Abstract
Delay in time to esophagectomy for esophageal cancer has been shown to have worse peri-operative and long-term outcomes. We hypothesized that COVID-19 would cause a delay to surgery, with worse perioperative outcomes, compared to standard operations. All esophagectomies for esophageal cancer at a single institution from March-June 2020, COVID-19 group, and from 2019 were reviewed and peri-operative details were compared between groups. Ninety-six esophagectomies were performed in 2019 vs 37 during March-June 2020 (COVID-19 group). No differences between groups were found for preoperative comorbidities. Wait-time to surgery from final neoadjuvant treatment was similar, median 50 days in 2019 vs 53 days during COVID-19 p = 0.601. There was no increased upstaging, from clinical stage to pathologic stage, 9.4% in 2019 vs 7.5% in COVID-19 p = 0.841. Fewer overall complications occurred during COVID-19 vs 2019, 43.2% vs 64.6% p = 0.031, but complications were similar by specific grades. Readmission rates were not statistically different during COVID-19 than 2019, 16.2% vs 10.4% p = 0.38. No peri-operative mortalities or COVID-19 infections were seen in the COVID-19 group. Esophagectomy for esophageal cancer was not associated with worse outcomes during the COVID-19 pandemic with minimal risk of infection when careful COVID-19 guidelines are followed. Prioritization is recommended to ensure no delays to surgery.
Keywords: COVID-19, Esophageal cancer, Esophagectomy, Delays of surgery
Abbreviations: COVID-19, Coronavirus Disease 2019
Graphical abstract
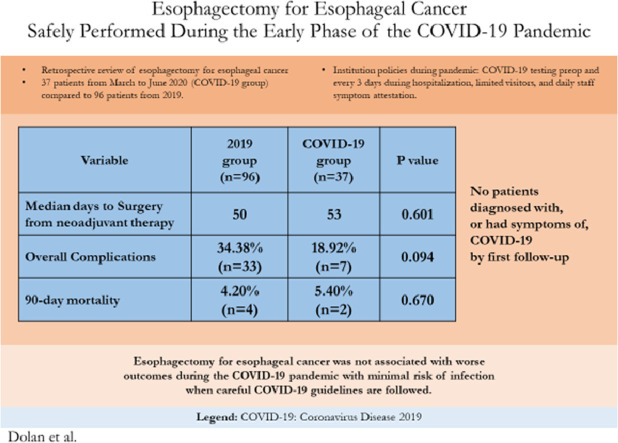
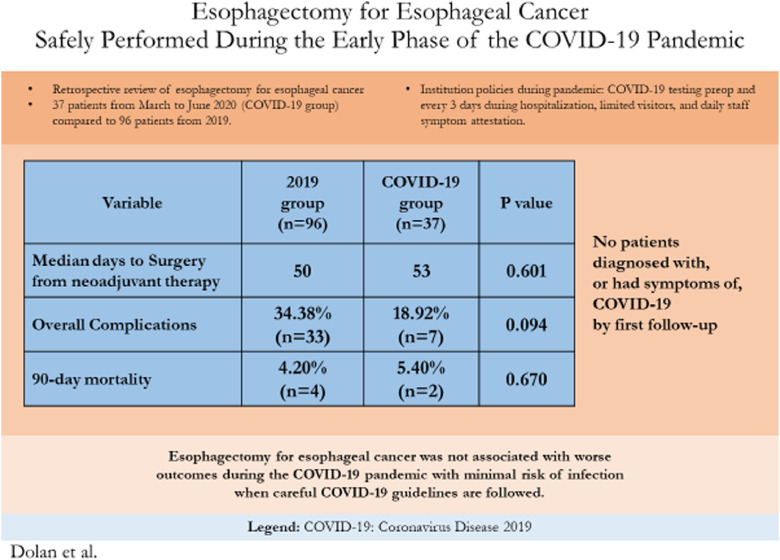
Summary of findings of Esophagectomies for Esophageal Cancer performed during the early months of the Coronavrius-19 pandemic compared to cases from 2019.
Key Findings for Esophagectomies Performed During the Early COVID-19 Pandemic.
Alt-text: Unlabelled box
Central Message.
Esophagectomy for esophageal cancer can safely be performed during the COVID-19 pandemic.
Alt-text: Unlabelled box
Perspective Statement.
Delays in esophagectomy for esophageal cancer lead to worsened long-term outcomes. With the ongoing challenges of the COVID-19 pandemic we demonstrate that, with appropriate precautions, esophagectomies can proceed with similar short-term outcomes and minimal risk of COVID-19 infection. Prioritization of surgical care for these patients is recommended.
Alt-text: Unlabelled box
INTRODUCTION
Coronavirus-19 has significantly affected healthcare globally. The safety of surgery and postoperative care for esophageal cancer patients who undergo esophagectomy during the pandemic is of particular concern due to the virus's multisystem effects that may impact the operation.1 , 2 During the coronavirus pandemic the screening, administration of chemotherapy, and surgical management of multiple cancers has been shown to be heavily impacted.3 Due to the aggressive nature of esophageal cancer, delays in treatment are not advised and esophageal cancer patients have priority when resources are available.4 However, patients who undergo neoadjuvant chemoradiation therapy typically require a brief recovery period before proceeding to definitive surgery. The ideal duration between neoadjuvant therapy and surgery remains under debate both in terms of peri-operative outcomes and long-term survival and is of paramount importance during the ongoing pandemic.5 , 6
We hypothesized that esophagectomies performed during the COVID-19 pandemic would be delayed with more complications and worse peri-operative complications and mortality compared to one year before the pandemic.
METHODS
The Institutional Review Board (IRB) or equivalent ethics committee of the Brigham and Women's Hospital approved the study protocol and publication of data. Patient written consent for the publication of the study data was waived by the IRB due to the retrospective nature of the study. Retrospective review of the charts of all patients who underwent esophagectomy for esophageal cancer at our institution from January 1, 2019, to December 31, 2019 (pre-COVID-19 group) and March 1, 2020 to June 30, 2020 (COVID-19 group) was performed. After June 30, 2020, the monthly rate of esophagectomies returned to pre-pandemic levels and differences were no longer expected to be observed. Esophagectomies performed for benign conditions were excluded. Demographic and comorbidity variables included were age, gender, body mass index (BMI), Barrett's esophagus, pre-operative atrial fibrillation, history of other cancer, congestive heart failure, coronary artery disease, chronic obstructive pulmonary disease, hypertension, diabetes, and smoking history. Clinical stage by endoscopic ultrasound, neoadjuvant treatment status, and time to surgery from either last neoadjuvant treatment or clinic visit (in the case of patients who did not undergo neoadjuvant therapy) were also collected. All operations were performed via a McKeown or Ivor Lewis technique. Surgical approach was categorized as open, minimally invasive (thoracoscopy or laparoscopy), or robotic based on the final technique when the operation was concluded. Overall approach was classified as open if both the thoracic and abdominal portions were via open technique, minimally invasive if both the thoracic and abdominal portions were via minimally invasive or robotic technique, or hybrid if there was a combination of minimally invasive and open techniques. Pathologic variables included histology, margins, lymph node details, microscopic invasion details, pathologic stage, and change of clinical stage to pathologic stage. Outcomes examined were initial intensive care unit length of stay, overall complications and complications by Clavien-Dindo grade, intensive care unit readmission, any additional procedures performed postoperatively, and mortality out to 90 days after surgery. Complications were graded on the Clavien-Dindo system of surgical complications; Grade III and higher was considered significant.7 Time to first follow-up was collected for the COVID-19 group.
Analysis
Values were compared between groups using Chi-square test, Wilcoxon rank-sum test, and Fisher's exact test, where appropriate. A p value equal to or less than 0.05 was considered significant. All statistical tests were performed with STATA version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP)
Institutional Changes to Respond to Coronavirus-19 infection
As part of our institutional pandemic response, all surgical patients were required to have a negative COVID-19 test within 72 hours before surgery and were directed to self-quarantine from the time of their COVID test until day of surgery. Hospital staff completed daily symptom attestations before work and were required to wear masks while in the hospital. Visitors were restricted to one two-hour visit with a single visitor between 1-8PM daily. No visitors were allowed in the ICU. Esophagectomy patients were in the same ICU as COVID-19 patients at the start of the study prior to creation of COVID-19 ICUs. Every patient was tested every 3 days for COVID-19 during their inpatient stay and each patient had a private room.
RESULTS
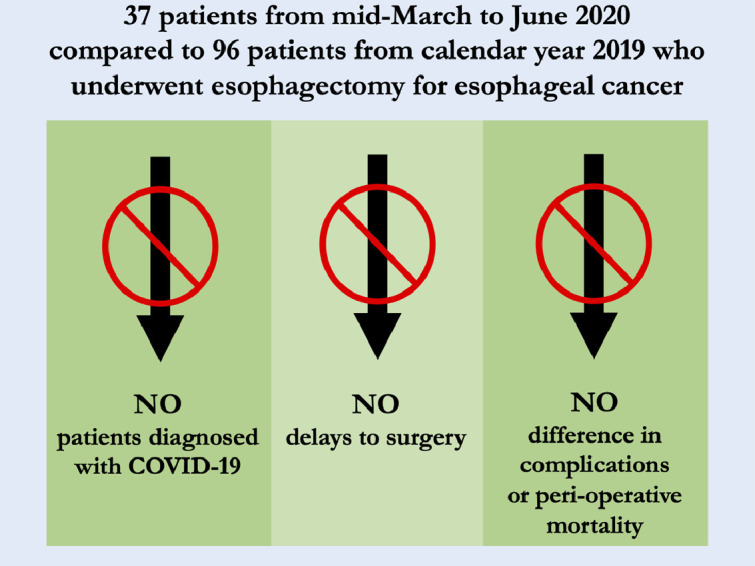
Thirty-seven esophagectomies were performed between March and June at our institution vs 96 in 2019. Four cases were done during mid-March to mid-April 2020 when the operating rooms were reserved only for emergency cases and the institution had the largest number of COVID-19 patients in the hospital. These cases were performed only after careful discussion with the patient, referring doctors, and operating room administrators. During this study, no patient was diagnosed with, or had COVID-19 symptoms during their hospitalization or by first follow-up.
Age, gender, BMI, Barrett's esophagus, hypertension, pre-operative atrial fibrillation, history of other cancer, congestive heart failure, coronary artery disease, chronic obstructive pulmonary disease, diabetes, and smoking history were similar between groups, Table 1 . Clinical stages were similar between groups. Both groups had similar rates of neoadjuvant chemoradiation (89.6% in 2019 vs 94.6% during COVID-19, p = 0.509). Median time to surgery from last neoadjuvant treatment was similar between groups (50 days in 2019 vs 53 days during COVID-19 p = 0.601).
Table 1.
Esophagectomy for Esophageal Cancer during COVID-19 Pandemic Compared to 2019: Pre-operative Demographics, Comorbidities, Clinical Staging, Neoadjuvant Treatment, and Time to Surgery
| 2019 Group |
COVID-19 Group |
p-value | ||||
|---|---|---|---|---|---|---|
| Variable | Number of patients (n total = 96) | % | Number of patients (n total = 37) | % | ||
| Age, years | Median (IQR) | 66.35 | 60.3, 71.7 | 66.34 | 59.4, 72.9 | 0.952 |
| Gender, Male | 78 | 81.25 | 32 | 86.49 | 0.612 | |
| Body Mass Index (BMI) | Median (IQR) | 28.0 | 24.4, 32.3 | 28.0 | 24.8, 32.3 | 0.914 |
| Barrett's esophagus | 27 | 28.13 | 5.00 | 13.51 | 0.112 | |
| Pre-operative Atrial Fibrillation | 14 | 14.58 | 3.00 | 8.11 | 0.396 | |
| History of Other Cancer | 18 | 18.75 | 10.00 | 27.03 | 0.344 | |
| Congestive Heart Failure | 4 | 4.17 | 0.00 | 0.00 | 0.576 | |
| Coronary Artery Disease | 13 | 13.54 | 4.00 | 10.81 | 0.779 | |
| Chronic Obstructive Pulmonary Disease | 12 | 12.50 | 5.00 | 13.51 | 1.00 | |
| Hypertension | 62 | 64.58 | 17.00 | 45.95 | 0.075 | |
| Diabetes | 15 | 15.63 | 8.00 | 21.62 | 0.447 | |
| Smoker | Never | 21 | 21.88 | 8.00 | 21.62 | 0.678 |
| Current | 19 | 19.79 | 10.00 | 27.03 | ||
| Former | 56 | 58.33 | 19.00 | 51.35 | ||
| Clinical Stage | Stage I | 6.0 | 6.3 | 3.0 | 8.1 | 0.708 |
| (EUS) | Stage II | 10.0 | 10.4 | 3.0 | 8.1 | 1.000 |
| Stage III | 40.0 | 41.7 | 23.0 | 62.2 | 0.052 | |
| Stage IVa | 9.0 | 9.4 | 2.0 | 5.4 | 0.727 | |
| Stage IVb | 2.0 | 2.1 | 1.0 | 2.7 | 1.000 | |
| Not Reported | 29.0 | 30.2 | 5.0 | 13.5 | 0.074 | |
| Neoadjuvant Chemoradiation Therapy | 86 | 89.58 | 35 | 94.59 | 0.509 | |
| Chemotherapy Only | 1 | 1.04 | 0 | 0 | ||
| Radiation Therapy Only | 0 | 0 | 0 | 0 | ||
| Surgery Only | 9 | 9.38 | 2 | 5.41 | ||
| Time to Surgery, days | Median (IQR) | 50 | 40, 67 | 53 | 37.5, 63.5 | 0.601 |
| from Last Dose of Neoadjuvant Treatment | ||||||
COVID-19, coronavirus disease 2019; COVID-19 group, esophagectomy patients from March-June 2020; 2019 group, esophagectomy patients from calendar year 2019; IQR, interquartile range for non-normal distribution data with 25th and 75th percentiles; BMI, body mass index in kg/m2; EUS, endoscopic ultrasound.
All cases were done via McKeown or Ivor Lewis technique in both groups with approximately 70% being performed with Ivor Lewis technique, Table 2 . There were trends of more minimally invasive operations during 2019 (83.3% vs 73.0%, p = 0.223) and more COVID-19 group operations completed via a hybrid surgical approach (21.6% patients during COVID-19 vs 8.3% in 2019, p = 0.070). More cases had a robotic abdominal approach in 2019 (19.8% vs 2.7% p=0.013) but conversion rates were similar between groups, Table 2. Median tumor size was larger during COVID-19 (4.2 cm vs 3.0 cm in 2019, p < 0.01). Rates of downstaging, from clinical stage to pathologic staging, and upstaging were no different between groups with approximately 60% of patients in both groups being down staged from their pre-operative clinical stage. Initial ICU stay was a median of 2 days in both groups but with more long duration stays in 2019, Table 3 . Median length of stay was shorter during COVID-19 (7 vs 9 days in 2019, p < 0.01). Overall complications and Grade IV, and V complications were no different between groups. Grade III complications were trended towards being more common in 2019 but did not reach significance (33.3% vs 16.2% COVID-19, p = 0.056). Anastomotic leak rate was 10.8% during COVID-19 vs 6.3% during 2019, p = 0.464. Additional postop procedure rate, 30-day mortality and 90-day mortality were similar between groups. Readmission rate was 16.2% during COVID-19 vs 10.4% in 2019, p = 0.38.
Table 2.
Esophagectomy for Esophageal Cancer during COVID-19 Pandemic Compared to 2019: Operative Details, Final Pathology and Staging
| 2019 Group |
COVID-19 Group |
p-value | ||||
|---|---|---|---|---|---|---|
| Variable | Number of patients (n total = 96) | % | Number of patients (n total = 37) | % | ||
| Surgical Technique | McKeown | 29.0 | 30.2 | 10.0 | 27.0 | 0.833 |
| Ivor Lewis | 67.0 | 69.8 | 27.0 | 73.0 | ||
| Approach - Overall | MIE | 80.0 | 83.3 | 27.0 | 73.0 | 0.223 |
| Open | 8.0 | 8.3 | 2.0 | 5.4 | 0.726 | |
| Hybrid- open and MIE | 8.0 | 8.3 | 8.0 | 21.6 | 0.070 | |
| Approach - Thoracic | MIE | 64.0 | 66.7 | 21.0 | 56.8 | 0.318 |
| Open | 12.0 | 12.5 | 4.0 | 10.8 | 1.000 | |
| Robotic | 20.0 | 20.8 | 12.0 | 32.4 | 0.179 | |
| Approach - Abdominal | MIE | 65.0 | 67.7 | 28.0 | 75.7 | 0.407 |
| Open | 12.0 | 12.5 | 8.0 | 21.6 | 0.189 | |
| Robotic | 19.0 | 19.8 | 1.0 | 2.7 | 0.013 | |
| Convert-to-open | Thoracic | 3.0 | 3.1 | 1.0 | 2.7 | 0.842 |
| Abdominal | 1.0 | 1.0 | 1.0 | 2.7 | ||
| Both | 1.0 | 1.0 | 0.0 | 0.0 | ||
| Tumor Size, cm | Median (IQR) | 3 | 1.8, 3.6 | 4.2 | 3.3, 5.5 | <0.01 |
| Histology | Adenocarcinoma | 74 | 77.08 | 31 | 83.78 | 0.11 |
| Squamous Cell Carcinoma | 12 | 12.5 | 6 | 16.22 | ||
| Other | 10 | 10.42 | 0 | 0 | ||
| Lymph nodes, Sampled | Median (IQR) | 21 | 16, 27 | 25 | 17, 30 | 0.265 |
| Lymph nodes, Positive | Median (IQR) | 0 | 0, 1.3 | 0 | 0, 2 | 0.64 |
| Lymphovascular Invasion Present | 18 | 18.75 | 11 | 29.73 | 0.24 | |
| Perineural Invasion Present | 12 | 12.50 | 9 | 24.32 | 0.113 | |
| Pathologic Stage at Surgery | Stage I | 51 | 53.13 | 16 | 43.24 | 0.336 |
| Stage II | 12 | 12.50 | 6 | 16.22 | ||
| Stage III | 8 | 8.33 | 3 | 8.11 | ||
| Stage IVa | 22 | 22.92 | 8 | 21.62 | ||
| Stage IVb | 2 | 2.08 | 4 | 10.81 | ||
| Stage IVb M1 | 1 | 1.04 | 0 | 0.00 | ||
| n = 67 | % | n = 32 | % | |||
| Overall Change in Stage | Downstage | 43 | 64.18 | 19.00 | 59.38 | 0.841 |
| (Clinical Stage to Final Pathologic stage) | Upstage | 5 | 7.46 | 3.00 | 9.38 | |
| Same stage | 19 | 28.36 | 10.00 | 31.25 | ||
COVID-19, coronavirus disease 2019; COVID-19 group, esophagectomy patients from March-June 2020; 2019 group, esophagectomy patients from calendar year 2019; IQR, interquartile range for non-normal distribution data with 25th and 75th percentiles; MIE, minimally invasive esophagectomy
Table 3.
Esophagectomy for Esophageal Cancer during COVID-19 Pandemic Compared to 2019: Peri-operative Morbidity and Mortality
| 2019 Group |
COVID-19 Group |
p-value | ||||
|---|---|---|---|---|---|---|
| Variable | Number of patients (n total = 96) | % | Number of patients (n total = 37) | % | ||
| Initial Intensive Care Unit Length of Stay | Median (IQR) | 2.0 | 1, 3 | 2.0 | 1, 2 | 0.019 |
| Length of Stay, days | Median (IQR) | 9 | 8, 12 | 7 | 7, 10 | <0.01 |
| Post-operative Complications | 33 | 34.38 | 7 | 18.92 | 0.094 | |
| Grade III | 32 | 33.33 | 6 | 16.22 | 0.056 | |
| Grade IV | 9 | 9.38 | 5 | 13.51 | 0.533 | |
| Grade V | 2 | 2.08 | 1 | 2.70 | 1 | |
| Anastomotic Leak | 6.0 | 6.3 | 4.0 | 10.8 | 0.464 | |
| Intensive Care Unit Readmission | 8.0 | 8.3 | 3.0 | 8.1 | 1.000 | |
| Additional Post-operative Procedure | 25.0 | 26.0 | 10.0 | 27.0 | 1.000 | |
| Major Reoperation | 12.0 | 12.5 | 5.0 | 13.5 | 1.000 | |
| Minor Reoperation | 13.0 | 13.5 | 5.0 | 13.5 | 1.000 | |
| Readmitted within 30 days | 10.0 | 10.4 | 6.0 | 16.2 | 0.38 | |
| 30-day Mortality | 2 | 2.08 | 0 | 0 | 1 | |
| 90-day mortality | 4 | 4.20 | 2 | 5.40 | 0.670 | |
Note: Complications were graded according to the Clavien-Dindo complication grading system. Minor reoperations consisted of tracheostomies, upper endoscopies, or bedside incision and drainage procedures. Major operations were those performed under general anesthesia to address significant complications.
COVID-19, coronavirus disease 2019; COVID-19 group, esophagectomy patients from March-June 2020; 2019 group, esophagectomy patients from calendar year 2019; IQR, interquartile range for non-normal distribution data with 25th and 75th percentiles
A summary of the key findings and their implications can be found in Figure 1 .
Figure 1.
Graphical Abstract of Esophagectomy for Esophageal Cancer Safely Performed During COVID-19 Pandemic showing summary of methods, results, and implications.
DISCUSSION
Esophagectomy for esophageal cancer was not associated with worse outcomes during the COVID-19 pandemic with minimal risk of infection when careful COVID-19 guidelines were followed. Our institution underwent an operating room shutdown in mid-March 2020 to mid-April 2020. Only emergencies and carefully considered cases were performed. As semi-elective operations were resumed there was a backlog of patients who were at risk significantly delayed surgery. Esophageal cancer cases were prioritized in the initial phase when the operating rooms were reopened due to their high-risk nature and to attempt to minimize the length of time from the completion of neoadjuvant therapy to surgery, if applicable. We found these efforts were successful.
With institutional protocols put in place before guidelines were more common, none of our patients were infected with COVID-19 during their post-operative admission or by their first follow-up appointment. This allowed us to avoid many of the pulmonary complications and mortality that had been reported elsewhere.8 Our institution did not set up full COVID-19-free surgical pathways (dedicated operating room, critical care, and inpatient wards) as noted in Glasbey et al. but dedicated critical care and inpatient wards were set up later in our study period.9
In the early portion of the pandemic there was debate regarding the possible aerosolization of the virus during laparoscopy.10, 11, 12 Larger tumor sizes were also seen during the pandemic, but conversion rate was similar to 2019. We do not believe these factors impacted our rate of open abdominal surgery as one surgeon customarily uses laparotomy for the abdominal portion of esophagectomies and the conversion rate was no different.
The increased, statistically nonsignificant, rate of anastomotic leaks seen during COVID-19 were not related to the pandemic and are within the range of recent studies.13, 14, 15 Upon inspection of our morbidity database from March 2020 through February 2021, 9 leaks occurred during the year with 4 during our study period for COVID-19. The annualized rate for leaks is 9.5% (9/95) compared to 5.2% (5/96) in 2019. For the COVID-19 cases, one case had technical intraoperative issues, 2 of the leaks were noted on upper gastrointestinal series done per standard post-operative esophagectomy protocols, and the final 2 patients had leaks noted 9 and 11 days after surgery which required additional procedural management. The causes for this increase in leak rate since COVID-19 started are currently under investigation.
Lastly, length of stay was shorter during the pandemic in part due to concerns for patients staying longer in the hospital but was accompanied by a higher readmission rate. The total readmission rate from March 2020 through February 2021 is 12.6% (12/95). Upon further review of the causes for readmission, none of the patients were readmitted for COVID-19 related issues. Examples include, delayed esophageal leak, failure to thrive, and dysphagia.
The lessons we learned in this early experience dealing with a respiratory pandemic were myriad. Due to the progressive nature of esophageal cancer, significant efforts to ensure timely scheduling of operations after completion of neoadjuvant treatment is necessary. Next, staying current on the developing literature, guidelines, and reports of disease transmission to update pre-admission, and during hospitalization, COVID-19 testing strategies should be done. Additionally, hospital staff adherence to strict mask and symptom reporting policies to prevent potential iatrogenic COVID-19 transmission are mandatory. Clear discussions with patients on how to prevent getting COVID-19 before admission as well as restricting visitors is essential to further reduce transmission risk. Once patients are in the hospital, every effort to perform esophagectomies by minimally invasive methods and keep the conversion rate to a minimum help maintain transmission safety and reduce complications of esophagectomy. Since strict visitor restrictions are necessary to prevent infection from non-patient and non-hospital staff sources, the hospital should provide increased social support services and technology for patients to communicate with outside family and friends. After discharge, close 2-week follow-up by either in-person visit or tele-medicine is key to help monitor patients postoperatively, remind them of best practices for preventing COVID-19 infection, and attempt to reduce readmission. Later in the pandemic, our successful and safe results of the first few brave patients were able to be communicated to subsequent patients. This helped reassure them that coming to the hospital for their surgery was safe.
Some limitations and possible biases may have been introduced. During resumption of semi-elective cases, patient selection bias may have occurred that may have not been captured in our data leading to decreased complication rates during COVID-19. The emphasis placed on rapid discharge to minimize COVID-19 exposure may have contributed to the higher, non-significant, readmission that was observed in the COVID-19 group. An additional limitation is the low number of patients operated on during he pandemic.
We expect no difference in long-term outcomes for the COVID-19 group compared to previous patients since there was no increased rate of complications or upstaging on pathology. Continued observation is warranted as the pandemic progresses to ensure esophageal cancer patients who need to be evaluated and treated receive appropriate care.16
CONCLUSIONS
During the ongoing COVID-19 pandemic, with appropriate case prioritization, preoperative testing, staff policies, and systemwide measures, esophagectomy for esophageal cancer was not associated with worse perioperative outcomes and minimal risk of infection with COVID-19 at our institution.
Footnotes
IRB approval: Protocol number: 2014P000998; Approval date: 3/13/20. Patient written consent for the publication of the study data was waived by the IRB due to the retrospective nature of the study.
Funding: No funding was provided for this study.
Disclosures: No relevant disclosures exist for the authors.
QR Code.

Supplementary Material
Dr. Dolan discusses the background, objectives, methods, and key findings of the study.
REFERENCES
- 1.Zaim S, Chong JH, Sankaranarayanan V, et al. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45 doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazzaroni MG, Piantoni S, Masneri S, et al. Coagulation dysfunction in COVID-19: The interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100745. [published online ahead of print, 2020 Aug 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for american seniors. JCO Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. PMID: 33253013 PMCID: PMC7713534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoracic Surgery Outcomes Research Network, Inc. M Antonoff, Backhus L, et al. COVID-19 guidance for triage of operations for thoracic malignancies: A consensus statement from Thoracic Surgery Outcomes Research Network. J Thorac Cardiovasc Surg. 2020;160:601–605. doi: 10.1016/j.jtcvs.2020.03.061. Epub 2020 Apr 9. PMID: 32689703PMCID: PMC7146695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JY, Correa AM, Vaporciyan AA, et al. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg. 2012;93:207–212. doi: 10.1016/j.athoracsur.2011.05.021. discussion 212-3. Epub 2011 Oct 1PMID: 21962263PMCID: PMC4041623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin Q, Xu H, Liu J, et al. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J Surg. 2018;59:11–18. doi: 10.1016/j.ijsu.2018.09.013. Epub 2018 Sep 24. PMID: 30261331. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. PMID: 15273542 PMCID: PMC1360123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. Epub 2020 May 29. Erratum in: Lancet. 2020 Jun 9;: PMID: 32479829 PMCID: PMC7259900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasbey JC, Nepogodiev D, Simoes JFF, et al. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2021;39:66–78. doi: 10.1200/JCO.20.01933. Epub 2020 Oct 6. PMID: 33021869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angioni S. Laparoscopy in the coronavirus disease 2019 (COVID-19) era. Gynecol Surg. 2020;17:3. doi: 10.1186/s10397-020-01070-7. Epub 2020 May 14. PMID: 32435173PMCID: PMC7224160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mintz Y, Arezzo A, Boni L, et al. The risk of COVID-19 transmission by laparoscopic smoke may be lower than for laparotomy: a narrative review. Surg Endosc. 2020;34:3298–3305. doi: 10.1007/s00464-020-07652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Du G. COVID-19 may transmit through aerosol. Ir J Med Sci. 2020;189:1143–1144. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manghelli JL, Ceppa DP, Greenberg JW, et al. Management of anastomotic leaks following esophagectomy: when to intervene? J Thorac Dis. 2019;11:131–137. doi: 10.21037/jtd.2018.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chidi AP, Etchill EW, Ha JS, et al. Effect of thoracic versus cervical anastomosis on anastomotic leak among patients who undergo esophagectomy after neoadjuvant chemoradiation. J Thorac Cardiovasc Surg. 2020;160:1088–1095. doi: 10.1016/j.jtcvs.2020.01.089. Epub 2020 Feb 20. PMID: 32299695. [DOI] [PubMed] [Google Scholar]
- 16.Kamarajah SK, Lin A, Tharmaraja T, et al. Risk factors and outcomes associated with anastomotic leaks following esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2020;33:doz089. doi: 10.1093/dote/doz089. PMID: 31957798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dr. Dolan discusses the background, objectives, methods, and key findings of the study.