Abstract
Background and aims
In June 2016, the Ontario, Canada government implemented the Ontario Naloxone Program for Pharmacies (ONPP), authorizing pharmacists to provide injectable naloxone kits at no charge to all Ontario residents. In March 2018, the program was amended to include intranasal naloxone and remove the requirement to present a government health card to the dispensing pharmacist. We examined whether these changes increased naloxone dispensing through the ONPP.
Design
Population‐based time–series analysis using interventional autoregressive integrated moving average models.
Setting
Ontario, Canada.
Participants
All Ontario residents between 1 July 2016 and 31 March 2020.
Measurements
Monthly rates of pharmacy naloxone dispensing.
Findings
Overall, 199 484 individuals were dispensed a naloxone kit during the study period. In the main analysis, the rate of pharmacy naloxone dispensing increased by 65.1% following program changes (55.6–91.8 kits per 100 000 population between February 2018 and May 2018; P = 0.01). In subgroup analyses, naloxone dispensing increased among individuals receiving opioid agonist therapy (OAT) (3374.9–7264.2 kits per 100 000 OAT recipients; P = 0.04) among individuals receiving other prescription opioids (192.8–381.8 kits per 100 000 population prescribed opioids; P < 0.01), among individuals with past opioid exposure (134.7–205.6 kits per 100 000 population with past opioid exposure; P < 0.01) and in urban centers (56.2–91.4 kits per 100 000 population; P < 0.01). We did not observe a clear impact on pharmacy‐dispensed naloxone to individuals with no or unknown opioid exposure (34.4–39.3 kits per 100 000 population with no/unknown opioid exposure; P = 0.42) and in rural regions (50.4–97.2 kits per 100 000 population; P = 0.09).
Conclusions
Changes to the Ontario Naloxone Program for Pharmacies to add intranasal naloxone and remove the requirement to present a government health card appeared to increase pharmacy‐based naloxone dispensing uptake in Ontario, Canada, particularly among individuals at high risk of inadvertent opioid overdose.
Keywords: Drug policy, harm reduction, health services research, naloxone, opioid, policy evaluation
Introduction
Overdoses and deaths involving opioids are a public health crisis in North America, with approximately 72 600 opioid‐related deaths occurring in Canada and the United States in 2018 alone [1, 2]. In Ontario, Canada's largest province, opioid‐related deaths increased by more than 400% between 2000 and 2018, from 1.9 to 10.2 deaths per 100 000 population [3, 4]. Importantly, in 2018, more than 90% of opioid‐related deaths in Canada were accidental and potentially preventable [5]. Although illicitly derived, non‐prescribed fentanyl analogues have contributed to most opioid‐related deaths since 2017 [1, 2], prescription opioids remain important drivers of opioid‐related harm in Ontario [5]. Because the majority of opioid overdoses are accidental and reversible with the prompt administration of the opioid antagonist naloxone [6, 7, 8, 9, 10, 11, 12, 13, 14], expanding access to take‐home naloxone kits has become a widely accepted harm‐reduction measure [15], with an estimated effectiveness of 96% for reversing opioid overdoses when administered by laypeople [16].
Pharmacist‐dispensed naloxone is a relatively novel strategy for optimizing the distribution of take‐home naloxone [17]. A 2016 scoping review of 16 international studies found that pharmacy‐dispensed naloxone research encompassed five domains, including pharmacist perceptions of naloxone, legal issues, pharmacist training, supply systems and costs and identification and recruitment of patients [18]. Evidence supporting the effectiveness of such programs is increasing, with findings from a recent US study showing that jurisdictions granting pharmacists direct authority to dispense naloxone had greater declines in opioid‐related deaths than jurisdictions implementing alternative naloxone strategies [19]. In 2016, the Ontario Ministry of Health and Long‐Term Care (MOHLTC) granted pharmacists the authority to provide injectable naloxone kits at no charge to all Ontario residents through the Ontario Naloxone Program for Pharmacies (ONPP) initiative [20]. Recent evaluations of the ONPP found that uptake was not uniform among Ontario residents between July 2016 and March 2018, with limited engagement by individuals prescribed high‐doses of opioids and those with a prior history of opioid‐related harm, and lower dispensing rates in rural relative to urban regions [21, 22]. In an effort to remove potential barriers to naloxone uptake through the ONPP, the program was amended in March 2018 to include intranasal naloxone and remove the requirement for residents to produce a valid health insurance card to the dispensing pharmacist as a condition of obtaining a naloxone kit [23]. We examined whether these changes increased the distribution of pharmacy‐based naloxone through the ONPP.
Methods
Setting
We conducted a population‐based time series analysis of all residents in Ontario between 1 July 1 and 31 March 2020.
Data sources
Data sources are summarized in Supporting information, Appendix S1. The use of data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act, which does not require Research Ethics Board review.
Study population and outcomes
For each month of the study period, we determined rates of naloxone dispensing by Ontario pharmacies per 100 000 Ontario residents, defined as the number of naloxone kits dispensed in a given month divided by the population of Ontario for that period. To determine if naloxone dispensing rates varied among individuals at differential risk of opioid‐related harm, we stratified the population of Ontario residents dispensed naloxone into four mutually exclusive, pre‐defined groups, using a pre‐defined hierarchical stratification approach that we applied in our previous work [21, 22]. First, we identified recipients of opioid agonist therapy (OAT) as individuals with any prescription for methadone or buprenorphine/naloxone dispensed on the naloxone claim date or in the 14 days preceding it. Secondly, we defined individuals taking other prescription opioids at the time they were dispensed a naloxone kit, defined by any non‐OAT opioid prescription with a supply overlapping the naloxone claim date. Thirdly, we defined individuals with a prior history of chronic prescription opioid exposure as those with either a prescription for a long‐acting non‐OAT opioid in the prior 4 years, five or more prescriptions for an immediate release non‐OAT opioid in the prior 4 years or any health service use for opioid‐use disorder or opioid‐related harm in the 5 years preceding the receipt of naloxone. We developed this definition to exclude individuals who were unlikely to have had chronic opioid exposure in the past. The remaining population was defined as those individuals with no or unknown opioid exposure history. We similarly applied the mutually exclusive stratification method for all Ontario residents to define population denominators in each month during the study period, and computed rates of naloxone dispensing per 100 000 population for each exposure group. Because we could not identify patients obtaining a naloxone kit without an Ontario health card in our administrative databases, we excluded these individuals from the stratified analysis. To explore regional variation in the impact of the March 2018 policy changes, we also stratified our analyses according to rural and urban regions of Ontario.
Statistical analysis
We used interventional autoregressive integrated moving average (ARIMA) models to examine the impact of including intranasal naloxone and removing the requirement to produce a health card on naloxone dispensing rates overall, and stratified by pre‐defined opioid exposure group and urban and rural regions, between July 2016 and March 2020 [24]. This study period encompasses all months for which data regarding the ONPP were available for analysis. Because we speculated that these changes would lead to immediate increases in the uptake of naloxone through community pharmacies, we tested for an immediate change in the rate of naloxone dispensing using a step function following implementation of these program changes (i.e. March 2018). We also included ramp intervention functions in all models to account for slope trends in rates of naloxone dispensing before and after the program changes and changes in naloxone dispensing associated with the May 2017 publication of Canadian guidelines for the use of opioids in chronic non‐cancer pain, which supported the provision of naloxone to patients at risk of overdose [25]. We used differencing to achieve a stationary time–series, and stationarity was confirmed using the augmented Dickey–Fuller test [26]. We examined autocorrelation function (ACF), partial autocorrelation function (PACF) and inverse correlation function (IACF) plots to guide appropriate moving average or autoregressive terms for the models. We assessed model fit using the ACF, PACF and IACF plots, white noise probability plots (Supporting information, Appendix S2) and Ljung–Box χ2 test for white noise [24, 27]. The analysis was not pre‐registered. All analyses were completed at ICES and St Michael's Hospital using SAS Enterprise Guide, version 6.1 (SAS Institute Inc., Cary, NC, USA).
Results
Overall, 199 484 individuals were dispensed a naloxone kit through the ONPP during the study period, 35 909 of whom (18.0%) were OAT recipients, 38 294 (19.2%) were prescription opioid recipients, 16 835 (8.4%) had past opioid exposure and 113 808 (57.1%) had no or unknown opioid exposure. The majority [n = 150 885 (75.6%)] of individuals accessed the ONPP after March 2018. During the 45‐month study period, 446 690 naloxone kids were dispensed (56.9% intranasal), and the monthly rate of pharmacy naloxone dispensing increased from 1.9 to 111.5 kits per 100 000 population. Prior to the March 2018 program changes, only the injectable formulation of naloxone was dispensed through Ontario pharmacies. The inclusion of intranasal naloxone in the pharmacy program in March 2018 shifted the pattern of naloxone dispensing, with the intranasal formulation representing 72.8% of all kits dispensed in March 2020. Similarly, the proportion of all naloxone kits dispensed without presentation of a personal health card increased from 0.0% to 37.3% between July 2016 and March 2020 (Fig. 1).
Figure 1.
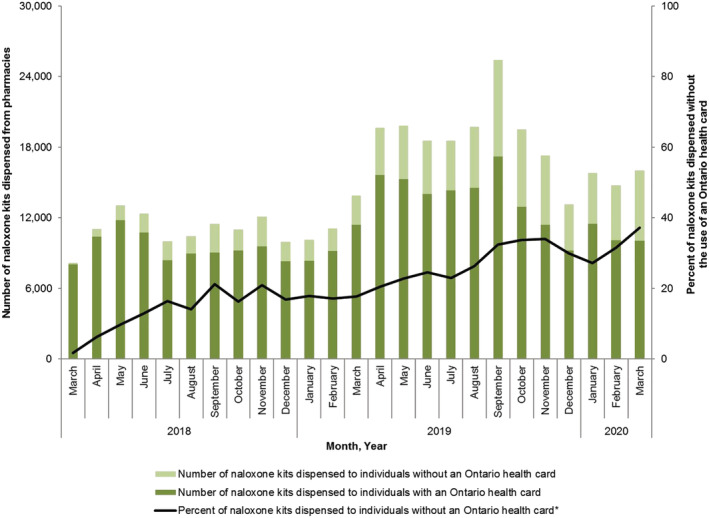
Number and percentage of pharmacy‐dispensed naloxone kits in Ontario, stratified according to whether provincial health card provided at time of dispensing. [Colour figure can be viewed at wileyonlinelibrary.com]
In our main analysis, the overall rate of naloxone dispensing increased immediately after the implementation of program changes in March 2018. Specifically, between February 2018 (the month preceding the policy change) and May 2018 (2 months following the policy change), the rate of pharmacy naloxone dispensing increased 65.1% (from 55.6 to 91.8 kits per 100 000 population; P = 0.01) (Fig. 2). In stratified analyses, we found immediate increases in rates of pharmacy naloxone dispensing between February 2018 and May 2018 among individuals receiving OAT (115.2% increase; 3374.9–7264.2 kits per 100 000; P = 0.04), prescription opioid recipients (98.0% increase; 192.8–381.8 kits per 100 000 population; P < 0.01), individuals with prior opioid exposure (52.6% increase; 134.7–205.6 kits per 100 000 population; P < 0.01) and in urban regions of Ontario (62.6% increase; 56.2–91.4 kits per 100 000 population; P < 0.01) (Figs 3 and 4). Conversely, we found no impact on pharmacy naloxone dispensing between February 2018 and May 2018 among individuals with no or unknown opioid exposure history 2018 (34.4 versus 39.3 kits per 100 000 population; P = 0.42) and in rural Ontario (92.9% increase; 50.4–97.2 kits per 100 000 population; P = 0.09).
Figure 2.
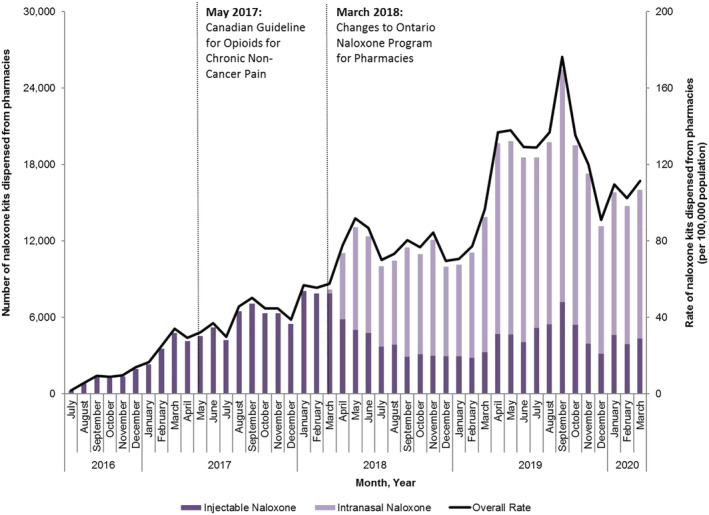
Number and rate (per 100 000 population) of pharmacy naloxone distribution in Ontario, July 2016–March 2020. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
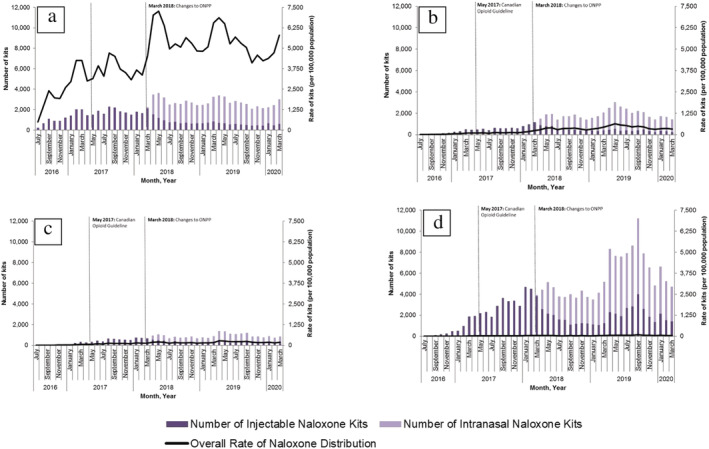
Number and rate (per 100 000 population) of pharmacy‐dispensed naloxone distribution in Ontario, stratified by opioid exposure group, July 2016–March 2020. (a) Prescription opioid agonist therapy recipients; (b) prescription opioid recipients; (c) people with past opioid exposure; (d) people with no/unknown opioid exposure. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
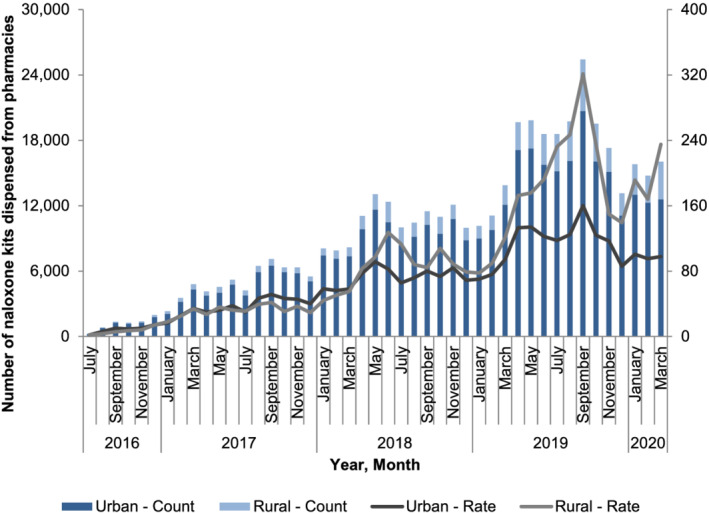
Number and rate (per 100 000 population) of pharmacy naloxone distribution in Ontario, by urban and rural region. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
In this population‐based study, we found that introduction of intranasal naloxone and the removal of the requirement to present a provincial health card were associated with an immediate increase in rates of pharmacy naloxone dispensing. This finding was consistent among OAT recipients, past and present opioid recipients and in urban regions of Ontario, with no immediate impact observed among individuals with no or unknown opioid exposure and rural regions of Ontario. Our findings highlight the importance of a policy intervention aimed at expanding naloxone formulation options and removing potentially stigmatizing requirements for identification in increasing access to naloxone through community pharmacies.
Our findings add to earlier research. Although several US studies have found that the passage of naloxone access laws significantly increased naloxone dispensing rates [17, 28, 29], ours is the first, to our knowledge, to quantify the effect of policy changes in an existing program targeting specific barriers to naloxone access. Moreover, our findings demonstrate that the program changes were associated with a 98.0% increase in naloxone dispensing among current recipients of prescribed opioids. This finding is important, because several US studies have found that individuals prescribed opioids may not feel comfortable availing themselves of naloxone through other distribution channels, such as harm reduction sites, and may be at low self‐perceived risk of inadvertent opioid overdose [30, 31, 32, 33]. However, changes to the ONPP did not appreciably increase naloxone dispensing rates to individuals with no or unknown opioid exposure history, a heterogeneous group that includes family and friends of individuals at risk of opioid overdose. Further research exploring barriers of naloxone access in this population is warranted, as prior international research has demonstrated that bystanders who have received naloxone training are more likely to recognize overdose scenarios and identify when naloxone administration is indicated compared to those with no such training [34, 35, 36].
Our study has some limitations. First, our study does not include naloxone distributed through other channels such as harm reduction sites and physicians' offices, and therefore underestimates total naloxone access by Ontario residents during the study period. However, we were specifically interested in quantifying the impact of changes to the ONPP on pharmacy‐based naloxone dispensing, which now represents the majority of naloxone distribution in Ontario [37]. Secondly, we cannot quantify separately the impact of including intranasal naloxone and removing the requirement to present an Ontario health card because both program changes were implemented in March 2018. However, we speculate that the availability of intranasal naloxone was the more impactful of the two changes, given that nearly two‐thirds of individuals continued to present a health card at the time of naloxone dispensing in March 2020, at which point intranasal naloxone represented 72.8% of all kits dispensed. A preference for the intranasal formulation probably reflects a greater sense of comfort and ease of use relative to injectable naloxone, and has been confirmed in a recent study of opioid recipients to be the preferred formulation of the drug [38]. Thirdly, some individuals may have been misclassified when deriving our exposure group definitions. However, our previous work summarizing the demographics of each exposure group suggests that any misclassification is probably minimal [21]. Finally, we did not have sufficient data to identify and account for seasonality in our models, or pharmacy characteristics that may influence pharmacy‐dispensed naloxone rates.
In summary, our study suggests that the addition of intranasal naloxone and removal of the requirement to present a government health card were associated with an increased rate of pharmacy‐based naloxone dispensing, including to individuals at risk of inadvertent opioid overdose. These findings can inform the development of pharmacy‐based naloxone programs in other jurisdictions by highlighting program components that were associated with a marked uptake in naloxone access through community pharmacies.
Declaration of interests
P.L. reports non‐financial support from Adapt Pharma through in‐kind donation of naloxone on an unrelated study. Adapt Pharma is not involved in the design or conduct of the project. M.M. reports receiving honoraria for serving as member on ad‐hoc advisory boards for Allergan and Novo Nordisk and receiving an honorarium from Celgene. No other authors report any conflicts of interest.
Author contributions
Tony Antoniou: Conceptualization; funding acquisition; writing‐original draft. Diana Martins: Formal analysis; project administration; writing‐review & editing. Tonya Campbell: Data curation; formal analysis; writing‐review & editing. Mina Tadrous: Conceptualization; funding acquisition; writing‐review & editing. Charlotte Munro: Conceptualization; funding acquisition; writing‐review & editing. Pamela Leece: Conceptualization; funding acquisition; writing‐review & editing. Muhammad Mamdani: Conceptualization; funding acquisition; writing‐review & editing. David N. Juurlink: Conceptualization; funding acquisition; writing‐review & editing. Tara Gomes: Conceptualization; funding acquisition; methodology; writing‐review & editing.
Supporting information
Data S1. Supporting information
Data S2. Supporting information
Acknowledgements
This study was funded by a grant from the Canadian Institutes for Health Research (grant no. 410281). This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. We thank Nancy Lum‐Wilson for her comments on the initial findings for this study and IMS Brogan Inc. for use of their Drug Information Database. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI.
Antoniou, T. , Martins, D. , Campbell, T. , Tadrous, M. , Munro, C. , Leece, P. , Mamdani, M. , Juurlink, D. N. , and Gomes, T. (2021) Impact of policy changes on the provision of naloxone by pharmacies in Ontario, Canada: a population‐based time–series analysis. Addiction, 116: 1514–1520. 10.1111/add.15324.
References
- 1. Centers for Disease Control and Prevention . Provisional Drug Overdose Death Counts. Available at: https://www.cdc.gov/nchs/nvss/vsrr/drug‐overdose‐data.htm (accessed 20 January 2020).
- 2. Government of Canada . Apparent Opioid‐Related Deaths: Surveillance of Opioid‐related Harms in Canada. Available at: https://health‐infobase.canada.ca/substance‐related‐harms/opioids/graphs?index=328 (accessed 17 August 2020).
- 3. Gomes T., Juurlink D. N. Understanding the implications of a shifting opioid landscape in Ontario. Healthcare Q 2019; 22: 6–11. [DOI] [PubMed] [Google Scholar]
- 4. Gomes T., Greaves S., Tadrous M., Mamdani M. M., Paterson J. M., Juurlink D. N. Measuring the burden of opioid‐related mortality in Ontario, Canada. J Addict Med 2018; 12: 418–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ontario Agency for Health Protection and Promotion (Public Health Ontario), Office of the Chief Coroner Ontario Forensic Pathology Service, Ontario Drug Policy Research Network . Opioid mortality surveillance report: analysis of opioid‐related deaths in Ontario July 2017–June 2018. Toronto, Ontario: Public Health Ontario; 2019.
- 6. World Health Organization (WHO) . Community Management of Opioid Overdose. Geneva: WHO; 2014. Available at: http://www.who.int/substance_abuse/publications/management_opioid_overdose/en/ (accessed 20 January 2020). [PubMed]
- 7. Beletsky L., Rich J. D., Walley A. Y. Prevention of fatal opioid overdose. JAMA 2012; 308: 18634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strang J., Best D., Man L., Noble A., Gossop M. Peer‐initiated overdose resuscitation: fellow drug users could be mobilised to implement resuscitation. Int J Drug Policy 2000; 11: 437–445. [DOI] [PubMed] [Google Scholar]
- 9. Walley A. Y., Xuan Z., Hackman H. H., Quinn E., Doe‐Simkins M., Sorensen‐Alawad A., et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 2013; 346: f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walley A. Y., Doe‐Simkins M., Quinn E., Pierce C., Xuan Z., Ozonoff A. Opioid overdose prevention with intranasal naloxone among people who take methadone. J Subst Abuse Treat 2013; 44: 241–247. [DOI] [PubMed] [Google Scholar]
- 11. Yokell M. A., Green T. C., Bowman S., McKenzie M., Rich J. D. Opioid overdose prevention and naloxone distribution in Rhode Island. Med Health RI 2011; 94: 240–242. [PMC free article] [PubMed] [Google Scholar]
- 12. Maxwell S., Bigg D., Stanczykiewicz K., Carlberg‐Racich S. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis 2006; 25: 89–96. [DOI] [PubMed] [Google Scholar]
- 13. Piper T. M., Stancliff S., Rudenstine S., Sherman S., Nandi V., Clear A., et al. Evaluation of a naloxone distribution and administration program in New York City. Subst Use Misuse 2008; 43: 858–870. [DOI] [PubMed] [Google Scholar]
- 14. Doe‐Simkins M., Walley A. Y., Epstein A., Moyer P. Saved by the nose: bystander‐administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health 2009; 99: 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wheeler E., Jones T. S., Gilbert M. K., Davidson P. J. Centers for Disease Control and Prevention (CDC). Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. Morb Mortal Wkly Rep 2015; 64: 631–635. [PMC free article] [PubMed] [Google Scholar]
- 16. McDonald R., Strang J. Are take‐home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016; 111: 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guy G. P. Jr., Haegerich T. M., Evans M. E., Losby J. L., Young R., Jones C. M. Vital signs: pharmacy‐based naloxone dispensing—United States, 2012–2018. Morb Mortal Wkly Rep 2019; 68: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen S., Van Hout M. C. What is known about community pharmacy supply of naloxone? A scoping review. Int J Drug Policy 2016; 32: 24–33. [DOI] [PubMed] [Google Scholar]
- 19. Abouk R., Pacula R. L., Powell D. Association between state laws facilitating pharmacy distribution of naloxone and risk of fatal overdose. JAMA Intern Med 2019; 179: 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canadian Pharmacists Association . Access to Naloxone Across Canada [Environmental Scan]. Ottawa, ON: Canadian Pharmacists Assocation; 2017. Available at: https://www.pharmacists.ca/cpha‐ca/assets/File/cpha‐on‐the‐issues/Environmental%20Scan%20‐%20Access%20to%20Naloxone%20Across%20Canada_Final.pdf (accessed 20 January 2020). [Google Scholar]
- 21. Choremis B., Campbell T., Tadrous M., Martins D., Antoniou T., Gomes T. The uptake of the pharmacy‐dispensed naloxone kit program in Ontario: a population‐based study. PLOS ONE 2019; 14: e0223589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antoniou T., McCormack D., Campbell T., Sutradhar R., Tadrous M., Lum‐Wilson N., et al. Geographic variation in the provision of naloxone by pharmacies in Ontario, Canada: a population‐based small area variation analysis. Drug Alcohol Depend 2020; 216: 108238. [DOI] [PubMed] [Google Scholar]
- 23. Ministry of Health and Long‐Term Care Ontario Public Drug Programs . Funding of Naloxone Nasal Spray Kits through the Ontario Naloxone Program for Pharmacies (ONPP) and Updates to the Existing Program: Updated Frequently Asked Questions for Pharmacists. Available at: http://www.health.gov.on.ca/en/pro/programs/drugs/opdp_eo/notices/fq_exec_office_20180321.pdf (accessed 20 January 2020).
- 24. Helfenstein U. The use of transfer function models, intervention analysis and related time series methods in epidemiology. Int J Epidemiol 1991; 20: 808–815. [DOI] [PubMed] [Google Scholar]
- 25. Busse J. W., Craigie S., Juurlink D. N., Buckley D. N., Wang L., Couban R. J., et al. Guideline for opioid therapy and chronic noncancer pain. Can Med Assoc J 2017; 189: E659–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dickey D. A., Fuller W. A. Distribution of the estimators for autoregressive time series with a unit root. J Am Stat Assoc 1979; 74: 427–431. [Google Scholar]
- 27. Ljung G. M., Box G. E. P. On a measure of lack of fit in time series models. Biometrika 1978; 65: 297–303. [Google Scholar]
- 28. Sohn M., Talbert J. C., Huang Z., Lofwall M. R., Freeman P. R. Association of Naloxone Coprescription Laws with naloxone prescription dispensing in the United States. JAMA Netw Open 2019; 2: e196215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J., Davis C. S., Cruz M., Lurie P. State naloxone access laws are associated with an increase in the number of naloxone prescriptions dispensed in retail pharmacies. Drug Alcohol Depend 2018; 189: 37–41. [DOI] [PubMed] [Google Scholar]
- 30. Dunn K. E., Barrett F. S., Fingerhood M., Bigelow G. E. Opioid overdose history, risk behaviors, and knowledge in patients taking prescribed opioids for chronic pain. Pain Med 2017; 18: 1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mueller S. R., Koester S., Glanz J. M., Gardner E. M., Binswanger I. A. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non‐cancer pain. J Gen Intern Med 2017; 32: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilder C. M., Miller S. C., Tiffany E., Winhusen T., Winstanley E. L., Stein M. D. Risk factors for opioid overdose and awareness of overdose risk among veterans prescribed chronic opioids for addiction or pain. J Addict Dis 2016; 35: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Behar E., Bagnulo R., Coffin P. O. Acceptability and feasibility of naloxone prescribing in primary care settings: a systematic review. Prev Med 2018; 114: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giglio R. E., Li G., DiMaggio C. J. Effectiveness of bystander naloxone administration and overdose education programs: a meta‐analysis. Inj Epidemiol 2015; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashrafioun L., Gamble S., Herrmann M., Baciewicz G. Evaluation of knowledge and confidence following opioid overdose prevention training: a comparison of types of training participants and naloxone administration methods. Subst Abuse 2016; 37: 76–81. [DOI] [PubMed] [Google Scholar]
- 36. Bagley S. M., Forman L. S., Ruiz S., Cranston K., Walley A. Y. Expanding access to naloxone for family members: the Massachusetts experience. Drug Alcohol Rev 2018; 37: 480–486. [DOI] [PubMed] [Google Scholar]
- 37. Ontario Drug Policy Research Network . Naloxone distribution across Ontario. Available at: https://odprn.ca/wp‐content/uploads/2019/05/Naloxone‐Distribution‐Report‐Final.pdf (accessed 20 January 2020).
- 38. Dunn K. E., Barrett F. S., Bigelow G. E. Naloxone formulation for overdose reversal preference among patients receiving opioids for pain management. Addict Behav 2018; 86: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Data S2. Supporting information


