hospitalar Tâmega e Sousa, Penafiel, Portugal.
References
- 1.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19 a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. JulEpub 2020 Apr 10. PMID: 32283144; PMCID: PMC7195158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao C., Liu X., Zhang H., Li Y., Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17:701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020:1–11. doi: 10.1038/s41379-020-0603-3. [published online ahead of print, 2020 Jun 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Jin C., Wu C.C., Zhao H., Liang T., Liu Z. Organizing pneumonia of COVID-19: time-dependent evolution and outcome in CT findings. PLoS ONE. 2020;15(11) doi: 10.1371/journal.pone.0240347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley B., Branley H.M., Egan J.J., Greaves M.S., Hansell D.M., Harrison N.K., Hirani N., Hubbard R., Lake F., Millar A.B., Wallace W.A., Wells A.U., Whyte M.K., Wilsher M.L. British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee; Thoracic Society of Australia; New Zealand Thoracic Society; Irish Thoracic Society. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]; Erratum in: Thorax 2008;63(11):1029.
- 7.Horby P., Lim W.S., Emberson J.R. Recovery: collaborative group, dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordier J.F. Cryptogenic organising pneumonia. Eur Respir J. 2006;28:422. doi: 10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]