Abstract
Background and Aim
We have previously shown that fecal microbial markers might be useful for non‐invasive diagnosis of colorectal cancer (CRC) and adenoma. Here, we assessed the application of microbial DNA markers, as compared with and in combination with fecal immunochemical test (FIT), in detecting CRC and adenoma in symptomatic patients and asymptomatic subjects.
Methods
We recruited 676 subjects [210 CRC, 115 advanced adenoma (AA), 86 non‐advanced adenoma, and 265 non‐neoplastic controls], including 241 symptomatic and 435 asymptomatic subjects. Fecal abundances of Fusobacterium nucleatum, a Lachnoclostridium sp. m3, Bacteroides clarus, and Clostridium hathewayi were quantified by quantitative PCR. Combining score of the four microbial markers (4Bac) and diagnostic prediction were determined using our previously established scoring model and cutoff values and FIT with a cutoff of 100 ng Hb/mL.
Results
4Bac detected similar percentages of CRC [85.3% (95%CI: 79.2–90.2%) vs 84.9% (68.1–94.9%)] and AA [35.7% (12.8–64.9%) vs 38.6% (29.1–48.8%)], while FIT detected more CRC [72.1% (63.7–79.4%) vs 66.7% (48.2–82.0%)] and AA [28.6% (8.4–58.1%) vs 16.8% (10.1–25.6%)], in symptomatic vs asymptomatic subjects, respectively. Focusing on the asymptomatic cohort, 4Bac was more sensitive for diagnosing CRC and AA than FIT (P < 0.001), with lower specificity [83.3% (77.6–88.0%) vs 98.6% (96.0–99.7%)]. FIT failed to detect any non‐advanced adenoma [0% (0.0–4.2%)] compared with 4Bac [41.9% (31.3–53.0%), P < 0.0001]. Combining 4Bac with FIT improved sensitivities for CRC [90.9% (75.7–98.1%)] and AA [48.5% (38.4–58.7%)] detection.
Conclusion
Quantitation of fecal microbial DNA markers may serve as a new test, stand alone, or in combination with FIT for screening colorectal neoplasm in asymptomatic subjects.
Keywords: colorectal cancer, fecal immunochemical test (FIT), microbial makers, neoplasia, screening
Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide. 1 As one of the most preventable forms of cancer, mortality rates of CRC have been shown to be significantly reduced in multiple CRC screening programs using fecal occult‐blood testing. 2 , 3 , 4 , 5 Specifically, a trial with 30‐year follow‐up showed that mortality rates reduced by 32% after annual screening and by 22% after biennial screening. 6 Reduction in the incidence of CRC by fecal occult‐blood test screening has not been conclusive. 7 , 8 , 9 This may be due to the limited sensitivity of fecal occult‐blood test for early stage CRC and pre‐malignant conditions. New non‐invasive molecular screening tests that are more sensitive than fecal occult‐blood test are warranted to further improve on the effectiveness of CRC screening.
In comparison of the tumor characteristics between symptomatic and asymptomatic CRCs, the presence of symptoms correlates with annular tumor morphology, larger tumor size, higher degree of luminal narrowing, and deeper invasion into intestinal wall. 10 Symptomatic CRCs were more often diagnosed in advanced stages compared with asymptomatic CRCs. 11 Post‐surgical outcomes, including amount of blood loss, duration of post‐operative hospital stay, and perioperative medical costs, were also significantly better in asymptomatic than symptomatic patients. 11 Therefore, screening leading to early detection of CRC, especially in asymptomatic subjects, improves clinical outcomes of patients.
Altered microbiome environment in the gut is associated with colorectal tumorigenesis. 12 , 13 , 14 We have shown that fecal microbial markers might be useful as non‐invasive tests for CRC and adenoma in our previous case–control studies. 14 , 15 , 16 By using metagenomics analysis to compare the fecal microbiome of CRC patients and healthy subjects, we identified 20 bacterial candidates that may serve as noninvasive biomarkers for CRC. 14 In order to translate these candidates into diagnostic biomarkers using simple, cost‐effective, and targeted methods, we then established probe‐based duplex quantitative polymerase chain reaction (PCR) (qPCR) assays for the quantification of bacterial marker candidates and shown that, among Fusobacterium nucleatum (Fn), Bacteroides clarus (Bc), Clostridium hathewayi (Ch), Roseburia intestinalis (Ri), and one undefined species (labeled as m7), Fn performed best in diagnosing CRC, and combination with Bc, Ch, and m7 further improved the diagnostic ability of Fn alone for CRC. 15 In a recent study, we identified a Lachnoclostridium sp. (m3) that showed good diagnostic performance for adenoma (including non‐advanced stage), although for CRC, it is not as good as Fn. A new panel composing of Fn, m3, Bc, and Ch was thereafter devised for detecting colorectal neoplasm, which is more sensitive than fecal immunochemical test (FIT), especially for early stage CRCs and pre‐malignant condition. 16 We have devised a scoring system using these markers, which shows areas under the receiver operating characteristic (ROC) curve (AUROCs) of over 0.9 for CRC and over 0.66 for adenoma (including non‐advanced stage). 16 Combining the microbial markers with FIT improves the diagnostic performance in detecting both CRC and advanced adenoma (AA). 16 However, because our previous studies have not differentiated between symptomatic from asymptomatic subjects, the effectiveness of the microbial markers in screening for CRC and adenoma is unknown.
The aim of this study is to evaluate the use of microbial markers in detecting adenoma and CRC among asymptomatic subjects in CRC screening programs and in subjects presented to clinics and hospitals with symptom. In this study, we assessed the application of microbial markers, as compared with and in combination with FIT, in detecting CRC and adenoma in symptomatic and asymptomatic subjects.
Methods
Design
This study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong and the Ethics Committee of Renji Hospital, Shanghai Jiaotong University. We performed a cross‐sectional retrospective study of Chinese subjects with and without CRC‐related symptoms before undergoing colonoscopy in Hong Kong and Shanghai, China. Diagnostic performances of FIT and fecal microbial markers were compared in CRC screening for the symptomatic and asymptomatic cohorts.
Participants
Fecal samples were collected from 676 Chinese subjects, consisting of 210 patients with CRC, 115 patients with AA, 86 patients with non‐advanced adenoma (NAA), and 265 non‐neoplastic controls, at the Prince of Wales Hospital, the Chinese University of Hong Kong between 2009 and 2014, and Renji Hospital, Shanghai Jiaotong University between 2014 and 2017. The exclusion criteria were (i) use of antibiotics within the past 3 months; (ii) on a vegetarian diet; (iii) had an invasive medical intervention within the past 3 months; (iv) had a past history of any cancer, or inflammatory disease of the intestine. Informed consents were obtained from all subjects.
Subjects presenting symptoms such as abdominal mass, abdominal pain, altered bowel habit, anemia, melena, rectal bleeding, or weight loss at recruitment were classified as symptomatic. Asymptomatic individuals were recruited from those aged 50 or above undergoing screening colonoscopy. The symptomatic cohort included 241 subjects, including 177 CRC, 14 AA, and 50 non‐neoplastic lesions. The asymptomatic cohort included 435 subjects, including 33 CRC, 101 AA, 86 NAA, and 215 non‐neoplastic lesions (Table 1).
Table 1.
Clinical characteristics of healthy subjects and colorectal adenoma/cancer patients
| All (n = 676) | Symptomatic cohort (n = 241) | Asymptomatic cohort (n = 435) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Control (n = 265) | NAA (n = 86) | AA (n = 115) | CRC (n = 210) | P value † | Control (n = 50) | AA (n = 14) | CRC (n = 177) | P value † | Control (n = 215) | NAA (n = 86) | AA (n = 101) | CRC (n = 33) | P value † |
| Age | 58.1 ± 7.7 | 60.2 ± 5.0 | 61.1 ± 6.8 | 67.0 ± 11.3 | <0.0001 | 53.9 ± 13.2 | 68.9 ± 9.5 | 67.8 ± 11.1 | <0.0001 | 59.1 ± 5.3 | 60.2 ± 5.0 | 60.0 ± 5.5 | 63.0 ± 11.7 | 0.006 |
| Gender | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Male | 110 (41.5%) | 49 (57.0%) | 66 (57.4%) | 124 (59.01%) | 0.0004 | 19 (38.0%) | 7 (50.0%) | 108 (61.0%) | 0.0139 | 91 (42.3%) | 49 (57.0%) | 59 (58.4%) | 16 (48.5%) | 0.0225 |
| Female | 155 (58.5%) | 37 (43.0%) | 49 (42.6%) | 86 (41.0%) | 31 (62.0%) | 7 (50.0%) | 69 (39.0%) | 124 (57.7%) | 37 (43.0%) | 42 (41.6%) | 17 (51.5%) | |||
| Location ‡ | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Proximal | — | 22 (25.0%) | 22 (25.0%) | 54 (25.7%) | 5 (41.7%) | 47 (26.6%) | 17 (22.4%) | 7 (21.2%) | ||||||
| Distal | — | 48 (54.5%) | 48 (54.5%) | 156 (74.3%) | 7 (58.3%) | 130 (73.4%) | 41 (53.9%) | 26 (78.8%) | ||||||
| both | — | 18 (20.5%) | 18 (20.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 18 (23.7%) | 0 (0%) | ||||||
| TNM stage § | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| I | — | — | — | 31 (14.8%) | — | — | — | 24 (21.2%) | — | — | — | — | 7 (13.6%) | — |
| II | — | — | — | 68 (32.4%) | — | — | — | 64 (12.1%) | — | — | — | — | 4 (36.2%) | — |
| III | — | — | — | 73 (34.8%) | — | — | — | 57 (48.5%) | — | — | — | — | 16 (32.2%) | — |
| IV | — | — | — | 28 (13.3%) | — | — | — | 22 (18.2%) | — | — | — | — | 6 (12.4%) | — |
NAA, non‐advanced adenoma; AA, advanced adenoma; CRC, colorectal cancer; TNM, tumor‐node‐metastasis.
27 AA and 1 non‐advanced adenoma with location information missing.
10 with unknown TNM staging.
Gender by chi‐square; age by one‐way ANOVA.
Definitions and clinical phenotypes
Patients were diagnosed by colonoscopic examination and histopathological review of any biopsies taken. Proximal tumors include those in the caecum, ascending colon, hepatic flexure, transverse colon, or splenic flexure; whereas distal tumors include those in the descending colon, sigmoid colon, or rectum. The CRC stage was assessed according to the seventh edition of the American Joint Committee on Cancer cancer staging manual. The AAs were adenomas 1 cm or larger in size, with a tubulovillous or villous component, or with high‐grade or severe dysplasia. Pathologists were blinded to the FIT or microbial marker results. Control subjects were individuals showing normal colorectal mucosae under screening colonoscopy.
Fecal sample collection, fecal immunochemical test, and DNA extraction samples were collected before or 1 month after colonoscopy, when gut microbiome should have recovered to baseline. 17 Subjects were asked to collect stool samples in standardized containers at home and store the samples in their home in −20°C freezer immediately. Frozen samples were then delivered to the hospitals in insulating polystyrene foam containers and stored at −80°C immediately until further analysis. The quantitative OC‐Sensor test was performed on an automatic OCsensor instrument (Eiken Chemical, Japan), using a positive cut‐off value equivalent to a concentration of 100 ng of hemoglobin per milliliter of blood (ng Hb/mL). Fecal DNA extraction was performed using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) followed by DNase‐free RNase treatment as our previous description. 15
Probe‐based relative quantification of microbial markers by duplex quantitative polymerase chain reaction
Fecal abundances of four microbial DNA markers (Fn, m3, Bc, and Ch) were quantified by qPCR. Primer and probe sequences targeting the markers and 16s rDNA internal control are as in our previous study. 15 , 16 Each probe carried a 5′ reporter dye FAM (6‐carboxy fluorescein) or VIC (4,7,2′‐trichloro‐7′‐phenyl‐6‐carboxyfluorescein) and a 3′ quencher dye TAMRA (6‐carboxytetramethyl‐rhodamine). Primers and hydrolysis probes were synthesized by Invitrogen (Carlsbad, CA). qPCR amplifications were performed on an ABI QuantStudio sequence detection system as previously described, with thermal cycler parameters of 95°C 10 min and (95°C 15 s, 60°C 1 min) × 45 cycles. 15 Positive controls of the markers and a negative control (H2O as template) were included within every experiment. Measurements were performed in triplicates for each sample. Relative abundance of each marker was calculated by using delta Cq method as compared with internal control (Power (2, −(Cqtarget − Cqcontrol))) and shown as Log value of “*10e6 + 1.”
Scoring algorithm and cutoff values
Combining score of four microbial markers (4Bac) using a logistic regression model (4Bac score = 0.23162*Fn + 0.13451*m3‐0.10075*Bc + 0.32841*Ch‐2.73836) and cutoff values (4Bac = 0.63, Fn = 7.43, and m3 = 0.363) were determined in our previous study. 16
Statistical analyses
Values were expressed as median (interquartile range) or mean ± SD as appropriate. The differences in bacterial abundances were determined by Mann–Whitney U‐test. Continuous clinical and pathological variables were compared by T‐test or one‐way ANOVA. ROC curves were used to evaluate the diagnostic value of bacterial markers/models in distinguishing CRC/adenoma and controls. Pairwise comparison of ROC curves was performed using a non‐parametric approach. 18 Occurrence rates between different groups and sensitivities by different markers/models were analyzed using the Fisher's exact test, while age in more than two groups were compared by chi‐square test. All tests were done by Graphpad Prism 5.0 (Graphpad Software Inc., San Diego, CA) or MedCalc Statistical Software V.18.5 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018). P < 0.05 was taken as statistical significance.
Results
Characteristics of the recruited symptomatic and asymptomatic subjects
We recruited 241 symptomatic and 435 asymptomatic subjects. As expected, significant differences were observed in age and gender among non‐neoplastic control subjects, patients with adenoma and patients with CRC in the entire recruited cohort, symptomatic group, and asymptomatic group, respectively (all P < 0.05; Table 1). In comparison between symptomatic and asymptomatic groups, the symptomatic non‐neoplastic controls were significantly younger than asymptomatic non‐neoplastic controls, while symptomatic patients with AA or CRC were significantly older as compared with the asymptomatic patients (all P < 0.05). No symptomatic NAA and 86 asymptomatic NAAs were recruited. There was no significant difference in gender between symptomatic and asymptomatic non‐neoplastic controls or AA patients or CRC patients. No significant difference was found in lesion location between symptomatic and asymptomatic patients with AA or CRC. There was also no significant difference in TNM staging between the recruited symptomatic and asymptomatic patients with CRC (Table 2).
Table 2.
Comparison of clinical characteristics between symptomatic and asymptomatic subjects
| Control | NAA | AA | CRC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symp(+) | Symp(−) | P | Symp(+) | Symp(−) | P | Symp(+) | Symp(−) | P | Symp(+) | Symp(−) | P | |
| Sample size | 50 | 215 | _ | 0 | 86 | _ | 14 | 101 | _ | 177 | 33 | _ |
| Age | 53.9 ± 13.2 | 59.1 ± 5.3 | <0.001 | _ | 60.2 ± 5.0 | _ | 68.9 ± 9.5 | 60.0 ± 5.5 | <0.001 | 67.8 ± 11.1 | 63.0 ± 11.7 | 0.0253 |
| Male gender | 19 (38.0%) | 91(42.3%) | 0.6344 | _ | 49(57.0%) | _ | 7 (50.0%) | 59 (58.4%) | 0.5761 | 108 (61.0%) | 16 (48.5%) | 0.1841 |
| Distal location | _ | _ | _ | _ | _ | _ | 7 (58.3%) | 41 (53.9%) | 0.3895 | 130 (73.4%) | 26 (78.8%) | 0.6654 |
| TNM: I | _ | _ | _ | _ | _ | _ | _ | _ | _ | 24 (21.2%) | 7(13.6%) | 0.3154 |
| II | _ | _ | _ | _ | _ | _ | _ | _ | _ | 64 (12.1%) | 4 (36.2%) | |
| III | _ | _ | _ | _ | _ | _ | _ | _ | _ | 57 (48.5%) | 16 (32.2%) | |
| IV | _ | _ | _ | _ | _ | _ | _ | _ | _ | 22 (18.2%) | 6 (12.4%) | |
NAA, non‐advanced adenoma; AA, advanced adenoma; CRC, colorectal cancer; TNM, tumor‐node‐metastasis.
Age by t test. Others by Fisher's exact test.
Fecal abundances of microbial markers show no difference between symptomatic and asymptomatic patients with colorectal cancer or advanced adenoma
To investigate the distribution of fecal microbial markers in symptomatic and asymptomatic patients with CRC or AA, we evaluate the fecal abundances of our previously identified four microbial markers (Fn, m3, Bc, and Ch) and their combined score (4Bac). In the overall recruited cohort, ROC curve analyses showed that Fn performed the best among the individual markers in diagnosing CRC (AUROC = 0.850), and 4Bac showed significantly improved diagnostic performance (AUROC = 0.904) as compared with the individual markers for CRC (all P < 0.0001). m3 alone performed the best in diagnosing adenoma (AUROC = 0.661), although not significantly as compared with 4Bac (AUROC = 0.639; P > 0.05) (Fig. 1a). This is consistent with our previous findings. 16 The levels of Fn, m3, and the combined 4Bac in symptomatic and asymptomatic groups are shown in Figure 1b. There is no significant difference in fecal abundances of Fn, m3, or 4Bac between symptomatic and asymptomatic patients with CRC or AA, while symptomatic non‐neoplastic control subjects show higher levels of Fn, m3, and 4Bac than asymptomatic non‐neoplastic controls (P < 0.05).
Figure 1.
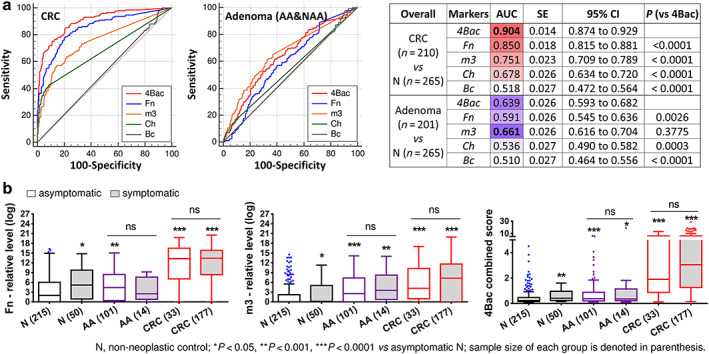
(a) Comparison of receiver operating characteristic curves of Fusobacterium nucleatum (Fn), Lachnoclostridium sp. (m3), Clostridium hathewayi (Ch), Bacteroides clarus (Bc), and their combination (4Bac) in distinguishing CRC and adenoma patients from non‐neoplastic controls in the overall recruited cohort not differentiating between symptomatic from asymptomatic subjects. (b) Relative abundances of fecal Fn, m3, and 4Bac in symptomatic and asymptomatic groups. Medians with interquartile ranges are shown in the box and whisker plots by Tukey method. N, non‐neoplastic control; AA, advanced adenoma; CRC, colorectal cancer; AUC, area under ROC; ns, not significant.
Using the same cutoff values as determined by our previous study, 16 Fn, m3, and 4Bac each showed no significant difference in sensitivity for detecting CRC and AA between symptomatic and asymptomatic subjects (all P > 0.05 by Fisher's exact test; Fig. 2a,b). 4Bac was more sensitive than Fn and m3 in detecting CRC in symptomatic and asymptomatic subjects, while m3 was more sensitive than 4Bac and Fn for AA in symptomatic and asymptomatic subjects (Fig. 2a,b). 4Bac detected 85.3% (95%CI: 79.2–90.2%) and 84.9% (68.1–94.9%) of CRC, and m3 detected 42.9% (17.7–71.1%) and 45.5% (74.5–85.5%) of AA in symptomatic and asymptomatic subjects, respectively (Fig. 2a,b). m3 is also more sensitive than 4Bac and Fn in detecting NAA in asymptomatic subjects (Fig. 2c). As expected, microbial markers (4Bac, Fn, and m3) showed higher specificity for asymptomatic controls than symptomatic controls (Fig. 2d). Of note, 4Bac [specificity = 83.3% (77.6–88.0%)] is also more specific than Fn [80.9% (75.0–86.0%)] and m3 [79.5% (73.0%–84.3%)] in detecting asymptomatic controls although not significantly. These results demonstrate that microbial markers are similarly useful in detecting both symptomatic and asymptomatic patients with CRC or AA, although less specific for symptomatic non‐neoplastic subjects. Moreover, 4Bac outperforms Fn and m3 in detecting CRC and in terms of specificity in asymptomatic subjects, while m3 is more sensitive in detecting adenomas in both symptomatic and asymptomatic subjects with compromised specificities.
Figure 2.
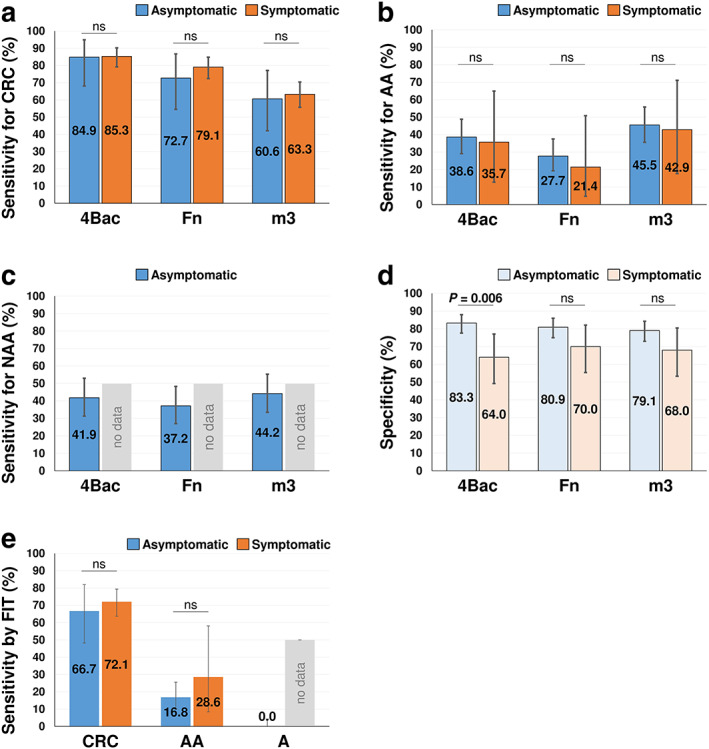
(a‑c) Diagnostic sensitivities of four microbial markers (4Bac), Fusobacterium nucleatum (Fn), and Lachnoclostridium sp. (m3) for colorectal cancer (CRC; panel a), advanced adenoma (AA; panel b), non‐advanced adenoma (NAA; panel c) in symptomatic and asymptomatic groups. (d) Diagnostic specificities of 4Bac, Fn, and m3 in symptomatic and asymptomatic groups. (e) Diagnostic sensitivities of fecal immunochemical test for CRC, AA, and A in symptomatic and asymptomatic groups. Data are shown as sensitivity/specificity (95% CI). ns, not significant
Fecal immunochemical test is more sensitive in detecting colorectal cancer or advanced adenoma in symptomatic subjects
We compared the performance of FIT in screening symptomatic and asymptomatic patients. FIT detected 72.1% (95%CI: 63.7–79.4%) versus 66.7% (48.2–82.0%) of CRC and 28.6% (8.4–58.1%) versus 16.8% (10.1–25.6%) of AA in symptomatic versus asymptomatic subjects, respectively (Fig. 2e). In the recruited subjects in this study, FIT failed to detect any NAAs. Although not statistically significant due to the limited sample sizes, these results show that, unlike microbial markers, FIT is more sensitive in detecting CRC and AA among symptomatic than asymptomatic subjects.
Combination of microbial markers for detecting colorectal cancer and adenoma in asymptomatic subjects
As shown in Figure 1a and consistent with previous findings, in the overall cohort with symptomatic and asymptomatic subjects, combination of microbial markers (4Bac) performs best for CRC detection while m3 alone outperforms 4Bac in detecting adenoma. Therefore, we proposed a step‐wise screening strategy with the microbial markers by applying 4Bac to detect CRC and then m3 alone to detect adenoma, which will comprise diagnostic specificity (78.5% by m3 vs 80.2% by 4Bac), in our previous study not differentiating between symptomatic from asymptomatic subjects. 16 However, focusing on the asymptomatic cohort in this study, 4Bac and m3 showed no difference in detecting adenoma by ROC curve analysis, with the same AUROC of 0.669 (95% CI: 0.621–0.715) (Fig. 3a). 4Bac performs best for detecting asymptomatic CRC as compared with the individual markers, although not significant as compared with Fn probably due to the limited sample size of CRC (AUROCs: 0.884 by 4Bac vs 0.856 by Fn, P > 0.05). The previously set cutoff value of 4Bac happens to maximize the Youden's index for both CRC and adenoma in the asymptomatic cohort. Therefore, 4Bac can be used for detecting both CRC and adenoma in asymptomatic subjects using the cutoff of 0.63.
Figure 3.
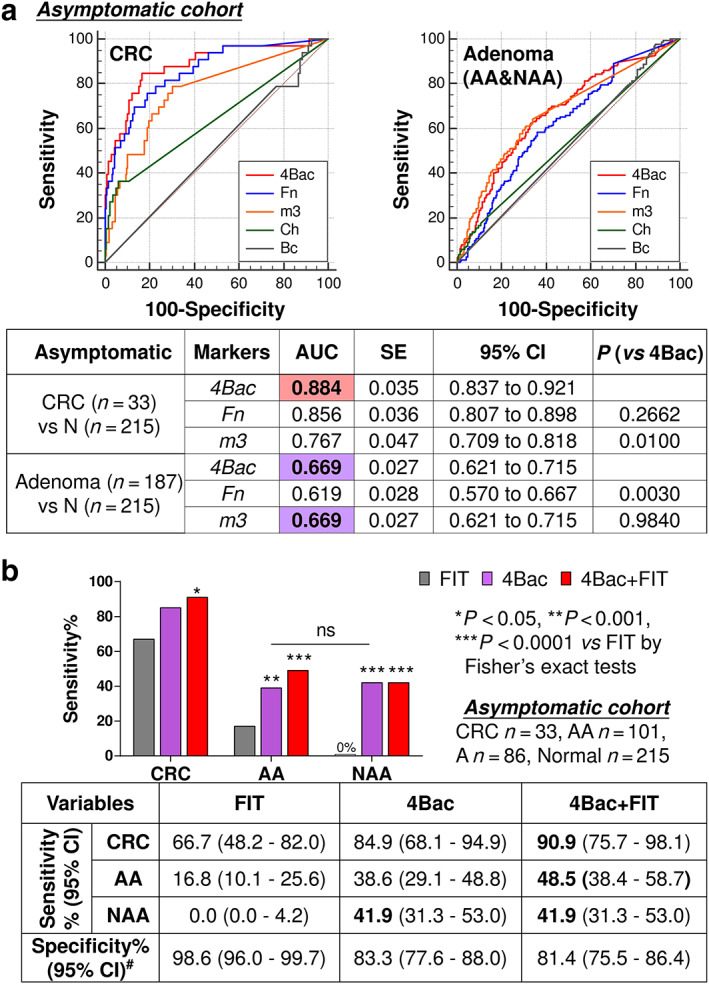
(a) Comparison of receiver operating characteristic curves of Fusobacterium nucleatum (Fn), Lachnoclostridium sp. (m3), Clostridium hathewayi (Ch), Bacteroides clarus (Bc), and their combination (4Bac) in distinguishing colorectal cancer (CRC) and adenoma patients from non‐neoplastic controls in asymptomatic subjects. (b) Diagnostic performances of fecal immunochemical test (FIT), 4Bac and their combination for CRC, advanced adenoma (AA), and non‐advanced adenoma (NAA) in asymptomatic subjects. Significant P values are shown for comparison with FIT. Comparing “4Bac + FIT” with 4Bac, P values are not significant and not shown. 4Bac shows no difference in detecting AA and A in asymptomatic subjects (ns, not significant). #Specificity is the true negative rate in non‐neoplastic controls. AUC, area under ROC.
Microbial markers are more sensitive than fecal immunochemical test in detecting colorectal cancer and adenoma in asymptomatic subjects
Focusing on the asymptomatic cohort, sensitivity and specificity of 4Bac and FIT in detecting CRC and AA are shown in Figure 3b. FIT was more specific for the diagnosis of CRC and AA than 4Bac [98.6% (96.0–99.7%) vs 83.3% (77.6–88.0%); P < 0.0001]. However, the sensitivity of FIT was lower than that of 4Bac in this group [CRC: 66.7% (48.2–82.0%) vs 84.9% (68.1–94.9%), P = 0.15; AA: 16.8% (10.1–25.6%) vs 38.6% (29.1–48.8%), P < 0.001 by Fisher's exact test]. Moreover, FIT failed to detect any NAA [0% (0.0–4.2%)]. 4Bac detected 41.9% (31.3–53.0%) of NAA (P < 0.0001 vs FIT), showing no difference with the sensitivity for AA. Therefore, microbial markers are superior to FIT in detecting asymptomatic patients with CRC or adenoma.
Combination of microbial markers and fecal immunochemical test shows improved diagnostic performance for colorectal cancer and advanced adenoma in asymptomatic subjects
We further assessed the combined diagnostic performance of microbial markers and FIT, with either one or both tests positive to yield a positive result. The combination of 4Bac with FIT significantly improve the diagnostic performance for both CRC and AA against FIT alone (Fig. 3b). Compared with 4Bac alone, 4Bac plus FIT show improved sensitivities to detect CRC [90.9% (75.7–98.1%) vs 84.9% (68.1–94.9%)] and for AA [48.5% (38.4–58.7%) vs 38.6% (29.1–48.8%)]. These results support the implementation of microbial markers in combination with FIT in colorectal neoplasm screening.
Discussion
In this study, we assess the performance of our identified microbial markers in the non‐invasive diagnosis of CRC and adenoma in asymptomatic compared with symptomatic subjects. We show that microbial markers demonstrate comparable performances in detecting CRC and AA among symptomatic and asymptomatic patients, while FIT performs better in detecting colonic lesions in symptomatic patients. Moreover, combination of microbial marker with FIT further improves the diagnostic performance in detecting asymptomatic patients with CRC and adenoma.
In this study, we have employed the same scoring method for calculating the combined score of the four microbial markers (4Bac) and the cutoff value determined in our previous study. 16 By doing this, we could avoid the overfitting concern by formulating a new algorithm based on samples recruited in this study. The scoring method was formulated based on both symptomatic and asymptomatic subjects involved. Although no difference was observed between symptomatic and asymptomatic patients with CRC and adenoma in the levels of 4Bac, Fn, and m3, symptomatic non‐neoplastic control subjects showed significantly higher levels of the microbial markers than asymptomatic non‐neoplastic control subjects. Using the same cutoff value, 4Bac showed a significantly lower specificity in screening the symptomatic subjects than the asymptomatic subjects (48.0% vs 82.8%, P < 0.0001). This will not affect the usefulness of the microbial markers in CRC screening as subjects with symptoms indicative of CRC will be referred to colonoscopy examination regardless of the levels of microbial markers. However, the involvement of symptomatic non‐neoplastic controls in training the scoring method results in the algorithm not optimal for distinguishing asymptomatic patients with colorectal neoplasm from asymptomatic non‐neoplastic subjects. A new scoring algorithm based on a large cohort of asymptomatic subjects is warranted to obtain better performance by the same microbial markers in diagnosing asymptomatic patients with colorectal neoplasm.
The role of Fn in promoting colorectal carcinogenesis and its usefulness in diagnosing CRC has been well demonstrated in previous studies conducted by us and others. 15 , 19 Fn has been shown to induce inflammation and modulate host immune response to promote tumor development. 20 , 21 Subjects with high levels of intestinal Fn may be at higher risk of developing CRC. Fecal abundance of m3 was identified to be significantly increased at pre‐cancer stage of CRC development in our recently published study. 16 However, the Lachnoclostridium species carrying m3 and its roles in colorectal tumorigenesis warrant further characterization in future studies.
Other limitations of the current retrospective case–control study include the relatively small sample size and combination of samples recruited from two independent Chinese cohorts from Hong Kong and Shanghai. With the limited sample sizes of symptomatic AA patients (n = 14) and asymptomatic CRC patients (n = 33), the recruited subjects may not accurately represent the real populations of the corresponding clinical entities. A validated study using a larger sample size is much needed. Future validation is also required in different cohorts representative of the CRC screening populations to evaluate the true performance of the markers. Nevertheless, our results show clearly that the 4Bac microbial markers can be used for colorectal neoplasm screening in symptomatic and asymptomatic subjects with similar sensitivity, although less specific for symptomatic non‐neoplastic subjects. Without considering the real disease prevalence in the symptomatic and asymptomatic populations, the microbial markers showed a positive predictive value (PPV) of 89.7% (85.6–92.7%) for CRC plus AA and a negative predictive value (NPV) of 47.8% (38.8–56.8%) for non‐neoplastic controls in the recruited symptomatic group, and a PPV of 74.1% (67.3–79.9%) for CRC, AA plus NAA, and an NPV of 60.5% (57.1–63.7%) for non‐neoplastic controls in the recruited asymptomatic group. However, a further study with careful consideration of the recruited sample sizes should be conducted to assess the accurate PPVs and NPVs for the microbial markers in symptomatic and asymptomatic populations. 22 The microbial markers are definitely better than FIT in detecting non‐advanced and AAs. Adding FIT to 4Bac has marginal but still statistically significant improvement for CRC. As shown in our previous study, our probe‐based duplex‐qPCR assays involve an internal control for relative quantification of the target markers, making the test reliable and convenient. 15 The simple and cost‐effective quantification approach offers significant promise for incorporation the fecal microbial markers into routine clinical practice to aid colorectal neoplasm screening.
In conclusion, FIT detects slightly more symptomatic than asymptomatic CRC and AA patients, while microbial DNA markers have similar sensitivities for CRC and adenoma in both symptomatic and asymptomatic subjects. Combination of microbial DNA markers with FIT improves diagnostic performance for in asymptomatic subjects both CRC and adenoma. Our results support the clinical application of the microbial markers, as an individual test or in combination with FIT, in screening of colorectal neoplasm.
Liang, J. Q. , Wong, S. H. , Szeto, C. H. , Chu, E. S. H. , Lau, H. C. , Chen, Y. , Fang, J. , Yu, J. , and Sung, J. J. Y. (2021) Fecal microbial DNA markers serve for screening colorectal neoplasm in asymptomatic subjects. Journal of Gastroenterology and Hepatology, 36: 1035–1043. 10.1111/jgh.15171.
Declaration of conflict of interest: All authors disclose no potential conflicts (financial, professional, or personal) that are relevant to the manuscript.
Author contributions: Study conception and design (J. J. Y. S.); Development of methodology (J. Q. L.); Acquisition of data (J. Q. L., S. W., C. H. S., E. S. H. C., H. L., Y. C., J. F., J. Y.); Analysis and interpretation of data (J. Q. L., J. J. Y. S.); Writing, review, and/or revision of the manuscript (J. Q. L., J. J. Y. S.); Study supervision (J. J. Y. S.).
Financial support: This study was supported by National Key R&D Program of China (2018YFC1312100, 2018YFC1312102 and 2017YFE0190700) and HRMF research fellowship scheme (02160037).
References
- 1. Allemani C, Matsuda T, Di Carlo V et al. Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018; 391: 1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hardcastle JD, Chamberlain JO, Robinson MH et al. Randomised controlled trial of faecal‐occult‐blood screening for colorectal cancer. Lancet 1996; 348: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 3. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal‐occult‐blood test. Lancet 1996; 348: 1467–1471. [DOI] [PubMed] [Google Scholar]
- 4. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J. Natl. Cancer Inst. 1999; 91: 434–437. [DOI] [PubMed] [Google Scholar]
- 5. Robinson MH, Hardcastle JD, Moss SM et al. The risks of screening: data from the Nottingham randomised controlled trial of faecal occult blood screening for colorectal cancer. Gut 1999; 45: 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaukat A, Mongin SJ, Geisser MS et al. Long‐term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013; 369: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 7. Mandel JS, Church TR, Bond JH et al. The effect of fecal occult‐blood screening on the incidence of colorectal cancer. N. Engl. J. Med. 2000; 343: 1603–1607. [DOI] [PubMed] [Google Scholar]
- 8. Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20‐year follow‐up. Gut 2012; 61: 1036–1040. [DOI] [PubMed] [Google Scholar]
- 9. Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut 2002; 50: 840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee MH, Hinshaw JL, Kim DH, Pickhardt PJ. Symptomatic versus asymptomatic colorectal cancer: predictive features at CT colonography. Acad. Radiol. 2016; 23: 712–717. [DOI] [PubMed] [Google Scholar]
- 11. Inada R, Nagasaka T, Watanabe A et al. Comparison of outcomes between symptomatic and asymptomatic patients with colorectal cancer: a propensity score‐matched analysis of surgical invasiveness, medical costs and oncological outcomes. BMJ Open Gastroenterol. 2017; 4: e000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Irrazabal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell 2014; 54: 309–320. [DOI] [PubMed] [Google Scholar]
- 13. Nakatsu G, Li X, Zhou H et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 2015; 6: 8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu J, Feng Q, Wong SH et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non‐invasive biomarkers for colorectal cancer. Gut 2017; 66: 70–78. [DOI] [PubMed] [Google Scholar]
- 15. Liang Q, Chiu J, Chen Y et al. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin. Cancer Res. 2017; 23: 2061–2070. [DOI] [PubMed] [Google Scholar]
- 16. Liang JQ, Li T, Chen YX et al. A novel fecal lachnoclostridium marker for the non‐invasive diagnosis of colorectal adenoma and cancer. Gut 2019; 0: 1–10 doi:.1136/gutjnl‐2019‐318532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jalanka J, Salonen A, Salojarvi J et al. Effects of bowel cleansing on the intestinal microbiota. Gut 2015; 64: 1562–1568. [DOI] [PubMed] [Google Scholar]
- 18. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 19. Zhang X, Zhu X, Cao Y, Fang JY, Hong J, Chen H. Fecal Fusobacterium nucleatum for the diagnosis of colorectal tumor: a systematic review and meta‐analysis. Cancer Med. 2019; 8: 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kostic AD, Chun E, Robertson L et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor‐immune microenvironment. Cell Host Microbe 2013; 14: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E‐cadherin/beta‐catenin signaling via its FadA adhesin. Cell Host Microbe 2013; 14: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steinberg DM, Fine J, Chappell R. Sample size for positive and negative predictive value in diagnostic research using case‐control designs. Biostatistics 2009; 10: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]


