Abstract
Objective
To assess the risk of adverse fetal outcomes after exposure to oral antifungal agents during pregnancy.
Search strategy
PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched up to October 2018.
Selection criteria
Cohort studies and case–control studies investigating fetal outcomes following maternal exposure to oral antifungal agents.
Data collection and analysis
Two reviewers independently assessed studies for inclusion, assessed risk of bias, and extracted data. Pooled estimates were calculated for the frequency of adverse fetal outcomes.
Main results
Overall, eight cohort studies and one case–control study were included. The oral antifungal agents used during pregnancy were fluconazole and itraconazole. The data indicated that oral fluconazole exposure during pregnancy might slightly increase the risk of congenital heart defects and limb defects relative to the general population; oral itraconazole during pregnancy might increase the risk of eye defects. No difference was found between oral fluconazole/itraconazole exposure and non‐exposure in the risk of other birth defects, spontaneous abortion, or stillbirth.
Conclusion
Oral fluconazole or itraconazole may not increase the risk of birth defects. Nonetheless, the risk of congenital heart defects and limb defects after fluconazole exposure and eye defects after itraconazole exposure should be cautiously investigated.
Keywords: Abortion, Birth defects, Fluconazole, Itraconazole, Meta‐analysis, Stillbirth
Short abstract
Exposure to oral antifungal drugs in early pregnancy may not increase the risk of overall birth defects, spontaneous abortion, or stillbirth.
1. INTRODUCTION
It is estimated that over 60% of healthy premenopausal women are colonized with candida, and 75% of all women will experience at least one episode of symptoms due to candida in their lifetime.1 Owing to increased levels of sex hormones, vulvovaginal candidiasis occurs more frequently, and may be prolonged and associated with more severe symptoms in pregnancy.2 In general, only topical azoles are recommended in pregnancy, but oral azoles are prescribed when topical treatment fails.
Nevertheless, the safety of oral antifungal agents during pregnancy is controversial. Some studies have reported birth defects among newborns of women who used antifungal agents during pregnancy,3, 4, 5 whereas others found no difference between antifungal agent exposure and non‐exposure groups. To our knowledge, there has been no systematic review of the safety of oral antifungal agents used in pregnancy. The aim of the present study was therefore to conduct a systematic review of observational studies and a meta‐analysis to provide an up‐to‐date and comprehensive assessment of the fetal safety of oral antifungal agents.
2. MATERIALS AND METHODS
2.1. Search strategy
The present systematic review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) and MOOSE (Meta‐analysis Of Observational Studies in Epidemiology) guidelines. PubMed, Embase, and CENTRAL databases were searched for studies investigating the fetal outcomes of oral antifungal agents in pregnancy published until October 31, 2018. The search terms were “fluconazole” (MeSH term) OR fluconazole (text word) OR “itraconazole” (MeSH) OR itraconazole (text) OR “ketoconazole” (MeSH) OR ketoconazole (text) OR “voriconazole” (MeSH) OR voriconazole (text) OR “antifungal agents” (MeSH) OR “antifungal agents” (text) AND “pregnancy” (MeSH) OR “gravidity” (MeSH) OR “pregnant women” (MeSH) OR “pregnancy (text) OR “pregnant” (text). In addition, the reference lists of all retrieved studies were checked for relevant studies.
2.2. Eligibility criteria
Cohort studies and case–control studies were included. In all eligible studies, the exposed group used oral antifungal agents during pregnancy and the control group did not. All eligible studies reported at least one of the following outcomes: birth defects, spontaneous abortion, and stillbirth. Studies with only an abstract were excluded because of the limited information available.
2.3. Study selection
The titles and abstracts of retrieved studies were independently reviewed by two researchers (DL and CZ) for potentially eligible studies. Final eligibility was determined by reading the whole text. In cases of disagreement, eligibility was decided by a third researcher (LZ).
2.4. Assessment of risk of bias
The risk of bias in the included studies was independently assessed by two researchers (Li Z and LW) using the Newcastle–Ottawa quality assessment scale.6 Any disagreement was resolved by a third researcher (Lingli Z).
2.5. Data collection
A standard form was designed to extract the following information: study design, details of the data source, eligibility, methods, study women, interventions, and outcomes. For each study included, two researchers (DL and CZ) independently extracted the data. Any discrepancies were resolved by a third researcher (Lingli Z).
2.6. Data analysis
Statistical analysis was conducted by using Review Manager version 5.3 (The Cochrane Collaboration, Copenhagen, USA). Cohort studies and case–control studies were analyzed separately. All outcomes were assessed as dichotomous variables. Estimates of outcomes were pooled by using the Mantel‐Haenszel method and presented as summary relative risk (RR) with 95% confidence intervals (CIs). Data were combined by fixed‐effect models or by random effect models if there was significant heterogeneity (I 2>50%) between estimates. Subgroup analysis was conducted to investigate the heterogeneity between adjusted data and non‐adjusted data. If substantial heterogeneity (I 2>75%) was observed, sensitivity analyses were used to investigate it.
3. RESULTS
3.1. Search results
Overall, 2204 reports were identified from the database search. After screening titles and abstracts, 2177 were excluded. Nine studies were included after full text screening (Fig. 1). Of these, eight were cohort studies (five retrospective and four prospective),7, 8, 9, 10, 11, 12, 13, 14 and one was a case–control study.15 Seven studies were conducted in Europe, and two in North America (Table 1). In total, the nine studies reported data from deliveries between 1989 and 2013, enrolling 14 534 pregnant women who used fluconazole and 1311 pregnant women who used itraconazole.
Figure 1.
Flow diagram of the study selection process.[Colour figure can be viewed at wileyonlinelibrary.com]
Table 1.
Characteristics of the studies included in the review
| Study | Design | Data source (country) | Duration | No. of participants | Exposure | Age, y | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| Exposure | Control | T1 | T2 | ||||||
| 7 | Prosp. cohort | Teratology Information Service centers (Italy) | Jan 1992 to Jun 1994 | 226 (fluco) | 452 | 226 | <20 (n=4); 20–24 (n=60); 25–29 (n=237); 30–34 (n=271); 35–39 (n=83); >40 (n=23) | Birth defects, spontaneous abortion, stillbirth | |
| 8 | Retrosp. cohort |
General Practice Research Database (UK) |
1999 |
234 (fluco) 88 (itraco) |
1629 | 323 | Undescribed | Birth defects, spontaneous abortion, stillbirth | |
| 9 | Retrosp. cohort |
North Jutland Pharmaco‐ Epidemiological Prescription Database (Denmark) |
1991–1996 | 165 (fluco) | 13 327 | 121 | 44 | 27.8 (13–47) | Birth defects, stillbirth |
| 10 | Prosp. cohort |
International Pharmacovigilance Department of the Manufacturer of Itraconazole (Belgium) |
1989–1998 | 199 (itraco) | 198 | 199 | 30.5 | Birth defects, spontaneous abortion, stillbirth | |
| 11 | Retrosp. cohort | Medical Birth Registry, Central Office of Civil Registration, Danish Healthcare Registries (Denmark) | 1991–2005 | 1079 (fluco) | 170 453 | 1079 | <25 (n=1257); 25–30 (n=1740); >30 (n=72 950) | Birth defects | |
| 12 | Prosp. cohort | European network of Teratology Information Service centers (Italy) | Jan 2002 to Oct 2006 | 206 (itraco) | 207 | 206 | 31.6 | Birth defects, spontaneous abortion | |
| 13 | Retrosp. cohort | Medical Birth Registry (Denmark) | 1996–2011 |
7352 (fluco) 687 (itraco) 72 (ketoco) |
968 236 | 8111 | 29.99 | Birth defects | |
| 14 | Retrosp. cohort | Medical Birth Registry (Denmark) | 1997–2013 |
5428 (fluco) 131 (itraco) |
21 506 (fluco‐matched); 524 (itraco‐matched) | <25 (n=20 928); 25–30 (n=77 654); >30 (n=2421) | Spontaneous abortion, stillbirth | ||
| 15 | Case control | National Birth Defects Prevention Study (USA) | 1997–2011 | Birth defects | |||||
Abbreviations: fluco, fluconazole; itraco, itraconazole; ketoco, ketoconazole; T1, trimester 1; T2, trimester 2.
3.2. Risk of bias in eligible studies
The overall quality of the studies was good. Four studies were categorized as having low risk of bias, and five as having medium risk of bias (Table 2).
Table 2.
Risk of bias in the studies
| Study design | Selection | Comparability | Outcome | Risk of biasa | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Representativeness of the exposed cohort | Non‐exposed cohort | Ascertainment of exposure | Outcome of interest not present at start | Comparability of cohorts based on design or analysis | Assessment of outcome | Follow‐up duration sufficient | Adequacy of follow‐up | |
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Low |
| 8 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | Medium |
| 9 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | Medium |
| 10 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | Medium |
| 11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Low |
| 12 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | Medium |
| 13 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Low |
| 14 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Low |
| Case–control | Adequate case definition | Representative‐ness of cases | Selection of controls | Definition of controls | Comparability of cases and controls based on design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non‐response rate | |
| 15 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | Medium |
Low, ≥7; medium, 5–7; high, ≤4.
All cohort studies had low risk of bias in terms of comparability. All cohort studies had good representativeness and ascertainment of exposure. Except for one study,10 the non‐exposed cohort was derived from the same population. The outcomes of interest of five studies were verified as not present at the start of the study. All studies used medical records to assess outcomes. The minimum duration of follow‐up was after delivery. Five studies reported a loss to follow‐up of less than 10%.
The case–control study had low risk of bias in selection and comparability. Regarding the risk of bias in outcomes, the study used non‐blind structured interviews to ascertain exposure; the risk of bias in the other categories of outcomes was low.
3.3. Outcomes from cohort studies
3.3.1. Fluconazole versus control
Five studies involving 1 163 149 pregnant women compared the risk of birth defects between pregnant women exposed to fluconazole and unexposed women. The pooled data showed no significant increase in risk (RR, 0.99; 95% CI, 0.46–2.12; I 2=0) (Fig. 2).
Figure 2.
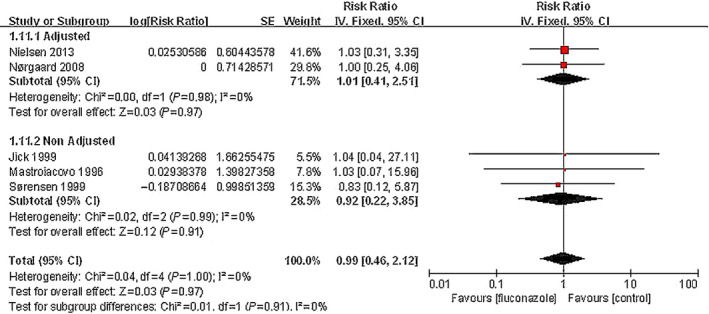
Risk of birth defects among pregnant women using fluconazole during pregnancy vs unexposed pregnant women.
Four studies reported specific categories of birth defects after maternal exposure to fluconazole during pregnancy (Table 3). Congenital heart defects were the most common type with a frequency of 1.52% (95% CI, 1.28–1.81), which was higher than the value for the general population published by EUROCAT (0.77%; 95% CI, 0.76–0.78).16 The second was limb defects with a frequency of 0.62% (95% CI, 0.48–0.78), which was slightly higher than the EUROCAT value (0.56%; 95% CI, 0.53–0.58). The frequencies of other birth defects were essentially similar to the constituent ratios of malformations published by EUROCAT.16
Table 3.
Types of congenital malformation (fluconazole)
| Malformation | Study | No. of cases | No. of women | Frequency, % (95% CI) | Ref. frequency, % (95% CI)a | P value |
|---|---|---|---|---|---|---|
| Congenital heart defect | 7, 11, 13 | 132 | 8665 | 1.52 (1.28–1.81) | 0.77 (0.76–0.78) | <0.05 |
| Limb defect | 7, 8, 11, 13 | 67 | 10 891 | 0.62 (0.48–0.78) | 0.56 (0.53–0.58) | <0.05 |
| Nervous system | 8, 11 | 3 | 1313 | 0.23 (0.05–0.67) | 0.26 (0.25–0.26) | ≥0.05 |
| Genital | 7, 11 | 3 | 1305 | 0.23 (0.05–0.67) | 0.22 (0.21–0.22) | ≥0.05 |
| Eye defect | 11 | 2 | 1079 | 0.19 (0.02–0.67) | 0.04 (0.03–0.04) | ≥0.05 |
| Urinary system | 7, 11 | 2 | 1305 | 0.15 (0.02–0.55) | 0.35 (0.34–0.35) | ≥0.05 |
| Cleft lip with or without palate | 13 | 10 | 7352 | 0.14 (0.07–0.25) | 0.08 (0.08–0.09) | ≥0.05 |
| Cleft palate | 13 | 5 | 7352 | 0.07 (0.02–016) | 0.06 (0.05–0.06) | ≥0.05 |
| Digestive system | 7, 11, 13 | 5 | 8657 | 0.06 (0.02–0.13) | 0.18 (0.17–0.18) | <0.05 |
| Respiratory | 8 | 1 | 1079 | 0.01 (0.00–0.05) | 0.04 (0.04–0.04) | ≥0.05 |
EUROCAT frequency in the general population.16
Two studies involving 27 612 pregnant women compared the frequency of spontaneous abortion between maternal exposure to fluconazole during pregnancy and non‐exposure. The pooled data showed no significant difference between the oral fluconazole group and non‐exposure group in the incidence of spontaneous abortion (RR, 1.15; 95% CI, 0.32–4.1; I 2=0%) (Fig. 3).
Figure 3.
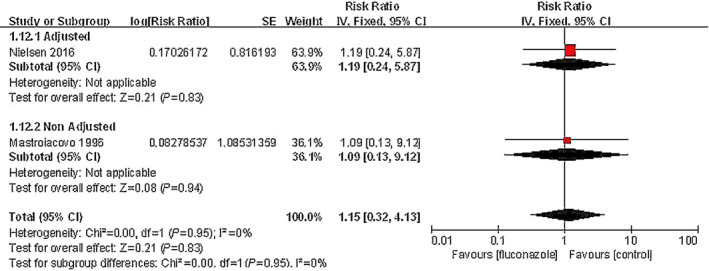
Risk of spontaneous abortion among pregnant women using fluconazole during pregnancy vs unexposed pregnant women.
Three studies involving 41 179 pregnant women reported stillbirth as an outcome. The pooled data showed no significant difference between the oral fluconazole group and non‐exposure group in the incidence of spontaneous abortion (RR, 1.09; 95% CI, 0.22–5.41; I 2=0%) (Fig. 4).
Figure 4.
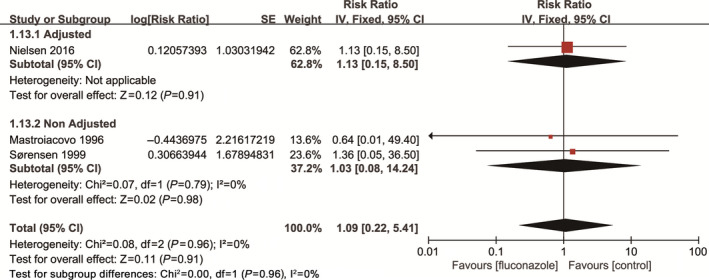
Risk of stillbirth among pregnant women using fluconazole during pregnancy vs unexposed pregnant women.
3.3.2. Itraconazole versus control
Four studies involving 971 450 pregnant women compared the overall risk of birth defects between maternal exposure to itraconazole and non‐exposure. The pooled data showed no significant difference (RR, 1.04; 95% CI, 0.32–3.34; I 2=0; Fig. 5). Four studies reported specific categories of birth defects after maternal exposure to itraconazole during pregnancy (Table 4). Limb defects and congenital heart defects were the most common type with a frequency of 0.82% (95% CI, 0.35–1.62) and 0.82% (95% CI, 0.35–1.61), respectively. The rate of eye defects was higher than the value published by EUROCAT, whereas the rates of other birth defects were essentially similar to the constituent ratios of malformation types published by EUROCAT.16
Figure 5.
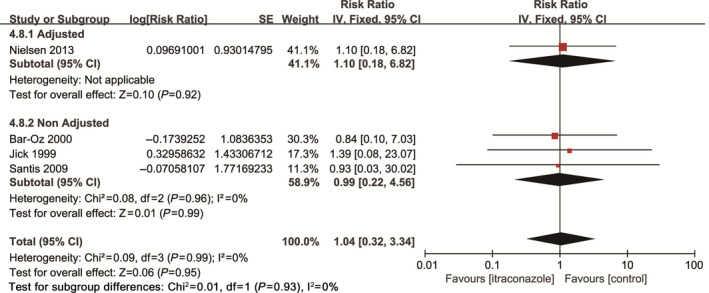
Risk of birth defects among pregnant women using itraconazole during pregnancy vs unexposed pregnant women.
Table 4.
Types of congenital malformation (itraconazole).
| Malformation | Study | No. of cases | No. of women | Frequency, % (95% CI) | Ref. frequency, % (95% CI)a | P value |
|---|---|---|---|---|---|---|
| Limb defect | 8, 10, 13 | 8 | 976 | 0.82 (0.35–1.62) | 0.44 (0.43–0.45) | ≥0.05 |
| Congenital heart defect | 8, 12, 13 | 8 | 981 | 0.82 (0.35–1.61) | 0.77 (0.76–0.78) | ≥0.05 |
| Genital | 13 | 4 | 687 | 0.58 (0.16–1.49) | 0.22 (0.21–0.22) | ≥0.05 |
| Eye defect | 10, 13 | 5 | 886 | 0.56 (0.18–1.32) | 0.04 (0.03–0.04) | <0.05 |
| Nervous system | 12 | 1 | 206 | 0.49 (0.01–2.70) | 0.26 (0.25–0.26) | ≥0.05 |
| Digestive system | 10, 13 | 2 | 886 | 0.23 (0.03–082) | 0.18 (0.17–0.18) | ≥0.05 |
| Urinary system | 12, 13 | 2 | 893 | 0.22 (0.03–0.81) | 0.35 (0.34–0.35) | ≥0.05 |
EUROCAT frequency in the general population.16
Three studies involving 1465 pregnant women compared the rate of spontaneous abortion between maternal exposure to itraconazole during pregnancy and non‐exposure. The pooled data showed no significant difference (RR, 1.44; 95% CI, 0.38–5.43; I 2=0) (Fig. 6).
Figure 6.
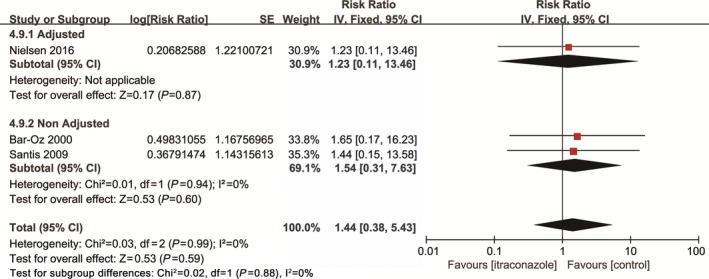
Risk of spontaneous abortion among pregnant women using fluconazole during pregnancy vs unexposed pregnant women.
One study involving 405 pregnant women reported stillbirth as an outcome. The data showed no significant difference between the oral itraconazole group and the non‐exposure group in the incidence of stillbirth.
3.4. Outcomes from case–control studies
Only one case–control study reported fetal outcomes following the administration of fluconazole during the first trimester of pregnancy.15 The study involved 31 645 cases of birth defects and 11 612 control women. Maternal exposure to fluconazole during the first trimester was significantly associated with cleft lip and cleft palate (OR, 5.53; 95% CI, 1.68–18.24) and dextro‐transposition of the great arteries (OR, 7.56; 95% CI, 1.22–35.45).
4. DISCUSSION
The present meta‐analysis suggests that both fluconazole and itraconazole are often used during pregnancy. The meta‐analysis found that the administration of fluconazole or itraconazole during pregnancy was not associated with an increased risk of overall birth defects, but it was associated with a possible increase in the risk of specific birth defects. The vast majority of the study data were collected for exposure during the first trimester of pregnancy, which is the most relevant time window with respect to potentially teratogenic exposures. The meta‐analysis also found that administration of fluconazole or itraconazole was not associated with an increased risk of spontaneous abortion or stillbirth.
In the review, the pooled estimate for incidence of birth defects from eight studies showed no difference between the fluconazole (RR, 0.99; 95% CI, 0.46–2.12) or itraconazole (RR, 1.04; 95% CI, 0.32–3.34) population and the non‐exposed population. This finding confirmed previous results from individual clinical observational studies.
Regarding specific types of birth defect observed in the fluconazole‐exposed population, the frequency of congenital heart defects was highest at 1.5% (95% CI, 1.28–1.81). This finding suggested a slight increase in the frequency of congenital heart defects as compared with the value reported by EUROCAT.16 This finding is in agreement with those of Mølgaard‐Nielsen et al.13 and Howley et al.15 Mølgaard‐Nielsen et al.'s research made a great contribution to the estimation of congenital heart defects, which means that our result is largely based on their outcome. The frequency of limb defects (0.62%; 95% CI, 0.48%–0.78%) among the fluconazole‐exposed population was slightly higher than that reported by EUROCAT (0.56%; 95% CI, 0.53–0.58). Although the frequency of eye defects in the itraconazole‐exposed population was not high (0.56%; 95% CI, 0.18–1.32), it was considerably higher than the rate reported by EUROCAT (0.04%; 95% CI, 0.03–0.04).
A previous systematic review identified and collected controlled studies evaluating birth defects associated with fluconazole exposure during the first trimester of pregnancy. Although the results did not indicate that maternal fluconazole is associated with an increased risk of birth defects,17 that review failed to include one case–control study, did not include studies published after 2014, and did not consider the results of spontaneous abortion or stillbirth. By contrast, the present review was based on an exhaustive search up until October 2018, and considered the results of different birth defects, spontaneous abortion, and stillbirth. In addition, studies on maternal exposure to itraconazole during pregnancy were included. As a result, the present meta‐analysis included five more studies than the previous review and provided relatively comprehensive evidence.
The study has some strengths. First, the results were reported in accordance with PRISMA and MOOSE guidelines. Second, comprehensive inclusion criteria, covering important indices affecting the fetus, were used. However, the study also has limitations. At the outset of the study, it was intended to study differences in the doses of antifungal agents used in pregnancy, but only a few studies reported these doses; thus, the data on of antifungal drug dosage were inadequate. In addition, the frequencies of specific kinds of birth defects were compared between maternal exposure to antifungal agents and an unexposed population based on EUROCAT data, because some studies reported only specific kinds of birth defect cases for the exposed group and did not state the number of cases in the non‐exposed group.
5. CONCLUSION
The current evidence suggests that the administration of oral antifungal agents in early pregnancy may not be associated with an increased risk of birth defects, spontaneous abortion, or stillbirth. Nonetheless, the risk of congenital heart defects and limb defects in the fluconazole‐exposed population and eye defects in the itraconazole‐exposed population should be cautiously investigated.
AUTHOR CONTRIBUTIONS
DL contributed to data acquisition, analysis, and interpretation; and wrote and revised the manuscript. CZ contributed to data acquisition and interpretation. LW contributed to data analysis and interpretation. Lingli Z and Li Z contributed to study conception and design; and revised the manuscript.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the affiliated institutions and organizations, and the Program for Yangtze River Scholars and Innovative Research Teams in University (No. IRT0935) and Group of People with Highest Risk of Drug Exposure of the International Network for the Rational Use of Drugs, China.
The copyright line for this article was changed on 23 June 2020 after original online publication.
REFERENCES
- 1. Sherrard J, Wilson J, Donders G, Mendling W, Jensen JS. 2018 European (IUSTI/WHO) International Union Against Sexually Transmitted Infections (IUSTI) World Health Organisation (WHO) Guideline on the Management of Vaginal Discharge. Int J STD AIDS. 2018;29:1258–1272. [DOI] [PubMed] [Google Scholar]
- 2. Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–1971. [DOI] [PubMed] [Google Scholar]
- 3. Pursley TJ, Blomquist IK, Abraham J, Andersen HF, Bartley JA. Fluconazole‐induced congenital anomalies in three infants. Clin Infect Dis. 1996;22:336–340. [DOI] [PubMed] [Google Scholar]
- 4. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: Report of an additional patient. Am J Med Genet Part A. 1997;72:253–256. [PubMed] [Google Scholar]
- 5. Lopez‐Rangel E, Van Allen MI. Prenatal exposure to fluconazole: An identifiable dysmorphic phenotype. Birth Defects Res A. 2005;73:919–923. [DOI] [PubMed] [Google Scholar]
- 6. Wells GASB, O'Connell D, Peterson J, et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of non‐randomized studies in meta‐analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 30, 2015.
- 7. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first‐trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645–1650. [DOI] [PubMed] [Google Scholar]
- 8. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221–222. [DOI] [PubMed] [Google Scholar]
- 9. Sorensen HT, Nielsen GL, Olesen C, et al. Risk of malformations and other outcomes in children exposed to fluconazole in utero. Br J Clin Pharmacol. 1999;48:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bar‐Oz B, Moretti ME, Bishai R, et al. Pregnancy outcome after in utero exposure to itraconazole: A prospective cohort study. Am J Obstet Gynecol. 2000;183:617–620. [DOI] [PubMed] [Google Scholar]
- 11. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: A Danish population‐based cohort study. J Antimicrob Chemother. 2008;62:172–176. [DOI] [PubMed] [Google Scholar]
- 12. De Santis M, Di Gianantonio E, Cesari E, Ambrosini G, Straface G, Clementi M. First‐trimester itraconazole exposure and pregnancy outcome: A prospective cohort study of women contacting teratology information services in Italy. Drug Saf. 2009;32:239–244. [DOI] [PubMed] [Google Scholar]
- 13. Mølgaard‐Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830–839. [DOI] [PubMed] [Google Scholar]
- 14. Mølgaard‐Nielsen D, Svanström H, Melbye M, Hviid A, Pasternak B. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58–67. [DOI] [PubMed] [Google Scholar]
- 15. Howley MM, Carter TC, Browne ML, Romitti PA, Cunniff CM, Druschel CM; National Birth Defects Prevention Study . Fluconazole use and birth defects in the National Birth Defects Prevention Study. Am J Obstet Gynecol. 2016; 214:657.e1–657.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. EUROCAT . Prevalence Tables. https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence;. Accessed December 20 2018.
- 17. Alsaad AM, Kaplan YC, Koren G. Exposure to fluconazole and risk of congenital malformations in the offspring: A systematic review and meta‐analysis. Reprod Toxicol. 2015;52:78–82. [DOI] [PubMed] [Google Scholar]


