Abstract
Background
To evaluate whether buccal bone thickness (BBT), implant diameter, and abutment/crown material influence the accuracy of cone‐beam computed tomography (CBCT) to determine the buccal bone level at titanium implants.
Methods
Two implant beds (i.e., narrow and standard diameter) were prepared in each of 36 porcine bone blocks. The implant beds were positioned at a variable distance from the buccal bone surface; thus, resulting in three BBT groups (i.e., >0.5 to 1.0; >1.0 to 1.5; >1.5 to 2.0 mm). In half of the blocks, a buccal bone dehiscence of random extent (“depth”) was created and implants were mounted with different abutment/crown material (i.e., titanium abutments with a metal‐ceramic crown and zirconia abutments with an all‐ceramic zirconia crown). The distance from the implant shoulder to the buccal bone crest was measured on cross‐sectional CBCT images and compared with the direct measurements at the bone blocks.
Results
While abutment/crown material and implant diameter had no effect on the detection accuracy of the buccal bone level at dental implants in CBCT scans, BBT had a significant effect. Specifically, when BBT was ≤1.0 mm, a dehiscence was often diagnosed although not present, that is, the sensitivity was high (95.8%), but the specificity (12.5%) and the detection accuracy (54.2%) were low. Further, the average measurement error of the distance from the implant shoulder to the buccal bone crest was 1.6 mm.
Conclusions
Based on the present laboratory study, BBT has a major impact on the correct diagnosis of the buccal bone level at dental titanium implants in CBCT images; in cases where the buccal bone is ≤1 mm thick, detection of the buccal bone level is largely inaccurate.
Keywords: alveolar process, cone‐beam computed tomography, dental implants, titanium, zirconium
1. INTRODUCTION
Cone‐beam computed tomography (CBCT) is used in implant dentistry primarily for treatment planning. 1 , 2 However, due to its capacity to register tissues in 3D, CBCT has been also widely used in several studies for assessing the buccal bone dimension at implants sites. 3 , 4 , 5 , 6 For example, a previous study assessed the fate of the buccal bone 10 years after early, delayed, or late implant installation by means of CBCT; 6 no significant differences were shown among the various timings of implant installation, with the buccal bone level being on average about 2 mm below the implant shoulder, but implants with an intrabony or a dehiscence defect at second stage surgery showed a significantly more apically located buccal bone level (i.e., at about 2.5 and 2.8 mm, respectively) compared with implants with no defect at second stage surgery (i.e., at about 1.8 mm). It is generally considered that the implant body should be completely surrounded with bone, while the presence of thin bone or a bone defect (i.e., a dehiscence or a fenestration) at the buccal aspect of the implant is considered a risk factor for esthetic and/or biological complications on the long‐term. 7 , 8 , 9
In this context, despite the fact, that CBCT is very accurate in terms of estimating distances of anatomical landmarks and dimensions of bone defects, 10 , 11 , 12 , 13 the accuracy of CBCT to assess bone in close proximity to dental implants is somehow questionable. The accuracy might be limited due to beam hardening artifacts from the metal of the implants and/or due to the reconstruction that may compromise image quality. 14 Indeed, several factors have been described to influence the accuracy of CBCT in regards to the implant site in general and in particular in regards to the condition of the buccal bone. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Two previously published laboratory studies in pig mandibles demonstrated that various modifiable (e.g., the resolution and the image reconstruction thickness, the CBCT unit) and non‐modifiable (e.g., the implant‐abutment material, the number of implants in the field of view) factors influence the accuracy of CBCT when assessing the buccal bone at implant sites. 19 , 20 Specifically, zirconia implant‐abutment restorations compared with titanium implant‐abutment restorations as well as multiple implants (i.e., two or three implants) compared with single implants increased the risk that the buccal bone condition was not correctly detected by 12 to 20 times and by three to 12 times, respectively; the range in the odds ratio is based on the data derived from two different CBCT units used in this specific study. 19 However, to date, there is no information available on whether the buccal bone thickness (BBT) affects the accuracy of CBCT to determine the buccal bone level at implants (i.e., the exact distance from the implant shoulder to the buccal bone crest).
The aim of the present laboratory study was to evaluate whether BBT influences the accuracy of CBCT to determine the buccal bone level at titanium implants, and whether the implant diameter and the abutment/crown material have an additional impact on the results.
2. MATERIALS AND METHODS
2.1. Specimen preparation
For the present study, 36 bone blocks of dry domestic pig jaws purchased from a local butcher were used. The bone blocks were obtained from the mandibular ramus with a horizontal cut at the height of the occlusal level and a vertical cut at the anterior aspect of the ramus, that is, the blocks contained the angle of the mandible. This is a region consisting of a cortical and cancellous bone structure similar to the residual alveolar process in humans. 23 , 24 The cranial cut surface of the bone blocks was used for implant installation and, hence, the lateral flat side of the blocks represented the buccal aspect (Fig. 1).
FIGURE 1.
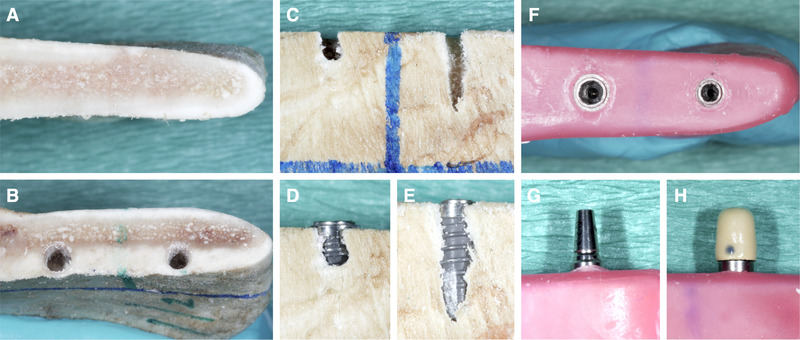
Preparation of the bone blocks and implant installation. The cranial cut surface (A) of the bone blocks was used for implant installation (B) and the flat side of the blocks was representing the buccal aspect. In half of the blocks of each group, a randomly sized buccal bone dehiscence was created with a round metal bur (C) and two implants were installed in each block (D and E). Before CBCT recording, the block was covered in a layer of pink wax (F) and the implants restored either with a titanium abutment (G) and metal‐ceramic crown H) or with a zirconia abutment and an all‐ceramic zirconia crown
2.2. Implant positioning and grouping according to the buccal bone thickness
Two implant beds (final drill: 3.3 and 3.8 mm in diameter, respectively, and 9 mm in length) were prepared 15 mm apart from each other (center‐to‐center) following the manufacturer's drilling protocol* by a single operator (DD) (Fig. 1). Implants were placed flush with the horizontal cut, but the positioning in relation to the buccal bone surface varied, thus, resulting in three groups of BBT after implant installation (Fig. 2): 1) Group 1 (n = 12): BBT >0.5 to 1.0 mm; 2) Group 2 (n = 12): BBT >1.0 to 1.5 mm; and 3) Group 3 (n = 12): BBT >1.5 to 2.0 mm.
FIGURE 2.
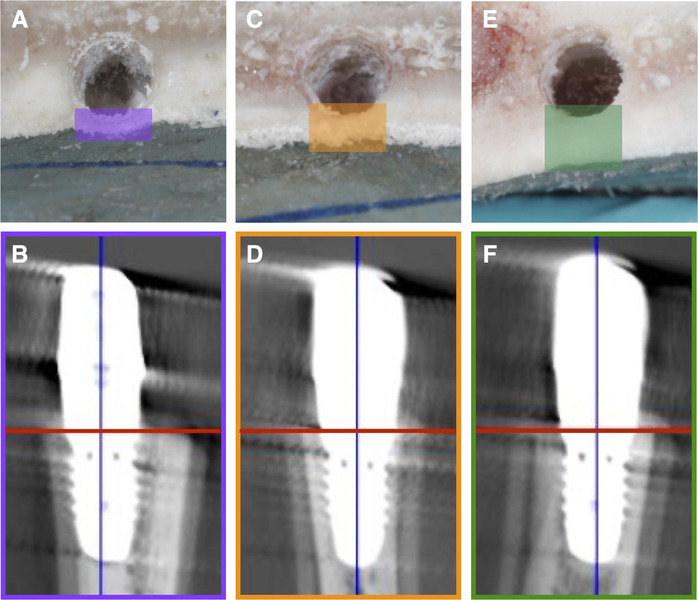
Implant positioning in relation to the buccal bone surface was grouped according to the buccal bone thickness (BBT) after implant installation: A and B) group 1 with a BBT >0.5 to 1.0 mm; C and D) group 2 with a BBT >1.0 to 1.5 mm; and E and F) group 3 with a BBT >1.5 to 2.0 mm
Previous pilot experiments have shown that when BBT was ≤0.5 mm, the buccal bone at implants was impossible to discern in CBCT with the current set‐up; thus, blocks with BBT ≤0.5 mm were intentionally not included.
In half of the blocks of each group, a buccal bone dehiscence was created with a round metal bur, randomly sized from 1 to 9 mm in extent (“depth”) by the same operator (DD). Dehiscence was created at least 4 weeks before any assessment. Then, two titanium implants* were installed in each block (diameter, 3.5 mm [narrow] and 4.1 mm [standard], respectively; length, 9 mm). Figure S1 (see online Journal of Periodontology) shows a flowchart of the study design.
2.3. CBCT recording
Before the CBCT scan, each block was positioned into a putty silicon† “platform” and individually adapted with the cranial cut surface containing the implants being horizontal, using a magnetic water‐level ruler. Further, each block was covered with a layer of pink wax‡ to imitate the soft tissues and provide a realistic x‐ray beam attenuation 25 (Fig. 1). Thereafter, CBCT scans§ were exposed with standardized settings (kV 90; mA 5; FOV 40 × 40 mm; voxel size, 0.125 mm; slice thickness, 1 mm). This specific CBCT unit is limited to a 180° rotation and a scout image was used to obtain the region of interest. Each block was scanned four times, always containing one implant: the first scan included the narrow diameter implant restored with a titanium abutment and metal‐ceramic crown (cobalt‒chromium alloy); the second scan included the same implant but restored with a zirconia abutment and an all‐ceramic zirconia crown; the third and fourth scans included the standard diameter implant and the two prosthetic options, respectively.
2.4. Evaluation of the buccal bone level
2.4.1. CBCT measurements
The raw data sets were exported and reconstructed into 3D volumes using appropriate software.** For evaluation of the buccal bone level, each implant was orientated centrally in a vertical and sagittal direction and the horizontal plane was positioned parallel to the cranial cut at the height of the implant shoulder. The image displaying a cross‐section of the bone and implant was finally exported as portable network graphics (i.e., PNG file) to an image‐processing program.†† Altogether, 144 images (i.e., 36 bone blocks × two implant diameters × two prosthetic restorations) were evaluated by two examiners (DD, SA). Both examiners judged blindly the presence of a buccal bone dehiscence, that is, the examiners did not know about the BBT group, implant diameter, and type of prosthetic restoration while judging the cross‐sections. Further, the judgment was performed in a dark room under standardized conditions; that is, both examiners used the same screen and the settings (i.e., brightness, contrast) were unchanged during the entire judgment period. In case of diagnosing a buccal bone dehiscence, the buccal bone level was determined by measuring the distance from the implant shoulder to the buccal bone crest (i.e., the deepest point of the dehiscence; Figure 3). For this purpose, the integrated measurement tool was used after calibration by means of the ruler included in the cross‐section images. One examiner (DD) repeated the assessment for 50% of the images.
FIGURE 3.
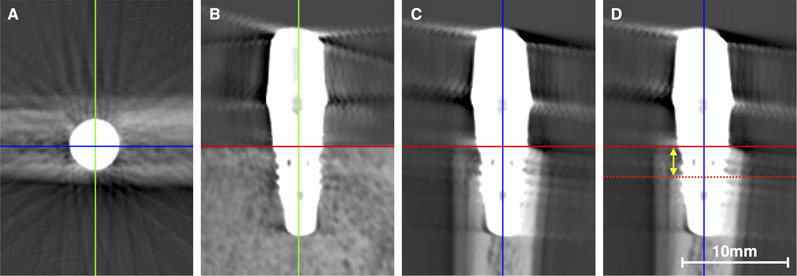
For evaluation of the buccal bone, each implant was orientated centrally in a vertical and sagittal direction (A and B; green and blue line) and the horizontal plane (red line) was positioned at the height of the implant shoulder (B and C). The image displaying the cross‐section of the implant (C and D) was judged blindly by two examiners on the presence or absence of a buccal bone dehiscence. In case a buccal bone dehiscence was diagnosed, the extent (yellow arrow) from the implant shoulder to the buccal bone crest (i.e., the deepest point of the dehiscence; red dotted line) was measured (D)
2.4.2. Direct measurements
After finishing the evaluation of the CBCT cross‐sections, the wax was removed from the blocks and the distance from the implant shoulder to the buccal bone crest was measured in triplicates by means of a digital caliper.‡‡ The mean value of these direct triplicate measurements was considered as the true buccal bone level and was used for the comparison with the CBCT measurements.
2.5. Statistical analysis
Two main outcome parameters 1) “correct diagnosis of presence/absence of a buccal bone dehiscence” and 2) “difference (in mm) between direct and CBCT measurements of the buccal bone level”, and three predictors (i.e., BBT, implant diameter, abutment/crown material) were defined. A binary logistic regression was performed to assess the effect of the predictors on the correct diagnosis of presence/absence of a buccal bone dehiscence based on all blocks/observations (n = 144). Further, in case of statistical significance for a given factor, the sensitivity (i.e., true positives divided by the real positive cases), the specificity (i.e., true negatives divided by the real negative cases), and the detection accuracy (i.e., sum of true positive and negative cases divided by the sum of real positive and negative cases; dACC) were calculated for the correct diagnosis of the presence/absence of a buccal bone dehiscence. A median regression was performed to assess the effect of the various predictors on the difference between the direct and CBCT measurements of the buccal bone level, based only on those blocks/observations with a dehiscence (n = 72). Further, Bonferroni correction was applied to the pairwise comparison of the significant variables and for both approaches, a quantile regression was additionally conducted to assess any potential interaction between BBT and implant diameter or abutment/crown material. Finally, the frequency distribution of 1) >0 to 0.5 mm, 2) >0.5 to 1 mm, 3) >1 to 1.5 mm, 4) >1.5 to 2 mm, and 5) >2 mm differences between direct and CBCT measurements of the buccal bone level were calculated. The intra‐observer repeatability and inter‐observer reproducibility regarding the CBCT measurements were tested with the intraclass correlation coefficient (ICC 2,1). Statistical analyses were performed using statistical software§§ and P values <0.05 were considered statistically significant.
3. RESULTS
An overview of the possible impact of the three predictors (i.e., BBT, implant diameter, abutment/crown material) on the two main outcome parameters (i.e., “correct diagnosis of the presence/absence of a buccal bone dehiscence” and “difference between direct and CBCT measurements of the buccal bone level”) is presented in Table 1. Specifically, for the first outcome parameter the frequency distribution of a correctly or wrongly diagnosed buccal bone condition is presented per predictor (i.e., BBT, implant diameter, abutment/crown material), and for the second outcome parameter the mean and standard deviation as well as the median and first/third quartile are presented.
TABLE 1.
Overview of the three predictors (i.e., BBT, abutment/crown material, and implant diameter) in relation to the two main outcome parameters “correct diagnosis of the presence/absence of a buccal bone dehiscence” and “difference between direct and CBCT measurements of the buccal bone level” (based on the observations with an actual dehisce)
| Predictor | Group | Main outcome parameter | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of the presence/absence of a buccal bone dehiscence | Difference between direct and CBCT measurements of the buccal bone level | ||||||||
| n a | Presence of a buccal bone dehiscence (n = 72) | Absence of a buccal bone dehiscence (n = 72) | n b | Mean ± SD (mm) | Median (1Q; 3Q) (mm) | ||||
| Correctly diagnosed | Wrongly diagnosed | Correctly diagnosed | Wrongly diagnosed | ||||||
| BBT | Group 1 | 48 | 23 | 1 | 3 | 21 | 24 | 1.61 ± 1.88 | 0.90 (0.51; 2.24) |
| Group 2 | 48 | 24 | 0 | 20 | 4 | 24 | 0.50 ± 0.63 | 0.34 (0.17; 0.50) | |
| Group 3 | 48 | 24 | 0 | 17 | 7 | 24 | 0.43 ± 0.36 | 0.34 (0.16; 0.59) | |
| Abutment/crown material | Ti/MCC | 72 | 36 | 0 | 19 | 17 | 36 | 0.93 ± 1.19 | 0.46 (0.24; 1.15) |
| Zirconia | 72 | 35 | 1 | 21 | 15 | 36 | 0.77 ± 1.36 | 0.44 (0.22; 0.66) | |
| Implant diameter | Narrow | 72 | 36 | 0 | 19 | 17 | 36 | 0.83 ± 1.39 | 0.35 (0.24; 0.95) |
| Standard | 72 | 35 | 1 | 21 | 15 | 36 | 0.86 ± 1.16 | 0.48 (0.22; 1.00) | |
1Q/3Q; First/third quartile; BBT, buccal bone thickness; CBCT, cone‐beam computed tomography; Ti/MCC, titanium/metal‐ceramic crown.
Numbers represent observations per group resulting in total in 144 observations per predictor (i.e., all blocks are considered for this outcome parameter).
Numbers represent observations per group resulting in total in 72 observations per predictor (i.e., only those blocks with a dehiscence are considered for this outcome parameter).
3.1. Correct diagnosis of presence/absence of a buccal bone dehiscence
Abutment/crown material (P = 0.829) and implant diameter (P = 0.829) did not have a significant impact on the correct diagnosis of presence/absence of a buccal bone dehiscence. In contrast, BBT had a significant impact, with group 1 presenting the lowest chances for a correct diagnosis (P < 0.001, compared with BBT groups 2 and 3). BBT group 2 or 3 had an odds ratio of 9 and 5, respectively, to be correctly diagnosed compared with BBT group 1, and there was no significant difference between BBT groups 2 and 3 (Table 2). No significant interaction between BBT and implant diameter or abutment/crown material was detected.
TABLE 2.
Effect of the three predictors (i.e., BBT, abutment/crown material and implant diameter) on the correct diagnosis of the presence/absence of a buccal bone dehiscence (binary logistic regression; based on 144 observations)
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| B coefficient | Odds ratio | Lower | Higher | P value | ||
| BBT | Group 1 versus 2 | 2.23 | 9.32 | 2.89 | 30.07 | <0.001 |
| Group 1 versus 3 | 1.60 | 4.96 | 1.86 | 13.26 | 0.001 | |
| Group 3 versus 2 | 0.63 | 1.88 | 0.51 | 6.90 | 0.342 | |
| Abutment/crown material | Ti/MCC versus zirconia | –0.93 | 0.91 | 0.39 | 2.12 | 0.829 |
| Implant diameter | Narrow versus standard | –0.93 | 0.91 | 0.39 | 2.12 | 0.829 |
The logistic regression model was statistically significant, χ2(4) = 21.490, P < 0.001, and correctly classified 77.1% of the cases.
Statistically significant values are indicated in bold.
BBT, buccal bone thickness; Ti/MCC, titanium/metal‐ceramic crown.
Among the observations in BBT group 1, in 23 out of 24 cases the presence of a dehiscence was correctly diagnosed resulting in a high sensitivity (i.e., 95.8%). Yet, the absence of a buccal bone dehiscence was correctly diagnosed only in three out of 24 cases resulting in a low specificity (i.e., 12.5%); i.e., in 21 cases a dehiscence was diagnosed although actually not present. Altogether, this resulted in a dACC of 54.2% (Table 1). In BBT groups 2 and 3 the wrong diagnoses were limited to the blocks presenting no buccal bone dehiscence; i.e., in BBT groups 2 and 3, in four and seven cases, respectively, a buccal bone dehiscence was diagnosed although actually not present. This resulted in a specificity of 83.3% and 70.8%, respectively, in a sensitivity of 100% for both groups, and the dACC was 91.7% and 85.4%, respectively.
3.2. Difference between direct and CBCT measurements of the buccal bone level
Abutment/crown material (P = 0.640) and implant diameter (P = 0.682) did not have a significant impact on the difference between direct and CBCT measurements of the buccal bone level, while CBCT measurements in the BBT group 1 were statistically significantly more imprecise compared with the BBT groups 2 and 3; any differences between BBT groups 2 and 3 were not significant (Table 3). BBT group 1 presented a mean difference between direct and CBCT measurements of 1.61 mm, which was three to four times larger than the mean differences observed in BBT groups 2 and 3 (Table 1). No significant interaction between BBT and implant diameter or abutment/crown material was observed.
TABLE 3.
Effect of the three predictors (i.e., BBT, abutment/crown material, and implant diameter) on the difference between direct and CBCT measurements in the buccal bone level among specimens with a buccal bone dehiscence (median regression; based on 72 observations)
| 95% CI | |||||
|---|---|---|---|---|---|
| Regression coefficient | Lower | Higher | P value | ||
| BBT | Group 1 | 0.00 | ‐ | ‐ | ‐ |
| Group 2 a | ‐0.63 | ‐1.05 | ‐0.21 | 0.004 | |
| Group 3 a | ‐0.57 | ‐0.99 | ‐0.15 | 0.008 | |
| Abutment/crown material | Ti/MCC versus zirconia | ‐0.08 | ‐0.42 | 0.26 | 0.640 |
| Implant diameter | Narrow versus standard | 0.07 | ‐0.27 | 0.41 | 0.682 |
Statistically significant values are indicated in bold.
BBT, buccal bone thickness; CBCT, cone‐beam computed tomography; Ti/MCC, titanium/metal‐ceramic crown.
Bonferroni correction for pairwise comparison was statistically significant compared with group 1.
In BBT group 1 in the 24 blocks with a dehiscence, the difference between direct and CBCT measurements was >1.5 mm in nine cases, and only in five cases ≤0.5 mm; in BBT groups 2 and 3 the difference between direct and CBCT measurements was ≤0.5 mm in 19 and 18 cases, respectively, out of 24 blocks each; in only one case in BBT group 2, the difference was >1.5 mm (see Table S1 in online Journal of Periodontology).
3.3. Intra‐observer repeatability and inter‐observer reproducibility
Evaluation of the reliability in the buccal bone level measurements in the CBCT scans showed a high degree of intra‐observer repeatability (ICC >0.86) and inter‐observer reproducibility (ICC >0.81). Deviations of >1 mm between the repeated measurements of the main examiner (DD) were mainly limited to the BBT group 1; that is, 4, 1, and 0 measurements deviated >1 mm in BBT groups 1, 2, and 3, respectively.
4. DISCUSSION
The present laboratory study evaluated the possible impact of several factors on the accuracy of CBCT to determine the buccal bone level at titanium implant sites. The results showed that BBT significantly influences the accuracy of CBCT to correctly detect a buccal bone dehiscence and to determine the buccal bone level. Specifically, when BBT was ≤1 mm, CBCT significantly overestimated the presence of a buccal bone dehiscence and was largely inaccurate regarding its extent. The implant diameter and the type of the abutment/crown material did not have an impact.
The finding that BBT influences the accuracy of CBCT at implant sites is in general supported by previous studies. 18 , 19 , 26 In another laboratory study with a very similar design as herein, a buccal bone wall thickness <1 mm significantly interfered with the ability to discern it. 19 Similarly, using bovine ribs and testing three different CBCT devices, a buccal bone thickness <1 mm had a probability of <40% to be identifiable, irrespective of the device. 26 Indeed, herein a buccal bone dehiscence was wrongly diagnosed in almost 90% of the cases (i.e., 21 out of 24 cases) when the BBT was ≤1 mm, yielding a rather low specificity (i.e., 12.5%). In this context, the very high sensitivity (i.e., 95.8%) should be interpreted with caution, since it is due to the fact that a buccal bone dehiscence was simply diagnosed almost in every case (i.e., in 44 out of 48 cases) even if existing in only 50% of the cases (i.e., in 24 cases). Consequently, the dACC, defined as the sum of correct diagnoses “presence of a dehiscence” and correct diagnoses “absence of a dehiscence,” divided by the sum of the cases, was only 54%. Hence, if the number of true dehiscence would have been even smaller, instead of the present 1:1 ratio of blocks with/without a dehiscence, dACC would have been even poorer. In contrast, when the BBT was >1 mm, the diagnosis “absence of a dehiscence” was only seldomly wrong and dACC was 92% and 85% in the groups with a BBT >1 and >1.5 mm, respectively.
A thin BBT (i.e., ≤1 mm) did not only negatively affect the dACC, it also had a negative impact on the measuring accuracy of the extent of the dehiscence. Specifically, the average measurement error between the direct and CBCT measurements in specimens with a BBT ≤1 mm was quite large (i.e., 1.6 mm) with a very large variation. For example, in six cases the measurements differed >2 mm, and in four of those cases the defect extent was overestimated. In contrast, these measurements were clearly more precise in groups with a BBT >1 and >1.5 mm; that is, an average measurement error of 0.5 and 0.6 mm, respectively, with a small variation was recorded. Specifically, in 19 and 18 cases (out of 24), respectively, the difference was <0.5 mm and only in one case >1.5 mm. Such an inaccuracy by CBCT regarding the extent of bone defects next to implants has been reported previously. For example, in an in vivo study in mini‐pig jaws, measurements of peri‐implant bone defects in CBCT images were on average 1.2 mm shorter compared with the bone level assessed on histological sections. 27 Similarly, in an in vivo study in the mandible of dogs, the comparison of bone level measurements at peri‐implantitis defects in CBCT and in histological slides, showed a smaller average difference but a large variation (i.e., 0.5 mm ± 1.5 mm). 28 These results seem to be in contrast, with another ex vivo study in pig jaws, where the average measurement error between direct and CBCT measurements regarding the extent of marginal box‐type buccal defects was negligible with a small variation (0.16 mm ± 0.14 mm). 29 However, in the latter study, the apical extent of the defects was formed as a step, that is, had a distinct/sharp edge and a depth of 2 to 3 mm; thus, such defects are not comparable with the appearance of common dehiscence defects, harboring a very thin crest.
Thus, although CBCT is very accurate in terms of estimating dimensions of pure bone defects, 10 , 11 , 12 , 13 it should be carefully used if a precise assessment of peri‐implant bone defects is needed. Based on the present study the results may be largely inaccurate especially in cases of thin alveolar crests/ridges. In perspective, this will be more relevant for anterior implants that often present with a buccal wall thickness <1 mm at the crestal aspect. Indeed, the majority of extraction sites in the anterior regions seem to present a thin buccal wall and such sites experience larger dimensional changes (i.e., volume loss in terms of “buccal collapse”) compared with posterior sites where the buccal bone is often thicker (i.e., >1 mm). 9 , 30 Although the relevance of the BBT and/or of the presence of a buccal bone dehiscence is not completely understood in terms of risk of biological peri‐implant complications, it seems reasonable to conclude that no intervention should be planned/executed purely on the basis of CBCT assessment of the buccal bone level.
As mentioned earlier, the inaccuracy of CBCT to estimate the bone level adjacent to implants is due to the inherent properties of the technology, exhibiting beam hardening artifacts when the x‐ray beam passes through dense materials. 14 Previous studies indicated that the denser the material of the implant and/or reconstruction (e.g., zirconia versus titanium) and/or a higher amount of dense material in the field of view (e.g., multiple versus single implants), the larger the amount of artifacts and the negative impact on the accuracy of CBCT. 19 , 20 , 31 , 32 For example, a zirconia implant with a zirconia supra‐structure or a titanium implant with a zirconia supra‐structure had significantly higher chances compared with a titanium implant and metal‐ceramic supra‐structure to interfere with a correct buccal bone assessment. 19 , 20 In contrast to these results, neither the abutment/crown material nor the implant diameter had any significant effect on the correct diagnosis herein. This may be due to differences among the various studies in the CBCT system and/or software version, or in the exposure (e.g., size of the field of view, voxel resolution) and/or reconstruction settings (e.g., slice thickness, grey levels). These factors have been previously shown to affect the accuracy of CBCT images in terms of bone parameters. 18 , 19 , 20 , 26 , 33
Further, in the present study, the assessment was based on a single central section from each implant/block. It may be argued that this is a limitation since using the entire data set, where one could scroll through the scan volume in all planes and also change contrast and brightness, may ease the visualization of peri‐implant bone defects. 34 However, it is still unclear whether using the entire scan volume actually increases the accuracy of CBCT, specifically in regards with the buccal bone level, compared with the assessment based on a single central section. Indeed, the vast majority of studies on this topic have used a similar approach, that is, analyses were made on the basis of single sections. Finally, the present results should be interpreted keeping in mind that only bone blocks were used; that is, despite the inclusion of a soft tissue simulation, a scan in the clinic includes the adjacent bone structures, teeth, and soft tissues, which may affect signal‐to‐noise and/or beam hardening differently, but most likely would aggravate artifact formation.
5. CONCLUSIONS
In conclusion, within the limitations of the present laboratory study, BBT significantly influences the accuracy of CBCT at titanium implant sites, while implant diameter and abutment/crown material do not. In particular, when BBT was ≤1 mm, CBCT significantly overestimated the presence of a buccal bone dehiscence at implant sites and was largely inaccurate regarding its extent. Therefore, CBCT should be carefully reconsidered as a reliable diagnostic tool for clinical decision‐making and/or for research purposes for monitoring peri‐implant bone level at the buccal aspect of titanium implants.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to conception and design of the study. Drs. Domic, Bertl, Ahmad, and Prof. Hellén‐Halme have been involved in data collection and data analysis; Drs. Domic, Bertl, Schropp and Dr/Prof. Stavropoulos have been involved in data interpretation. All authors participated in drafting the manuscript and revising it critically and have given final approval of the version to be published.
Supporting information
Figure S1. Study flowchart; BBT, buccal bone thickness.
Table S1. Differences between direct and CBCT measurements of the buccal bone level among those implant sites with a buccal bone dehiscence (n = 72).
ACKNOWLEDGMENTS
The authors thank Thommen Medical (Grenchen, Switzerland) for donating the implants and related components. The authors report no conflicts of interest related to this study.
Domic D, Bertl K, Ahmad S, Schropp L, Hellén‐Halme K, Stavropoulos A. Accuracy of cone‐beam computed tomography is limited at implant sites with a thin buccal bone: A laboratory study. J Periodontol. 2021;92:592–601. 10.1002/JPER.20-0222
Footnotes
Thommen Medical, Grenchen, Switzerland.
Provil Novo, Heraues Kulzer, Germany.
Tenax Wax, SS White Group, Gloucester, England.
Veraviewepocs3D F40 J. Morita, Kyoto, Japan.
i‐Dixel, J. Morita Mfg., Kyoto, Japan.
Adobe Photoshop CC, Version 19.1.5, Adobe Systems, San Jose, CA.
Biltema, Helsingborg, Sweden.
SPSS version 24, IBM, Chicago, IL.
REFERENCES
- 1. Bornstein MM, Horner K, Jacobs R. Use of cone beam computed tomography in implant dentistry: current concepts, indications and limitations for clinical practice and research. Periodontol 2000. 2017;73:51‐72. [DOI] [PubMed] [Google Scholar]
- 2. Rios HF, Borgnakke WS, Benavides E. The use of cone‐beam computed tomography in management of patients requiring dental implants: an American Academy of Periodontology best evidence review. J Periodontol. 2017;88:946‐959. [DOI] [PubMed] [Google Scholar]
- 3. Chappuis V, Rahman L, Buser R, Janner SFM, Belser UC, Buser D. Effectiveness of contour augmentation with guided bone regeneration: 10‐year results. J Dent Res. 2018;97:266‐274. [DOI] [PubMed] [Google Scholar]
- 4. Jung RE, Benic GI, Scherrer D, Hämmerle CH. Cone beam computed tomography evaluation of regenerated buccal bone 5 years after simultaneous implant placement and guided bone regeneration procedures–a randomized, controlled clinical trial. Clin Oral Implants Res. 2015;26:28‐34. [DOI] [PubMed] [Google Scholar]
- 5. Kaminaka A, Nakano T, Ono S, Kato T, Yatani H. Cone‐beam computed tomography evaluation of horizontal and vertical dimensional changes in buccal peri‐implant alveolar bone and soft tissue: a 1‐year prospective clinical study. Clin Implant Dent Relat Res. 2015;17(suppl 2):e576‐85. [DOI] [PubMed] [Google Scholar]
- 6. Schropp L, Wenzel A, Spin‐Neto R, Stavropoulos A. Fate of the buccal bone at implants placed early, delayed, or late after tooth extraction analyzed by cone beam CT: 10‐year results from a randomized, controlled, clinical study. Clin Oral Implants Res. 2015;26:492‐500. [DOI] [PubMed] [Google Scholar]
- 7. Buser D, Martin W, Belser UC. Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants. 2004;19(Suppl):43‐61. [PubMed] [Google Scholar]
- 8. Chen ST, Darby IB, Reynolds EC. A prospective clinical study of non‐submerged immediate implants: clinical outcomes and esthetic results. Clin Oral Implants Res. 2007;18:552‐562. [DOI] [PubMed] [Google Scholar]
- 9. Ferrus J, Cecchinato D, Pjetursson EB, Lang NP, Sanz M, Lindhe J. Factors influencing ridge alterations following immediate implant placement into extraction sockets. Clin Oral Implants Res. 2010;21:22‐29. [DOI] [PubMed] [Google Scholar]
- 10. Correa LR, Spin‐Neto R, Stavropoulos A, Schropp L, da Silveira HE, Wenzel A. Planning of dental implant size with digital panoramic radiographs, CBCT‐generated panoramic images, and CBCT cross‐sectional images. Clin Oral Implants Res. 2014;25:690‐695. [DOI] [PubMed] [Google Scholar]
- 11. Fokas G, Vaughn VM, Scarfe WC, Bornstein MM. Accuracy of linear measurements on CBCT images related to presurgical implant treatment planning: a systematic review. Clin Oral Implants Res. 2018;29(suppl 16):393‐415. [DOI] [PubMed] [Google Scholar]
- 12. Schropp L, Stavropoulos A, Gotfredsen E, Wenzel A. Comparison of panoramic and conventional cross‐sectional tomography for preoperative selection of implant size. Clin Oral Implants Res. 2011;22:424‐429. [DOI] [PubMed] [Google Scholar]
- 13. Stavropoulos A, Wenzel A. Accuracy of cone beam dental CT, intraoral digital and conventional film radiography for the detection of periapical lesions. An ex vivo study in pig jaws. Clin Oral Investig. 2007;11:101‐106. [DOI] [PubMed] [Google Scholar]
- 14. Schulze R, Heil U, Gross D, et al. Artefacts in CBCT: a review. Dentomaxillofac Radiol. 2011;40:265‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benic GI, Sancho‐Puchades M, Jung RE, Deyhle H, Hämmerle CH. In vitro assessment of artifacts induced by titanium dental implants in cone beam computed tomography. Clin Oral Implants Res. 2013;24:378‐383. [DOI] [PubMed] [Google Scholar]
- 16. Bohner LOL, Tortamano P, Marotti J. Accuracy of linear measurements around dental implants by means of cone beam computed tomography with different exposure parameters. Dentomaxillofac Radiol. 2017;46:20160377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gröbe A, Semmusch J, Schöllchen M, et al. Accuracy of bone measurements in the vicinity of titanium implants in CBCT data sets: a comparison of radiological and histological findings in minipigs. Biomed Res Int. 2017;2017:3848207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamburoğlu K, Murat S, Kılıç C, et al. Accuracy of CBCT images in the assessment of buccal marginal alveolar peri‐implant defects: effect of field of view. Dentomaxillofac Radiol. 2014;43:20130332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liedke GS, Spin‐Neto R, da Silveira HED, Schropp L, Stavropoulos A, Wenzel A. Factors affecting the possibility to detect buccal bone condition around dental implants using cone beam computed tomography. Clin Oral Implants Res. 2017;28:1082‐1088. [DOI] [PubMed] [Google Scholar]
- 20. Liedke GS, Spin‐Neto R, da Silveira HED, Schropp L, Stavropoulos A, Wenzel A. Accuracy of detecting and measuring buccal bone thickness adjacent to titanium dental implants‐a cone beam computed tomography in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:432‐438. [DOI] [PubMed] [Google Scholar]
- 21. Raskó Z, Nagy L, Radnai M, Piffkó J, Baráth Z. Assessing the accuracy of cone‐beam computerized tomography in measuring thinning oral and buccal bone. J Oral Implantol. 2016;42:311‐314. [DOI] [PubMed] [Google Scholar]
- 22. Razavi T, Palmer RM, Davies J, Wilson R, Palmer PJ. Accuracy of measuring the cortical bone thickness adjacent to dental implants using cone beam computed tomography. Clin Oral Implants Res. 2010;21:718‐725. [DOI] [PubMed] [Google Scholar]
- 23. Powell K, Atkinson PJ, Woodhead C. Cortical bone structure of the pig mandible. Arch Oral Biol. 1973;18:171‐180. [DOI] [PubMed] [Google Scholar]
- 24. Štembírek J, Kyllar M, Putnová I, Stehlík L, Buchtová M. The pig as an experimental model for clinical craniofacial research. Lab Anim. 2012;46:269‐279. [DOI] [PubMed] [Google Scholar]
- 25. Schropp L, Alyass NS, Wenzel A, Stavropoulos A. Validity of wax and acrylic as soft‐tissue simulation materials used in in vitro radiographic studies. Dentomaxillofac Radiol. 2012;41:686‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. González‐Martín O, Oteo C, Ortega R, Alandez J, Sanz M, Veltri M. Evaluation of peri‐implant buccal bone by computed tomography: an experimental study. Clin Oral Implants Res. 2016;27:950‐955. [DOI] [PubMed] [Google Scholar]
- 27. Corpas LS, Jacobs R, Quirynen M, Huang Y, Naert I, Duyck J. Peri‐implant bone tissue assessment by comparing the outcome of intra‐oral radiograph and cone beam computed tomography analyses to the histological standard. Clin Oral Implants Res. 2011;22:492‐499. [DOI] [PubMed] [Google Scholar]
- 28. Golubovic V, Mihatovic I, Becker J, Schwarz F. Accuracy of cone‐beam computed tomography to assess the configuration and extent of ligature‐induced peri‐implantitis defects. A pilot study. Oral Maxillofac Surg. 2012;16:349‐354. [DOI] [PubMed] [Google Scholar]
- 29. Mengel R, Kruse B, Flores‐de‐Jacoby L. Digital volume tomography in the diagnosis of peri‐implant defects: an in vitro study on native pig mandibles. J Periodontol. 2006;77:1234‐1241. [DOI] [PubMed] [Google Scholar]
- 30. Huynh‐Ba G, Pjetursson BE, Sanz M, et al. Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin Oral Implants Res. 2010;21:37‐42. [DOI] [PubMed] [Google Scholar]
- 31. Kuusisto N, Vallittu PK, Lassila LV, Huumonen S. Evaluation of intensity of artefacts in CBCT by radio‐opacity of composite simulation models of implants in vitro. Dentomaxillofac Radiol. 2015;44:20140157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steiger‐Ronay V, Krcmaric Z, Schmidlin PR, Sahrmann P, Wiedemeier DB, Benic GI. Assessment of peri‐implant defects at titanium and zirconium dioxide implants by means of periapical radiographs and cone beam computed tomography: an in‐vitro examination. Clin Oral Implants Res. 2018;29:1195‐1201. [DOI] [PubMed] [Google Scholar]
- 33. Wang D, Künzel A, Golubovic V, et al. Accuracy of peri‐implant bone thickness and validity of assessing bone augmentation material using cone beam computed tomography. Clin Oral Investig. 2013;17:1601‐1609. [DOI] [PubMed] [Google Scholar]
- 34. González‐Martín O, Veltri M. Cone beam analysis of the buccal bone associated with a dental implant: a tridimensional assessment case report. Quintessence Int. 2017;48:339‐344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flowchart; BBT, buccal bone thickness.
Table S1. Differences between direct and CBCT measurements of the buccal bone level among those implant sites with a buccal bone dehiscence (n = 72).


