Abstract
Background and Aims
The global growth of mobile phone use has led to new opportunities for health interventions, including through text messaging. We aimed to estimate the effects of text messaging interventions on alcohol consumption among risky drinkers.
Methods
Systematic review and meta‐analysis of reports on randomized controlled trials (RCTs) published in English. Searches were conducted on 23 May 2019 in PubMed; PubMed Central; CENTRAL; CDSR; DARE; NHS‐EED; Scopus; PsycINFO; PsycARTICLES; CINAHL; and Web of Science. Measurements included number of episodes of heavy drinking (HED) per month and weekly alcohol consumption (WAC) in grams. Trials among risky drinkers who were not receiving co‐interventions were included in the review (n = 3481, mean age 29 years, 41% female). Data were extracted from reports and authors were contacted for additional data.
Results
Ten trials were included and all analyses were based on random‐effects models. Primary analyses, including seven trials (n = 2528) for HED and five trials (n = 2236) for WAC, found that the interventions may reduce self‐reported HED [−0.33 episodes per month; 95% confidence interval (CI) = −0.79, 0.12] and WAC (−18.62 g per week; 95% CI = −39.61, 2.38), although both estimates included the null. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) quality of evidence was judged to be low for both HED and WAC, primarily due to risk of attrition and performance bias, heterogeneity and influence of pilot trials on estimates.
Conclusions
Text messaging alcohol interventions may reduce alcohol consumption compared with no or basic health information; however, there are doubts about the overall quality of the evidence.
Keywords: Alcohol consumption, brief interventions, meta‐analysis, risky drinking, telemedicine, text messaging
Introduction
Rationale
Alcohol consumption is a leading risk factor for non‐communicable diseases, which are responsible for 70% of deaths globally each year, of which cardiovascular diseases, cancer, respiratory diseases and diabetes account for more than 80% [1]. Alcohol also causes injuries, road traffic accidents and violence [2]. This means that alcohol consumption continues to be a leading cause of death, with approximately 4.5% of deaths globally attributable to alcohol and 25% of all deaths in the age group 20–49 years [3]. While there is evidence suggesting that a small amount of alcohol may have a protective effect on myocardial infarction, the overall risk of alcohol consumption outweighs any potential benefit, hence the conclusion that there is no safe dose [4].
mHealth and text messaging
In 2019, it was estimated that 97% of the global population resided in an area with a mobile cellular signal [5]. This global growth of mobile phone subscriptions has led to new opportunities for health promotion, and the field of mobile health (mHealth) has grown substantially over the past decade [6]. Continuous contact with individuals, interactivity, and cost reductions are some of the potential benefits associated with mHealth interventions.
One way in which mHealth interventions can be deployed is through text messaging, which is a technology ubiquitous in mobile phones. The technology runs on networks utilizing earlier standards, such as the Global System for Mobile (GSM) communications, which are generally more available and cheaper than later standards (3G and 4G). Thus, interventions utilizing text messaging potentially have great reach globally among those who could benefit from health behaviour change.
In alcohol research, text messaging has been used both as a stand‐alone intervention and in combination with other digital media such as websites [7, 8, 9]. Reviews of mHealth interventions for alcohol, which have included text messaging, have indicated positive but mixed findings of their efficacy [10, 11]; however, these reviews have had a wide scope and have not included meta‐analyses. Thus, direct guidance is limited with respect to the effectiveness of text messaging as a stand‐alone alcohol intervention. Therefore, this systematic review and meta‐analysis aimed to estimate the effects of text messaging interventions on alcohol consumption among risky drinkers.
Methods
This systematic review and meta‐analysis included reports of randomized trials estimating the effects of stand‐alone text messaging interventions on alcohol consumption among risky drinkers in comparison to no or basic health information. A review protocol, developed according to Preferred Reporting Items For Systematic Review and Meta‐Analysis (PRISMA)‐P [12], was published in advance of this systematic review [13] (PROSPERO: CRD42019117431, IRRID: PRR1–10.2196/12898), and this report includes the items recommended by the PRISMA statement [14].
Information sources and search
On 23 May 2019 we searched PubMed (1982–present), PubMed Central (1989–present), Cochrane Central Register of Controlled Trials (CENTRAL, 1994–present); Cochrane Database of Systematic Reviews (CDSR, 2012–present); Database of Abstracts of Reviews of Effects (DARE, 1997–present); National Health Service Economic Evaluation Database (NHS‐EED, 1997–present); Scopus (1969–present); PsycINFO (1983–present); PsycARTICLES (1985–present); Cumulative Index to Nursing and Allied Health Literature (CINAHL, 2000–present); Web of Science (1991–present); International Standard Randomised Controlled Trial Number (ISRCTN, 2006–present) registry; ClinicalTrials.gov (2005–present); and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP, 2006–present).
Grey literature was sourced from the OpenGrey database (1999–present), and PROSPERO (2012–present) was searched to identify systematic reviews of relevance.
Search strategies can be found in Supporting information, Appendix A.
Eligibility criteria
Only randomized controlled trials (RCTs), including cluster RCTs, evaluating text messaging without co‐interventions were eligible for inclusion. We included reports in English and put no restriction on publication date.
Participants
Trials including risky drinkers (including both harmful and hazardous [15]), identified by a screening tool in any population (e.g. students, general population and primary care patients), were included. No restriction on age was made. Trials which included participants who were obviously receiving care for their alcohol problems, e.g. patients in a treatment programme, were not included. Trials were excluded if participants were mandated to take part.
Interventions
Interventions consisted of a series of text messages sent to participants’ mobile phones over a number of weeks. For an intervention to be included, at least two messages should have been sent per week (on average). The content of the messages should be focused on behaviour change, thus excluding studies where text messages were used only to schedule or remind participants of other activities. Only trials where a text message intervention was the sole intervention were considered; therefore, trials of interventions where text messages were combined with other interventions (e.g. therapy or pharmaceutical treatment) were excluded.
Comparators
There were four types of control conditions permitted for inclusion:
-
1.
Minimal or no contact, including waiting list.
-
2.
Basic health information on alcohol provided no more than once a week.
-
3.
Referral to information sources such as websites, or recommended to contact primary health‐care services, with reminders no more than once a week.
-
4.
Intervention focusing on something other than alcohol consumption; for instance, physical activity or smoking.
Outcomes
Trials were included if they planned to report one of two common alcohol consumption outcomes:
-
1.
Number of episodes of heavy drinking during the past month [heavy episodic drinking (HED)].
-
2.
Weekly alcohol consumption (WAC) measured in grams or standard units of alcohol.
Report selection, data collection and risk of bias
M.B. initially screened the titles and abstracts for duplicates and removed reports that were clearly deemed irrelevant for the objective. Each member of the data collection team (M.B., K.Å., P.B.) independently analyzed the full text of the remaining reports and assessed eligibility. The final decision on which reports to include was made through discussion among team members.
A standardized data collection form could not be used, due to outcomes being reported with great variety. Instead, M.B. extracted data from reports and K.Å. and P.B. reviewed and checked the extraction in independent sessions. Authors were contacted for additional data as needed.
The Cochrane Collaboration's tool was used to assess risk of bias in individual trials [16, 17]. M.B. judged each potential source of bias for each report, then K.Å. and P.B. reviewed these judgements independently. No trials were excluded based on risk of bias, but sensitivity analyses were conducted without those judged to be at high risk of attrition bias. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [18] was used by M.B. and J.M. to judge risk of bias among studies and to assess the quality of the body of evidence for each outcome. Trial registration databases and protocols were searched to ensure that trials, and trial outcomes, were reported as planned, supported by funnel plots and Egger's tests.
Data items
The following items were extracted from the reports:
-
1.
Mean and dispersion for HED and WAC.
-
2.
Number randomized, group sizes, number of follow‐up responses and trial design.
-
3.
Age, gender, baseline consumption of trial participants.
-
4.
Number of weeks the intervention lasted, average weekly frequency of text messages, rationale behind content of messages.
-
5.
The type of control condition used.
-
6.
The type and source of financial support.
All six clusters were used for the narrative description of the trials (Table 1 and Supporting information, Appendix B), with 1 and 2 used for meta‐analyses.
Table 1.
Summary of included trials evaluating the effect of text messaging interventions on alcohol consumption outcomes
| Source/methods | Participants a | Interventions | Controls | Outcomes b (follow‐up rate) |
|---|---|---|---|---|
| Suffoletto 2012 [19] Pilot randomized controlled trial |
• Young adults recruited from emergency departments • Intervention n = 15 Control n = 30 • Mean age: 21 y • Female: 64% • Mean HED past month: 5.2 |
12‐week programme with number of messages varying depending on input from participant Pre‐weekend planning and post‐weekend assessment through text messages with real‐time feedback |
Post‐weekend assessment message only for 12 weeks (n = 15) No messages (n = 15) |
• 3‐month: HED (86.7%) WAC c (86.7%) |
| Crombie 2013 [20] Pilot randomized controlled trial |
• Disadvantaged men reached through community outreach programme • Intervention n = 34 Control n = 33 • Mean age: 34.2 y d • Females: 0% • Mean HED past month: 5.88 |
36 messages over 28 days Tailored to the target group and constructed to take advantage of the conventional pattern of heavy weekend drinking |
34 messages over 28 days General health information not mentioning alcohol |
• 3‐month: HED (96%) WAC (96%) |
| Suffoletto 2014 [21] Suffoletto 2015 [22] Randomized controlled trial |
• Young adults recruited from emergency departments • Intervention n = 384 Control n = 381 • Mean age: 22 y • Female: 65.4% • Mean AUDIT‐C score: 6.3 |
12‐week programme with number of messages varying depending on input from participant Pre‐weekend planning and post‐weekend assessment through text messages with real‐time feedback |
Post‐weekend assessment message only for 12 weeks (n = 196) No messages (n = 185) |
• 3‐month: HED (78.2%) WAC c (78.2%) • 6‐month: HED (63.5%) WAC c (63.5%) • 9‐month: HED (54.9%) WAC c (54.9%) |
| Bock 2016 [23] Pilot randomized controlled trial |
• Community college students recruited by posted flyer • Intervention n = 31 Control n = 29 • Mean age: 21.8 y • Females: 61.7% • Mean HED past 2 weeks: 3.91 |
36 messages sent over 6 weeks Facts about alcohol, strategies to limit alcohol use and alcohol‐related‐risks, and motivational messages |
36 messages sent over 6 weeks General motivational content without reference to alcohol or harm reduction |
• 6‐week (93.3%) • 12‐week (88.3%) HED/WAC not available in report |
| Muench 2017 [24] Pilot randomized controlled trial |
• On‐line help‐seekers • Intervention n = 127 Control n = 30 • Mean age: 43.2 y • Female: 74.9% • Mean HED per week: 3.4 HED |
85 messages over 12 weeks + weekly assessment Four different versions: • Loss‐framed • Gain‐framed • Statistically tailored • Tailored adaptive |
Weekly assessment only | • 3‐month:HED (83%)WAC (83%) |
| Crombie 2018 [25] Randomized controlled trial |
• Disadvantaged men reached through community outreach programme • Intervention n = 411 Control n = 414 • Mean age: 34.6 y • Females: 0% • Mean HED past month: 6.58 |
112 messages over 12 weeks Tailored to the target group and the drinking culture of disadvantaged young men |
89 messages over 12 weeks General health information not mentioning alcohol |
• 6‐month: HED (89.3%) WAC (89.3%) • 15‐month: HED (85.6%) WAC (85.6%) |
| Merrill 2018 [26] Pilot randomized controlled trial |
• College students invited by e‐mail • Intervention n = 34 Control n = 34 • Mean age: 19 y • Female: 70.6% • Mean HED past month: 3.77 |
28 messages over 28 days Feedback based on descriptive norms (what others do) and injunctive norms (what others approve of) |
28 messages over 28 days Text messages with fun facts unrelated to alcohol |
•1 month: HED (100%) WAC c (100%) |
| Sharpe 2018 [27] Randomized controlled trial |
• Inpatients admitted for injury • Intervention n = 299 Control n = 299 • Mean age: 34 y • Female: 28.6% •Mean AUDIT‐C score: 6.85 |
16 messages over 4 weeks Feedback and reflection on drinking, recommendation to cut down and linked to existing services, tips and strategies, support and encouraging content |
One message acknowledging participation in the trial and indicated they would be contacted in 3 months |
• 3 months: HED c (89.3%) • 6 months: HED c (82.8%) • 12‐month: HED c (72.1%) WAC not available |
|
Thomas 2018 [28] Randomized controlled trial |
• University and college students invited through e‐mail • Intervention n = 460 Control n = 436 • Mean age: 25.4 y • Female: 56.8% • Mean WAC: 165.6 g of alcohol |
62 messages sent over 6 weeks Messages throughout the week to support behaviour change, post‐weekend assessment through texting with feedback |
Recommended to assess their drinking at website, no further contact | • 3‐month: HED (45.3%) WAC (91.1%) |
y = years;
HED = heavy episodic drinking, WAC = weekly alcohol consumption;
made available by request from corresponding author;
converted from categorical (25–29 n = 20, 30–34 n = 11, 35–39 n = 18, 40–44 n = 15). AUDIT = Alcohol Use Disorders Identification Test.
Summary measures
For HED, individuals are typically asked to report the number of times that they drank more than a certain number of units of alcohol on the same occasion (country‐dependent) during the past month, or it may be inferred from time‐line follow‐back approaches. We converted all data to monthly assessments, converting fixed‐response options to numerical measures (e.g. once or twice a week: (1 + 2)/2 × 4 = 6).
For WAC, both a time‐line follow‐back period approach and a frequency–intensity approach were used, and for both we converted standard drinks data to grams per week for each trial.
Synthesis of results
In trials where outcomes were assessed more than once, we used data from the first post‐intervention analysis in the primary meta‐analysis. Subgroup analyses were conducted for different time‐frames: 1–3 months, 4–6 months and 7 + months also using subsequent follow‐up data. Length of follow‐up was defined based on time elapsed since randomization.
After designing the protocol, it was found that several included trials were identified as feasibility or pilot trials by their authors. As it became evident that these strongly shaped the synthesized outcomes, we added a stratified analysis of the primary outcomes separating pilot and full‐scale trials.
In all meta‐analyses, we used random‐effects models with inverse variance weighting. Heterogeneity was assessed using the I 2 statistic χ2 tests at the recommended P‐value cut‐off of 0.1 [17]. We used R version 3.6.1 with the meta package version 4.9‐7 for all analyses.
Results
Record selection
The search for records was conducted on 23 May 2019. Figure 1 shows a PRISMA flow diagram of the record selection process. The search of PubMed (474), PubMed Central (250), CDSR (4), CENTRAL (428), DARE NHS‐EED (12), Scopus (219), PsycINFO (149), PsycARTICLES (28), CINAHL (173), Web of Science (646) and OpenGrey (7) yielded a total of 2390 records. Citation searching identified only two other candidates.
Figure 1.
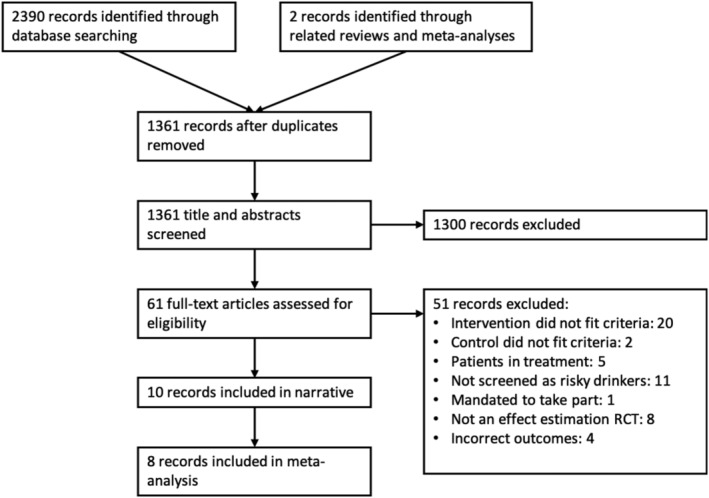
Preferred Reporting Items For Systematic Review and Meta‐Analysis (PRISMA) flow diagram of record selection process
Among 373 trial registry entries found during the search [ICTRN (102), ClinicalTrials.org (144), ICTRP (100), other (27)], a total of 14 entries were relevant with respect to the eligibility criteria for this review. Eight of these entries were for the included trials and six items were for ongoing trials.
Study characteristics
Of the 10 reports included in this review, five were pilot trials and five full‐scale trials (as described by the authors). Two reports presented data from the same full‐scale trial but for different follow‐up intervals [22, 29]. A summary of the trials can be found in Table 1, and a summary of data availability can be found in Table 2.
Table 2.
Data availability from the trials included in the systematic review
| Source | HED | WAC | Comment |
|---|---|---|---|
| Suffoletto 2012 [19] | In report | By request | Two arms fitted the criteria for control which were combined using weighted means |
| Crombie 2013 [20] | Not available | Not available | Standard deviations of outcome measures were not available. We decided against imputing standard deviations as it was a pilot trial with few participants, thus the actual sample standard deviations could potentially be very different from those reported in other included trials |
| Suffoletto 2014 [21] | In report | By request | Two arms fitted the criteria for control which were combined using weighted means |
| Suffoletto 2015 [22] | In report | By request | Two arms fitted the criteria for control which were combined using weighted means. Data were not included in the primary meta‐analyses as they came from the same trial participants as in Suffoletto [21], thus records would not be independent for statistical analysis purposes |
| Bock 2016 [23] | Not available | Not available | |
| Muench 2017 [24] | In report | In report | There were four intervention arms and one control arm. The intervention arms were combined using weighted means |
| Crombie 2018 [25] | In report | In report | Data from the 6‐month follow‐up were included in the primary meta‐analyses |
| Merrill 2018 [26] | In report | By request | WAC was made available by request but could not be used without additional data not available at the time of analysis |
| Sharpe 2018 [27] | By request | Not available | Data on the third item of the AUDIT‐C questionnaire was made available by request. As was planned in the protocol, categorical answers were converted to numeric: never = 0, less than monthly = 0.5, monthly = 1, weekly = 4, daily or almost daily = 22.5. Data from the 3‐month follow‐up was included in the primary meta‐analyses |
| Thomas 2018 [28] | In report | In report | As was planned in the protocol, categorical answers for HED were converted to numeric: never = 0, less than monthly = 0.5, monthly = 1, 2–3 times per month = 2.5, once or twice a week = 6, 3 times or more per week = 14 |
HED = heavy episodic drinking; WAC = weekly alcohol consumption.
Participants (n = 3481) were, on average, 29 years of age and 41% were female. Study populations included emergency department visitors, inpatients, college students, disadvantaged men and on‐line help‐seekers. With the exception of on‐line help‐seekers, recruitment was proactive among populations not primarily seeking help with alcohol consumption. All the interventions consisted of a series of text messages sent over an average of 8 weeks (ranging from 4 to 12). The average frequency of messages sent was approximately 6.3 messages per week.
The text messages were designed to support behaviour change, typically including: self‐assessment and feedback on alcohol consumption, information addressing drinking culture in the target population, facts about alcohol, strategies to limit alcohol consumption, motivational content including benefits and consequences, normative feedback and linking out to additional support. Six of the interventions included a broad set of components [20, 23, 24, 25, 27, 28], one focused primarily on normative feedback [26] and one intervention (included in three reports) focused on assessment and feedback [19, 21, 22].
For a longer description of each trial and intervention, please see Supporting information, Appendix B.
Results of individual trials and synthesis of results
Pooled results with respect to HED are presented in Fig. 2 and for WAC in Fig. 3 (with stratified analyses separating pilot from full‐scale trials). A risk of bias summary for each outcome is presented in Figs 4 and 5; details can be found in Supporting information, Appendix C. Subgroup analyses of different follow‐up intervals can be found in Supporting information, Appendix D. Here we present findings for each outcome, taking into consideration effect size estimates, risk of bias and overall quality of the presented body of evidence (in accordance with the GRADE framework [18]).
Figure 2.
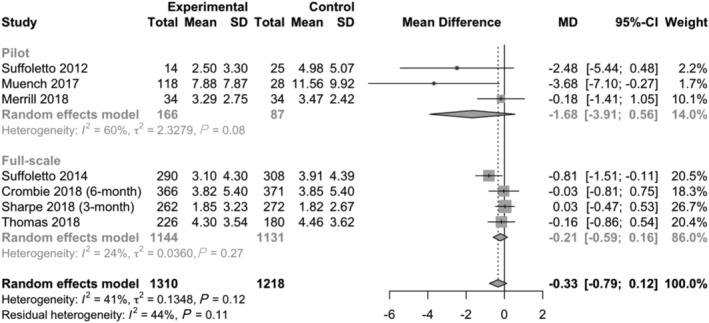
Results from individual trials and overall effect estimate with respect to number of heavy drinking episodes per month. Primary planned analysis which includes the first post‐intervention follow‐up interval from each included trial (SD = standard deviation; MD = mean difference; CI = confidence interval). Stratified by pilot and full‐scale trials
Figure 3.
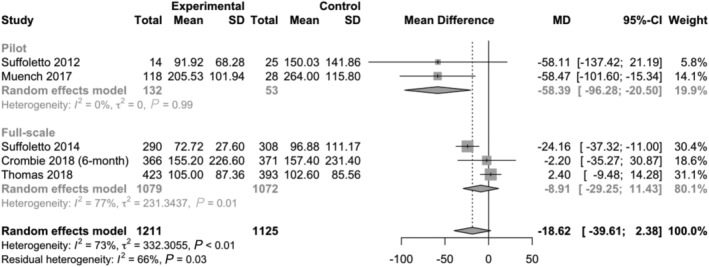
Results from individual trials and overall effect estimate with respect to grams of alcohol per week. Primary planned analysis which includes the first post‐intervention follow‐up interval from each included trial (SD = standard deviation; MD = mean difference; CI = confidence interval). Stratified by pilot and full‐scale trials
Figure 4.
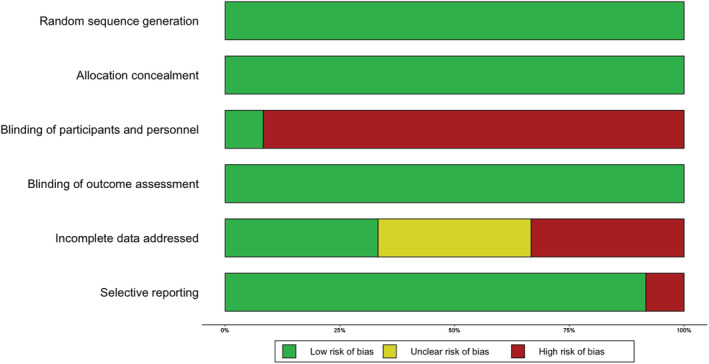
Risk of bias summary: risk of bias broken down for each criterion across all included trials [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 5.
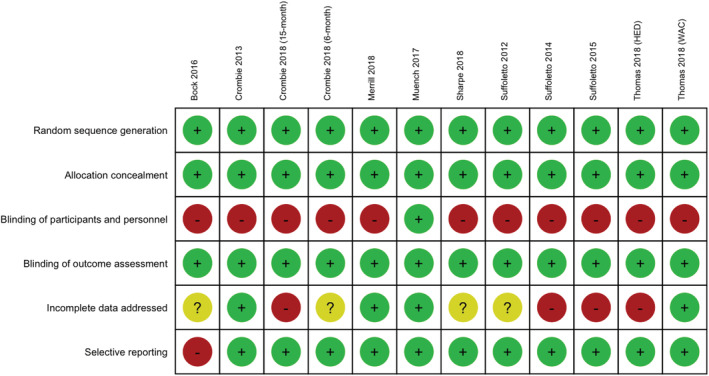
Risk of bias plot: risk of bias broken down for each criterion and each included trial. [Colour figure can be viewed at wileyonlinelibrary.com]
The prevalence of risky drinking, a planned secondary outcome, was not studied consistently with our protocol to enable meta‐analysis to be undertaken; only two trials (Sharpe [27] and Crombie [25]) measured prevalence of risky drinking following criteria specified by authors in the reports (Sharpe [27] used AUDIT‐C ≥ 3 for females and ≥ 4 for males, and Crombie [25] used ≥ 3 episodes of heavy drinking per month).
Heavy episodic drinking
The primary meta‐analysis of HED (seven trials, n = 2528) found a weighted mean difference of −0.33 episodes per month (95% CI = −0.79, 0.12) in favour of the text messaging interventions. When removing pilot trials from the primary analysis, the overall effect size for HED was lower (−0.21 episodes per month, 95% CI = −0.59, 0.16). In both cases, the CIs suggest that effect sizes may be more than twice as large as estimated, but they also include zero, thus we cannot rule out null findings.
The GRADE quality of the body of evidence for HED was judged to be low. First, small pilot trials shaped the overall outcome estimates. Secondly, high risk of performance bias due to lack of blinding was prevalent in all trials except Muench [24]. Thirdly, risk of attrition bias was high in the full‐scale trials by Suffoletto [21] and Thomas [28], which together have a weight of 41% in the primary analysis of HED. A sensitivity analysis removing these two trials resulted in a similar overall effect estimate [confidence interval (CI) = −0.28 episodes per month, 95% CI = −0.96, 0.39].
HED was not reported as planned in Bock [23] (ClinicalTrials.gov NCT02507115), which warrants some concern about publication bias. However, as the trial included few participants it was not judged to impact the overall quality of evidence for HED. A funnel plot (Fig. 6) and Egger's test (P‐value = 0.049) revealed marginally statistically significant asymmetry, but we did not judge this to warrant further downgrade of the quality of evidence.
Figure 6.
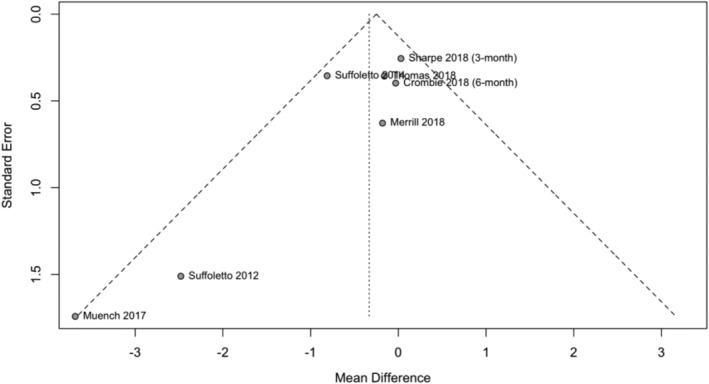
Funnel plot investigating publication bias of heavy episodic drinking. Egger's test revealed marginally statistically significant asymmetry (P‐value = 0.049)
The subgroup analyses of different follow‐up intervals (Supporting information, Appendix D) revealed no apparent reduction of effect over time: −0.43 episodes per month (95% CI = –0.99, 0.13) at 1–3 months, −0.39 episodes per month (95% CI = −1.03, 0.24) at 4–6 months and −0.36 episodes per month (95% CI = −1.09, 0.36) at 7 + months. The GRADE quality of the body of evidence for 4–6‐ and 7 +‐month follow‐up intervals was considered low, as all included trials in these subgroups had high or unclear risk of bias due to incomplete data; however, no pilot trials were included.
Weekly alcohol consumption
The primary meta‐analysis of WAC (five trials, n = 2236) found a weighted mean difference of −18.62 g per week (95% CI = −39.61, 2.38), in favour of the text messaging interventions. When removing pilot trials, the overall effect size for WAC was lower (−8.91 g per week, 95% CI = −29.25, 11.43). As was the case for HED, CIs suggest that null findings cannot be ruled out; however, nor can estimates more than twice as large.
The GRADE quality of the body of evidence for WAC was judged to be low. First, pilot trials strongly shaped overall effect estimates and heterogeneity was evident when analyzing WAC (I 2 = 73%, P‐value < 0.01). Secondly, there was high risk of performance bias prevalent in all trials except Muench [24]. Thirdly, risk of attrition bias was judged to be high for Suffoletto [21]. Removing this trial in a sensitivity analysis revealed similar effect estimates (−18.97 g per week, 95% CI = −48.95, 11.02), being more reliant upon pilot trials. A funnel plot (Fig. 7) and Egger's test (P‐value = 0.39) revealed no evidence of asymmetry.
Figure 7.
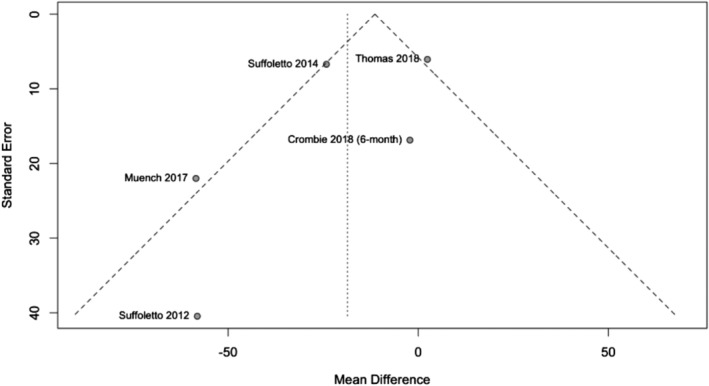
Funnel plot investigating publication bias of weekly alcohol consumption. Egger's test revealed no statistically significant asymmetry (P‐value = 0.39)
Subgroup analyses of WAC for different follow‐up intervals (Supporting information, Appendix D) revealed some modest reductions of effect over time: −23.45 g per week (95% CI = –48.72; 1.83) at 1–3 months, −15.71 g per week (95% CI = −31.10, −0.31) at 4–6 months, and −18.75 g per week (95% CI = −36.38, −1.12) at 7 + months. All included trials in these subgroups had high or unclear risk of bias due to incomplete data; however no pilot trials were included, thus the GRADE quality of body of evidence for these subgroup analyses was judged to be low.
Discussion
Summary of evidence
The meta‐analyses in this review provided low‐quality evidence of text messaging interventions reducing HED by 0.33 episodes per month, and low‐quality evidence of text messaging interventions reducing WAC by 18.62 g per week. As the CIs did not rule out null findings, any possible benefits are likely to be small and imprecisely estimated. Concerns about attrition and performance bias, heterogeneity and the degree to which pilot trials shaped effect estimates were reasons for downgrading the quality of evidence using GRADE.
Comparison to previous literature
There have been no published meta‐analyses of text messaging alcohol interventions to which we could directly compare the findings herein. However, two recent meta‐analyses with a broader scope, including a diverse set of digital alcohol interventions, are informative.
An individual patient data meta‐analysis (IPDMA) of digital interventions included 19 trials of both guided and unguided interventions in non‐student populations [30]. The overall analysis suggested that the unguided interventions reduced WAC (−32.30 g per week, 95% CI = −58.80, −5.90). There was evidence of heterogeneity among the included trials (I 2 = 55.5%, P‐value < 0.001) and, as here, outcomes were self‐reported.
A Cochrane Review also took a broader view of digital interventions and was last updated in 2017 [31]. A total of 42 trials were included in the analyses of WAC, and revealed an effect of −22.84 g per week (95% CI = −15.36, −30.31) in favour of the interventions. Heterogeneity was again marked (I 2 = 77.6%, P‐value < 0.0001), and sensitivity analyses removing trials with high risk of performance bias due to lack of blinding halved the estimates. Analyses of HED, including 15 trials, suggested an effect of −0.24 episodes per month (95% CI = −0.35, −0.13) in favour of the interventions.
The effect sizes found in the IPDMA were somewhat higher than in this study and the Cochrane Review. The reduction in HED found in this review, particularly in full‐scale trials, is very similar to the findings in the Cochrane Review. There is thus some consistency across these studies, both with respect to the substantive findings and in the limitations of the literatures reviewed.
Limitations
The issue of variability of outcomes in brief alcohol intervention research is well established [31, 32]. As an indication of the magnitude of the problem, the Outcome Reporting in Brief Intervention Trials: Alcohol (ORBITAL) project [33], which aims to produce a core outcome set for brief alcohol interventions, identified 2641 different outcomes used, measured in approximately 1560 different ways, in 405 trials of brief alcohol interventions [32]. Due to this variability we were not able to include all trials in both outcome analyses, clearly a limitation of this review. While the ORBITAL project is not yet complete, we recommend that researchers consider adhering to the core outcome set to ensure that the synthesis of results from trials can be conducted efficiently.
Risk of bias due to lack of blinding of study participants was regarded as high in all included trials. Even trials that used non‐alcohol related text messages as a control condition cannot claim blinding, as participants were aware of the nature of the study. Blinding of participants was unlikely to be an option in the included trials; however future trials may consider using different designs which reduce the likelihood of bias. For example, in a factorial design, effects of the components of an intervention could be estimated, allowing participants to be informed that everyone will receive the intervention but that different versions of the intervention is being tested.
The uncertainties due to lack of blinding are also related to another form of bias that is highly relevant to this literature yet does not feature directly in the tool used; the outcomes are both self‐reported. It is plausible that bias may be differential by randomization arm, due to intervention participants down‐playing the extent of their drinking for social desirability reasons more so than control participants [34, 35, 36]. Studies in alcohol treatment contexts find self‐report to be valid [37], although data in brief intervention trials give cause for concern [38]. There is a need for further study of this important issue, although it is worth noting that dedicated on‐line alcohol studies have not identified such problems [39, 40], and objective measures of alcohol consumption are not sufficiently available to overcome reliance on self‐report.
The eligibility criteria required some homogeneity with respect to intervention delivery; i.e. a series of text messages were sent over several weeks. However, this did not place restrictions on intervention content. Including interventions with different content in meta‐analyses may be viewed as a strength, as it allows for effect sizes to be computed which are marginalized over different content; however, such marginalization arguments should be tempered, as there was a limited number of trials included here.
Prevalence of risky drinking was a planned secondary outcome which was not possible to analyze, as it was scarcely reported. We decided to not request these data from authors of the included trials as it was a secondary outcome, and this decision should be considered a limitation of this review. Similarly, planned subgroup analyses with respect to age and gender could not be conducted due to data not being available. Additional planned sensitivity above those already reported were not necessary; e.g. no cluster RCTs were included and all reports included intention‐to‐treat data. Finally, it was planned that two team members independently would extract data from studies, although due to the variety of ways outcome measures were reported, our standardized form could not be used. Instead, M.B. extracted data and K.Å. and P.B. independently checked the extracted data.
Conclusions
Implications for practice
The effect estimates for HED found in this meta‐analysis correspond to approximately one less episode of heavy drinking every 3 months. Such an effect is small but not trivial at the population level. WAC estimates similarly identify a small reduction which could nonetheless potentially have a meaningful effect on population level incidence of cardiovascular and other non‐communicable diseases [3, 4]. Helping individuals to reduce their alcohol consumption is important to do, and it should be noted that trials are mainly concerned with group‐level estimates (as is this review), and they may be masking important heterogeneous effects of interventions in subgroups [41].
An important factor when considering the synthesized effect sizes is that, with the exception of Muench [24], the included trials all proactively recruited participants by offering participation to individuals not primarily seeking help with alcohol. Additionally, the interventions were unguided and relied upon widely available and cheap technology. Thus, small effect sizes may be indicative of potential benefit if they are free from bias.
Finally, and beyond the primary analyses, the absence of clear attenuation in effects over time is somewhat surprising; while this is clearly what is anticipated for brief interventions [42], it is hypothetically possible that repeated exposure to text messages over time facilitates more enduring effects.
Implications for research
When comparing the findings of this study of text messaging interventions with those on digital interventions more broadly, it is noteworthy that estimated effects are similar across reviews. From a research perspective this leaves unanswered questions with respect to heterogeneity in effect estimates, and also underlines concern regarding bias in such trials stemming from the lack of blinding of participants.
Only one of the trials included in this review, Muench [24], was judged to be at low risk of performance bias, as participants were, with some certainty, blinded. A similar lack of blinding of participants was also evident in the IPDMA discussed earlier [30], with only one of the 19 included trials judged to have a low risk of performance bias. Similarly, few of the included trials in the aforementioned Cochrane Review [31] (13 trials, 23%) were judged to have a low risk of performance bias; all others were judged to be at high risk due to non‐blinding of participants.
Similar effect sizes in the three different reviews may be due to research artefacts rather than intervention effects. It should therefore be emphasized that not only is there a need for more full‐scale trials of text messaging interventions to better interrogate possible benefit, but future trials could seek to implement blinding [43] in such a way that information given to participants at the time of study entry does not allow participants to become aware of their allocated condition or the precise nature of the study and their role in it.
Declaration of interests
MB and PB own a private company (Alexit AB) which develops and disseminates eHealth applications to health organizations and professionals in both the private and public sector. Among the applications disseminated are digital lifestyle interventions that have been developed and researched within the LiiR group. Alexit AB had no part in the funding, planning, execution or analysis of this systematic review.
Author contributions
Marcus Bendtsen: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; validation; visualization; writing‐original draft; writing‐review & editing. Jim McCambridge: Formal analysis; investigation; methodology; validation; writing‐original draft; writing‐review & editing. Katarina Åsberg: Data curation; formal analysis; investigation; validation; writing‐original draft; writing‐review & editing. Preben Bendtsen: Conceptualization; formal analysis; investigation; methodology; validation; writing‐original draft; writing‐review & editing.
Supporting information
Appendix A Search strategies.
Appendix B Description of included trials.
Appendix C Risk of bias judgment.
Appendix D Planned subgroup analyses.
Acknowledgements
We would like to extend our gratitude to Dr Brian Suffoletto, Dr Sarah Sharpe and Dr Jennifer Merrill, who supplied data upon request for this review. We also thank the anonymous reviewers for their input which improved this review.
Bendtsen, M. , McCambridge, J. , Åsberg, K. , and Bendtsen, P. (2021) Text messaging interventions for reducing alcohol consumption among risky drinkers: systematic review and meta‐analysis. Addiction, 116: 1021–1033. 10.1111/add.15294.
References
- 1. Global Burden of disease (GBD) 2015 Risk factors collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016; 388; 10.1016/S0140-6736(15)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) Global Status Report on Alcohol and Health 2018. Geneva, Switzerland: WHO, p. 2018.
- 3. Griswold M. G., Fullman N., Hawley C., Arian N., Zimsen S. R. M., Tymeson H. D. et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018; 392: 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood A. M., Kaptoge S., Butterworth A. S., Willeit P., Warnakula S., Bolton T. et al. Risk thresholds for alcohol consumption: combined analysis of individual‐participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018; 391: 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Telecommunication Union (ITU) . Measuring Digital Development: Facts and Figures 2019. ITU Publications; 2019. (Accessed 10 October 2019). [Google Scholar]
- 6. World Health Organization (WHO) mHealth New Horizons For Health Through Mobile Technologies. Geneva, Switzerland: WHO; 2011.
- 7. Bernstein M. H., Stein L. A. R., Neighbors C., Suffoletto B., Carey K. B., Ferszt G. et al. A text message intervention to reduce 21st birthday alcohol consumption: Evaluation of a two‐group randomized controlled trial. Psychology of Addictive Behaviors 2018; 32: 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haug S., Paz Castro R., Kowatsch T., Filler A., Dey M., Schaub M. P. Efficacy of a web‐ and text messaging‐based intervention to reduce problem drinking in adolescents: results of a cluster‐randomized controlled trial. J Consult Clin Psychol 2017; 85: 147–159. [DOI] [PubMed] [Google Scholar]
- 9. Tahaney K. D., Palfai T. P. Text messaging as an adjunct to a web‐based intervention for college student alcohol use: A preliminary study. Addictive Behaviors 2017; 73: 63–66. [DOI] [PubMed] [Google Scholar]
- 10. Kazemi D. M., Borsari B., Levine M. J., Li S., Lamberson K. A., Matta L. A. A systematic review of the mHealth interventions to prevent alcohol and substance abuse. J Health Commun 2017; 22: 413–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berman A. H., Gajecki M., Sinadinovic K., Andersson C. Mobile interventions targeting risky drinking among university students: a review. Curr Addict Rep 2016; 3: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M. et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bendtsen M. Text messaging interventions for reducing alcohol consumption among harmful and hazardous drinkers: protocol for a systematic review and meta‐analysis. J Med Internet Res Protoc 2019; 8: e12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reid M. C., Fiellin D. A., O'Connor P. G. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med 1999; 159: 1681–1689. [DOI] [PubMed] [Google Scholar]
- 16. Higgins J. P. T., Altman D. G., Gotzsche P. C., Juni P., Moher D., Oxman A. D. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J. et al. Cochrane Handbook for Systematic Reviews of Interventions, 2nd edn. version 6.0. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 18. Atkins D., Best D., Briss P. A., Eccles M., Falck‐Ytter Y., Flottorp S. et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 1490: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suffoletto B., Callaway C., Kristan J., Kraemer K., Clark D. B. Text‐message‐based drinking assessments and brief interventions for young adults discharged from the emergency department. Alcohol Clin Exp Res 2012; 36: 552–560. [DOI] [PubMed] [Google Scholar]
- 20. Crombie I., Falconer D., Irvine L., Williams B., Ricketts I., Humphris G. et al. Reducing alcohol‐related harm in disadvantaged men: development and feasibility assessment of a brief intervention delivered by mobile telephone. Public Health Res 2013; 1: 1–138. [PubMed] [Google Scholar]
- 21. Suffoletto B., Kristan J., Callaway C., Kim K. H., Chung T., Monti P. M. et al. A text message alcohol intervention for young adult emergency department patients: a randomized clinical trial. Ann Emerg Med 2014; 64: e4–e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suffoletto B., Kristan J., Chung T., Jeong K., Fabio A., Monti P. et al. An interactive text message intervention to reduce binge drinking in young adults: a randomized controlled trial with 9‐month outcomes. PLOS ONE 2015; 10: e0142877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bock B. C., Barnett N. P., Thind H., Rosen R., Walaska K., Traficante R. et al. A text message intervention for alcohol risk reduction among community college students: TMAP. Addict Behav 2016; 63: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muench F., van Stolk‐Cooke K., Kuerbis A., Stadler G., Baumel A., Shao S. et al. A randomized controlled pilot trial of different mobile messaging interventions for problem drinking compared to weekly drink tracking. PLOS ONE 2017; 12: e0167900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crombie I. K., Irvine L., Williams B., Sniehotta F. F., Petrie D. J., Jones C. et al. Text message intervention to reduce frequency of binge drinking among disadvantaged men: the TRAM RCT. Public Health Res 2018; 6: 1–156. [PubMed] [Google Scholar]
- 26. Merrill J. E., Boyle H. K., Barnett N. P., Carey K. B. Delivering normative feedback to heavy drinking college students via text messaging: a pilot feasibility study. Addict Behav 2018; 83: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharpe S., Kool B., Whittaker R., Lee A. C., Reid P., Civil I. et al. Effect of a text message intervention to reduce hazardous drinking among injured patients discharged from a trauma ward: a randomized controlled trial. NPJ Digit Med 2018; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas K., Müssener U., Linderoth C., Karlsson N., Bendtsen P., Bendtsen M. Effectiveness of a text messaging‐based intervention targeting alcohol consumption among university students: randomized controlled trial. J Med Internet Res Mhealth Uhealth 2018. e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suffoletto B., Callaway C. W., Kristan J., Monti P., Clark D. B. Mobile phone text message intervention to reduce binge drinking among young adults: study protocol for a randomized controlled trial. Trials 2013; 14: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riper H., Hoogendoorn A., Cuijpers P., Karyotaki E., Boumparis N., Mira A. et al. Effectiveness and treatment moderators of internet interventions for adult problem drinking: an individual patient data meta‐analysis of 19 randomised controlled trials. PLoS Med 2018; 15: e1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaner E. F., Beyer F. R., Garnett C., Crane D., Brown J., Muirhead C. et al. Personalised digital interventions for reducing hazardous and harmful alcohol consumption in community‐dwelling populations. Cochrane Database Syst Rev 2017; 9: CD011479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shorter G. W., Bray J. W., Giles E. L., O'Donnell A. J., Berman A. H., Holloway A. et al. The variability of outcomes used in efficacy and effectiveness trials of alcohol brief interventions: a systematic review. J Stud Alcohol Drugs 2019; 80: 286–298. [PubMed] [Google Scholar]
- 33. Shorter G. W., Heather N., Bray J. W., Giles E. L., Holloway A., Barbosa C. et al. The ‘outcome reporting in brief intervention trials: alcohol’ (ORBITAL) framework: protocol to determine a core outcome set for efficacy and effectiveness trials of alcohol screening and brief intervention. Trials 2017; 18: 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cunningham J. A., Kypri K., McCambridge J. Exploratory randomized controlled trial evaluating the impact of a waiting list control design. BMC Med Res Methodol 2013; 13: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCambridge J., Kypri K., Elbourne D. Research participation effects: a skeleton in the methodological cupboard. J Clin Epidemiol 2014; 67: 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCambridge J., Saitz R. Rethinking brief interventions for alcohol in general practice. BMJ 2017; 356: j116. [DOI] [PubMed] [Google Scholar]
- 37. Babor T. F., Steinberg K., Anton R., Del Boca F. Talk is cheap: measuring drinking outcomes in clinical trials. J Stud Alcohol 2000; 61: 55–63. [DOI] [PubMed] [Google Scholar]
- 38. Noknoy S., Rangsin R., Saengcharnchai P., Tantibhaedhyangkul U., McCambridge J. RCT of effectiveness of motivational enhancement therapy delivered by nurses for hazardous drinkers in primary care units in Thailand. Alcohol Alcohol 2010; 45: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kypri K., Wilson A., Attia J., Sheeran P., Miller P., McCambridge J. Social desirability bias in the reporting of alcohol consumption: a randomized trial. J Stud Alcohol Drugs 2016; 77: 526–531. [DOI] [PubMed] [Google Scholar]
- 40. McCambridge J., Wilson A., Attia J., Weaver N., Kypri K. Randomized trial seeking to induce the Hawthorne effect found no evidence for any effect on self‐reported alcohol consumption online. J Clin Epidemiol 2019; 108: 102–109. [DOI] [PubMed] [Google Scholar]
- 41. Bendtsen M. Heterogeneous treatment effects of a text messaging smoking cessation intervention among university students. PLOS ONE 2020; 15: e0229637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moyer A., Finney J. W., Swearingen C. E., Vergun P. Brief interventions for alcohol problems: a meta‐analytical review of controlled investigations in treatment‐seeking and non‐treatment‐seeking populations. Addiction 2002; 97: 279–292. [DOI] [PubMed] [Google Scholar]
- 43. Kypri K., Wilson A., Attia J., Sheeran P. J., McCambridge J. Effects of study design and allocation on self‐reported alcohol consumption: randomized trial. Trials 2015; 16: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A Search strategies.
Appendix B Description of included trials.
Appendix C Risk of bias judgment.
Appendix D Planned subgroup analyses.


