Abstract
Background
Nasal high flow (NHF) has demonstrated efficacy in relieving dyspnea in various patients with hypoxemic and hypercapnic respiratory failure. It may also reduce dyspnea in patients with acute severe asthma in the emergency department (ED). The aim of the study was to compare the efficacy of NHF with conventional oxygen therapy (COT) in improving dyspnea in acute severe asthma patients with hypoxemia in the ED.
Methods
This pilot nonblinded randomized controlled trial was conducted involving 37 patients aged ≥ 18 years with acute severe asthma and hypoxemia in the ED of Siriraj Hospital, Bangkok, Thailand (TCTR20180926003). The participants were randomly allocated to receive either COT (n = 18) or NHF (n = 19) for 120 minutes. The primary outcome was comparing the intervention effects on the patients’ degree of dyspnea measured using the modified Borg scale (MBS). The secondary outcomes were comparing the interventions based on the numeric rating scale (NRS) of dyspnea, the dyspnea scale assessing accessory muscle use, vital signs, and blood gas results.
Results
The intention‐to‐treat analysis included 37 patients (COT group n = 18 and NHF group n = 19). The baseline mean MBS was 7.8 in both groups. At 120 minutes, the mean (±SD) MBSs in patients receiving COT and NHF were 3.3 (±2.5) and 1.4 (±2.5), respectively (mean difference = 1.9 [95% CI = 0.2 to 3.8], p = 0.043). The trends in NRS and dyspnea score results were similar to those of MBS. Respiratory rates were lower with NHF (mean difference = 4.7 [95% CI = 1.5 to 7.8], p = 0.001). No between‐ or within‐group differences in blood gas results were found.
Conclusion
Nasal high flow reduced the severity of dyspnea and respiratory rate in hypoxemic patients with acute severe asthma in the ED.
Asthma is a chronic inflammatory airway disease characterized by airway hyperresponsiveness and often reversible airflow obstruction. 1 It is one of the most common airway diseases in both children and adults. 1 In Thailand, the prevalence of childhood asthma is currently 10% to 13%. 2 , 3 Although the prevalence in adults is lower at 4%, and the mortality rate is decreasing, the disease has not been well controlled with a high rate of exacerbations, emergency department (ED) visits, and hospital admissions. 4 , 5
Acute severe asthma, or more commonly referred to as an asthma exacerbation, is characterized by an acute obstruction of expiratory airflow due to airway inflammation, bronchospasm, and hypersecretion, resulting in increased work of breathing. If not reversed, the respiratory muscles may fatigue, therefore leading to hypercapnia, severe hypoxemia, and consequently respiratory failure. Initial management of acute severe asthma in the ED, as recommended by The Global Initiative for Asthma (GINA) guideline, includes early administration of supplemental oxygen as needed as well as medications such as nebulized bronchodilators and systemic corticosteroid. 1 Despite aggressive initial management, in some patients with respiratory failure or hypoxemia, invasive ventilatory support may be required. Otherwise, oxygen is usually given via nasal cannula or nonrebreather mask. Noninvasive ventilation (NIV), which is a promising modality for chronic obstructive pulmonary disease (COPD), has had limited success in treating acute severe asthma. 6
Nasal high flow (NHF) is another oxygen‐delivering method of inspired fraction (FiO2) up to 1.0 via a purposely made high‐flow nasal cannula across a range of flows from 2 to 60 L/min. Conditioning of delivered gas by warming to 37° C and fully saturating with water offers patients comfort during the therapy while high flow provides positive airway pressure and decreases rebreathing from anatomic dead space, which leads to a reduced respiratory effort. 7 , 8 , 9 , 10 Many studies have evaluated the use of NHF in critical patients with hypoxemic respiratory failure of multiple etiologies. 11 , 12 , 13 , 14 Its use has also been effective in EDs for patients with all‐cause hypoxemic respiratory failure, cardiogenic pulmonary edema, and do‐not‐intubate status. 15 , 16 , 17 To date, there have been many published studies demonstrating the benefit of NHF in decreasing the arterial partial pressure of carbon dioxide (PaCO2) in patients with hypercapnic respiratory failure secondary to COPD. 18 , 19 , 20
In acute severe asthma, NHF appears to be a physiologically favorable treatment. A decrease in respiratory rate, as has been demonstrated in many previous studies, may allow patients to increase their expiratory time, thereby decreasing dynamic hyperinflation. In addition, heated and humidified air facilitates mucociliary clearance and could deliver more comfort. This may also help reduce bronchoconstriction induced by cold air. 21 In pediatric patients with acute severe asthma, NHF has been proven to improve clinical severity effectively, compared with both conventional oxygen therapy (COT) 22 , 23 and NIV. 24 A decrease in PaCO2 and an increase in the pH level using NHF therapy were also shown in children and adolescents, but the evidence in adult patients is lacking. 24 The trial investigators hypothesized that NHF may provide similar respiratory support benefits in adults with asthma. Therefore, this preliminary study was conducted to explore the efficacy of NHF compared with COT for adult patients with acute severe asthma with hypoxemia in the ED as improvement in subjective dyspnea ratings, physiologic variables, and arterial blood gas results.
METHODS
Study Design and Settings
The trial investigators designed this pilot study to determine the preliminary level of effectiveness before undertaking a confirmatory randomized controlled trial. This single‐centered, nonblinded, randomized controlled trial was conducted at the ED of Siriraj Hospital, the largest tertiary university hospital in Bangkok, Thailand, with over 20,000 Emergency Severity Index (ESI) triage Level I and II ED visits annually. The annual ED visit rate with dyspnea was approximately 3,600, of which 5% to 10% were due to acute severe asthma. The study protocol was approved by the Siriraj Institutional Review Board and was registered in the Thai Clinical Trials Registry (ID: TCTR 20180926003). Patients were recruited between March 22, 2019, and February 25, 2020. All participants or their next of kin provided written informed consents prior to enrollment. We made no changes to the trial protocol after the trial initiation.
Selection of Participants
Eligible participants were adults aged ≥ 18 years previously diagnosed with asthma 1 presenting to the ED with symptoms compatible with an acute exacerbation and hypoxemia defined as a pulse oximetry reading (SpO2) at room air of less than 95%. An acute exacerbation was defined as having all the following symptoms: a progressive increase in shortness of breath, cough and wheezing, or chest tightness. 1 Patients were excluded if they had respiratory failure defined as having a respiratory rate > 35 breaths/min or SpO2 < 90% despite oxygen supplement or signs of increased work of breathing, a Glasgow Coma Scale (GCS) score of <13, contraindications to the use of equipment with positive airway pressure and a previous diagnosis of COPD or smoking more than 5 pack‐years, and lung cancer. Also excluded were patients concurrently diagnosed with other lower respiratory tract conditions that may have changed the course of disease, such as pneumonia and pulmonary edema.
Study Protocol and Interventions
After arrival in the ED, all patients were assessed consecutively for eligibility by an ED physician. If acute severe asthma was suspected, the physician gave standard treatment as per recommendation, 1 which included up to three doses of nebulized beta‐agonist and anticholinergics every 20 minutes. Oxygen therapy was given via nebulization during a 60‐minute run‐in period. Systemic corticosteroid and magnesium sulfate were given and investigations such as laboratory analysis and chest radiographs were ordered at the discretion of the treating physician. This physician then notified a project investigator, who confirmed and reassessed eligibility at 60 minutes after initial management. This run‐in period was designed to exclude patients whose severity of acute attack was too mild or too severe, who might not have completed the study protocol. If eligibility was confirmed, the project investigator enrolled the patient, obtained informed consent, randomized the interventions, and completed data collection. A computer‐generated, mixed‐block (block size of 2 and 4) randomization for sequence assignment (1:1 ratio) was performed using sealed opaque envelopes.
Enrolled patients were randomized to receive either COT or NHF for 120 minutes. COT was given by a standard oxygen nasal cannula or nonrebreather mask. NHF was delivered by a high‐flow blower‐humidifier AIRVO 2 via Optiflow medium‐sized high‐flow nasal cannula (Fisher & Paykel Healthcare, Auckland, New Zealand). The initial NHF rate was 35 L/min and could be adjusted from 30 to 60 L/min according to the participant’s level of comfort. FiO2 was adjusted in both groups to achieve a steady state SpO2 of 95% to 99% or 90% to 92% if the patients’ baseline SpO2 at room was less than 92%.
During the trial, all participants received standard treatments and interventions, including continued doses of bronchodilators, systemic corticosteroid, and magnesium sulfate if not given prior to reassessment, antibiotics, intravenous fluids, and other treatments as deemed appropriate by the treating physician. After the trial, standard of care for acute severe asthma and other treatments were delivered at the discretion of the treating physicians.
Outcome Measurements
The primary outcome was the degree of dyspnea measured by the modified Borg scale (MBS). This is a validated category ratio scale ranging from 0 to 10 points that has been used to assess the severity of dyspnea in acute severe asthma. 25 , 26 , 27
The secondary outcomes were the numeric rating scale (NRS) of dyspnea, a dyspnea score measured by visual analog scale (VAS), comfort score, effects on vital signs (respiratory rate, SpO2, heart rate, and mean arterial pressure), blood gas analysis, NHF‐associated adverse event rate, escalation to invasive ventilation within 24 hours, ED disposition, and length of stay in the ED and hospital.
Patient‐reported dyspnea scale scores and physiologic variables were collected at the start of intervention and at 30, 60, and 120 minutes after starting treatment. The comfort score was assessed after the intervention was complete.
The NRS is a validated dyspnea scale ranging from 0 (“no shortness of breath”) to 10 (“worst shortness of breath”) points 28 used to assess convergent validity with MBS. The dyspnea scale is a VAS recorded by the trial investigators to assess the severity of dyspnea by measuring the degree of accessory muscle use. The clinical researchers evaluated this score by observing the retraction of the sternocleidomastoid muscles. This VAS is measured on a horizontal line labeled as absent (0 mm), mild (25 mm), moderate (50 mm), severe (75 mm), and very severe (100 mm). 27 The comfort scale is also a ratio measure, ranging from 0 (no comfort) to 10 (maximum comfort). 16 , 17 At each time point, study physicians measured dyspnea scale prior to asking participants to record their reported scores on the record form.
Data Analysis
Because there were no previous trials comparing NHF with COT in adult patients with acute severe asthma at the start of the trial, the trial investigators designed the trial as a pilot study using a stepped rule of thumb to recruit 50 patients in total. This was based on our hypothesis that the target effect size (d) would be small (0.1 ≤ d <0.3), and the future main trial would be powered at 90%. 29 However, due to the coronavirus disease 2019 pandemic, trials involving NHF use outside negative‐pressure rooms had to be suspended. It was therefore decided to terminate the study after only 37 patients had participated. Consequently, all statistical analyses performed should be considered exploratory analyses with an overall type I error rate of 5%.
Continuous variables are presented as mean (±SD) or median (interquartile range) as appropriate. Categorical variables are described as frequencies and percentages. Differences between groups were analyzed with the Student’s t‐test or Mann‐Whitney U‐test for continuous variables as appropriate.
All statistical analyses were performed on an intention‐to‐treat (ITT) basis. For the primary analysis of each quantitative outcome, a linear mixed model using restricted maximum likelihood estimation was fitted to compare repeated measures at three time points (30, 60, and 120 minutes) between the two groups. Analysis of covariance (ANCOVA) in the linear mixed model adjusted for the baseline value of each outcome measurement. Fixed effects were treatment, time, baseline outcome measurement value, time‐by‐treatment interaction term, and time‐by‐baseline outcome measurement interaction term, and the random effect was patient identity. An appropriate variance–covariance matrix structure was chosen based on Bayesian Information Criteria. To control the overall type I error rate due to multiple comparisons, the Tukey‐Kramer method was applied. Missing data were handled by last observation carried forward. Per‐protocol analyses by the linear mixed model were also performed. Linear mixed‐model diagnostics for assumptions of linearity, normal distribution of residuals, and homoscedasticity were checked using residuals plots and histograms. For pre‐ and posttreatment blood gas results, a paired t‐test was used to compare within each group between pre‐ and posttreatment results, and an unpaired t‐test was used to compare pretreatment results between COT and NHF groups as well as posttreatment results. Furthermore, the postintervention between‐group comparison was adjusted for the pretreatment outcome measurement by ANCOVA. A further sensitivity analysis was performed excluding patients with chronic lung disease that could have altered the clinical course, especially for blood gas results. Analyses were performed using SAS studio 9.2 (Cary, NC) and SPSS 18.0 (Chicago, IL).
RESULTS
Characteristics of Study Subjects
A total of 290 patients were consecutively assessed for eligibility between March 2019 and February 2020. Of these, 29 patients were excluded for the following reasons: meeting the criteria of respiratory failure (n = 17), GCS < 13 (n = 8), concurrent COPD (n = 3), and advanced lung cancer (n = 1). All patients received three doses of nebulized bronchodilators before being reassessed at the end of the 60‐minute run‐in period. After reassessment, 224 patients were further excluded; six had a respiratory rate ≥ 35 breaths/min, two hundred had SpO2 ≥ 95%, and eighteen had been diagnosed with pneumonia. A total of 37 patients were randomized, 18 in the COT group and 19 in the NHF group (Figure 1). One participant from each group had worsening clinical symptoms and was intubated after initial assessment and before 30 minutes. Therefore, they could not contribute any patient‐reported data for the analysis. Consequently, we carried forward their previous MBS and NRS scale scores, all of which were the maximum score of 10, to all the following time points after they were intubated for the ITT analysis.
Figure 1.
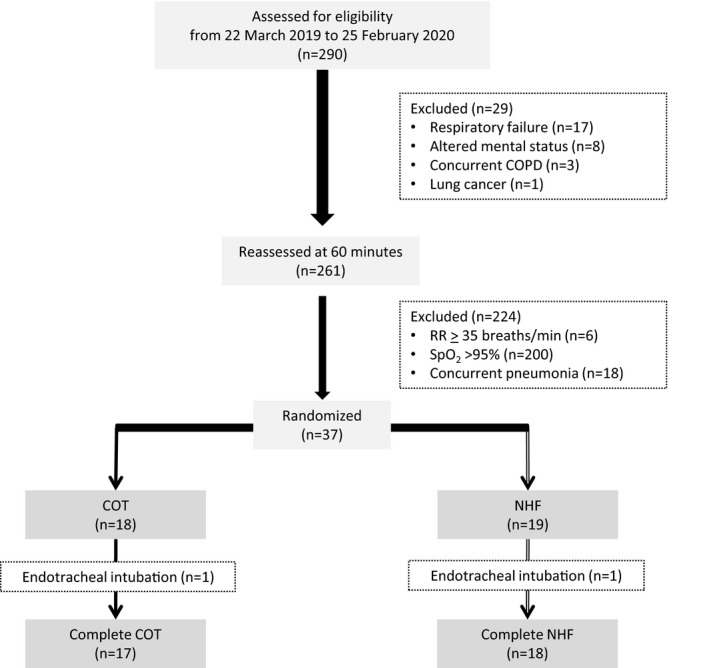
Flow of study participants. COPD = chronic obstructive pulmonary disease; COT = conventional oxygen therapy; NHF, nasal high flow; RR = respiratory rate; SpO2 = oxygen saturation by pulse oximetry.
Baseline characteristics of participants are presented in Table 1. The overall mean age was 63.5 years. Most of the included participants were female (83.8%). Medications currently used to control asthma and the mean baseline asthma control test score 30 , 31 were similar between participants randomized to the COT and NHF groups. Vital signs on arrival in the ED and at the start of intervention, as well as the results of the MBS, NRS, and dyspnea scale at the start of intervention, were also comparable between the two groups. All patients in both groups received systemic corticosteroid prior to enrollment. Participants randomized to the COT group received a mean (±SD) oxygen flow rate of 3.1 (±0.5) L/min through standard nasal cannula. NHF settings were a mean (±SD) flow rate of 36.8 (±4.2) L/min and a mean (±SD) FiO2 of 0.4 (±0.1).
Table 1.
Characteristics of Patients
| Variable at baseline |
COT (n = 18) |
NHF (n = 19) |
|---|---|---|
| Age (years) | 63.2 ± 21.8 | 63.7 ± 16.9 |
| Female sex | 15 (83.3) | 16 (84.2) |
| Comorbidities | ||
| Diabetes mellitus | 2 (11.1) | 5 (26.3) |
| Hypertension | 5 (27.8) | 10 (52.6) |
| Hyperlipidemia | 3 (16.7) | 2 (10.5) |
| Chronic kidney disease | 0 (0) | 3 (15.8) |
| Cardiovascular disease | 1 (5.6) | 2 (10.5) |
| Current medication | ||
| ICS | 2 (11.1) | 2 (10.5) |
| LABA | 1 (5.6) | 4 (21.1) |
| SABA | 10 (55.6) | 10 (52.6) |
| ICS + LABA | 13 (72.2) | 15 (78.9) |
| Anticholinergics + SABA | 5 (27.8) | 7 (36.8) |
| Methylxanthine | 2 (11.1) | 4 (21.1) |
| Leukotriene receptor antagonist | 3 (16.7) | 10 (52.6) |
| Procaterol HCl | 3 (16.7) | 4 (21.1) |
| Duration before intervention (minutes) | 70.5 (65–91.5) | 94 (67–103) |
| Oxygen flow before intervention | 4.8 ± 1.5 | 4.9 ± 1.5 |
| Initial vital signs | ||
| Respiratory rate (breaths/min) | 33.6 ± 4.0 | 33.0 ± 4.4 |
| Pulse oximetry (%) | 90.5 ± 1.8 | 90.3 ± 1.8 |
| Heart rate (beats/min) | 114.6 ± 19.2 | 117.6 ± 17.2 |
| Mean arterial pressure (mm Hg) | 111.6 ± 13.3 | 114.6 ± 21.1 |
| Parameter before intervention | ||
| MBS | 7.8 ± 2.0 | 7.8 ± 1.9 |
| NRS | 7.7 ± 2.0 | 7.8 ± 1.9 |
| Dyspnea scale | 5.6 ± 2.1 | 5.6 ± 2.1 |
| Respiratory rate (breaths/min) | 31.8 ± 4.4 | 30.4 ± 4.0 |
| Pulse oximetry (%) | 91.7 ± 1.1 | 91.7 ± 1.9 |
| Heart rate (beats/min) | 108.7 ± 19.7 | 112.7 ± 18.2 |
| Mean arterial pressure (mm Hg) | 106.4 ± 14.6 | 109.5 ± 18.9 |
| Baseline asthma control test score | 14.9 ± 5.1 | 14.6 ± 4.1 |
Data are presented as n (%), mean ± SD, or median (range).
COT = conventional oxygen therapy; ICS = inhaled corticosteroid; MBS = modified Borg scale; LABA = long‐acting beta2‐agonist; NHF = nasal high flow; NRS = numeric rating scale; SABA = short‐acting beta2‐agonist.
Primary Outcomes
The mean MBSs of both groups at each measured time point are shown in Table 2 and Figure 2A. At the end of the intervention, the mean (±SD) MBS with NHF was 1.4 (±2.5) and the mean (±SD) MBS with COT was 3.3 (±2.5) (mean difference = 1.9 [95% CI = 0.2 to 3.8], p = 0.043). No significant between‐group differences were seen at 30 and 60 minutes after initiation of treatment. The per‐protocol analyses showed similar results (Data Supplement S1, Table S1, available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14187/full).
Table 2.
Primary Outcome and Secondary Outcomes
| At 30 Minutes | At 60 Minutes | At 120 Minutes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COT (n = 18) | NHF (n = 19) | Difference (95% CI); p‐value | COT (n = 18) | NHF (n = 19) | Difference (95% CI); p‐value | COT (n = 18) | NHF (n = 19) | Difference (95% CI); p‐value | ||
| Patient‐reported outcomes | ||||||||||
| MBS | 5.9 ± 2.3 | 5.2 ± 2.1 | 0.7 (–1.3 to 2.6); p = 0.27 | 4.2 ± 2.8 | 2.8 ± 2.2 | 1.4 (–0.5 to 3.3); p = 0.27 | 3.3 ± 2.5 | 1.4 ± 2.5 | 1.9 (0.2 to 3.8); p = 0.043 | |
| NRS | 5.8 ± 2.4 | 5.1 ± 2.0 | 0.9 (–1.1 to 2.8); p = 0.76 | 4.2 ± 2.8 | 2.7 ± 2.3 | 1.6 (–0.4 to 3.5); p = 0.17 | 3.2 ± 2.5 | 1.4 ± 2.5 | 1.9 (0.3 to 3.8); p = 0.044 | |
| Comfort score* | — | — | — | — | — | — | 6.1 ± 1.6 | 9.1 ± 1.3 | –3.0 (–4.0 to –2.0); p < 0.0001 | |
| Physician‐reported outcome and physiologic variables | ||||||||||
| Dyspnea score | 3.8 ± 2.2 | 2.9 ± 1.6 | 0.6 (–1.0 to 2.2); p = 0.90 | 3.1 ± 2.3 | 1.7 ± 2.1 | 1.4 (–0.4 to 2.9); p = 0.25 | 3.1 ± 2.5 | 1.4 ± 2.5 | 1.8 (0.4 to 3.6); p = 0.04 | |
| Respiratory rate | 28.2 ± 5.3 | 25.1 ± 2.7 | 2.4 (–0.8 to 5.5); p = 0.26 | 26.1 ± 4.4 | 22.4 ± 2.4 | 2.9 (–0.3 to 6.0); p = 0.09 | 25.1 ± 4.2 | 19.7 ± 2.9 | 4.7 (1.5 to 7.8); p = 0.001 | |
| Pulse oximetry | 96.3 ± 2.5 | 97.7 ± 2.0 | –1.4 (–3.3 to 0.6); p = 0.35 | 97.2 ± 2.4 | 98.0 ± 1.8 | ‐0.8 (–2.8 to 1.2); p = 0.86 | 97.3 ± 2.6 | 98.5 ± 1.3 | –1.2 (–3.2 to 0.8); p = 0.49 | |
| Heart rate | 106.3 ± 16.1 | 105.7 ± 20.2 | 3.2 (–7.8 to 14.1); p = 0.96 | 104.6 ± 13.8 | 103.0 ± 19.3 | 4.3 (–6.6 to 15.2); p = 0.85 | 100.1 ± 11.5 | 101.2 ± 19.3 | 1.3 (–9.6 to 12.2); p = 0.99 | |
| Mean arterial pressure | 94.8 ± 12.1 | 99.0 ± 15.5 | –2.6 (–12.2 to 6.9); p = 0.97 | 91.5 ± 11.6 | 93.2 ± 13.2 | –0.1 (–9.7 to 9.4); p = 0.99 | 86.7 ± 9.4 | 97.3 ± 16.6 | –9.1 (–18.6 to 0.5); p = 0.07 | |
Data are presented as mean ± SD. Covariance structure of first‐order autoregressive was the most parsimonious for most outcomes except heterogenous autoregressive for the dyspnea scale
COT = conventional oxygen therapy; MBS = modified Borg scale; NHF = nasal high flow; NRS = numeric rating scale.
COT(n = 17), NHF (n = 18).
Figure 2.
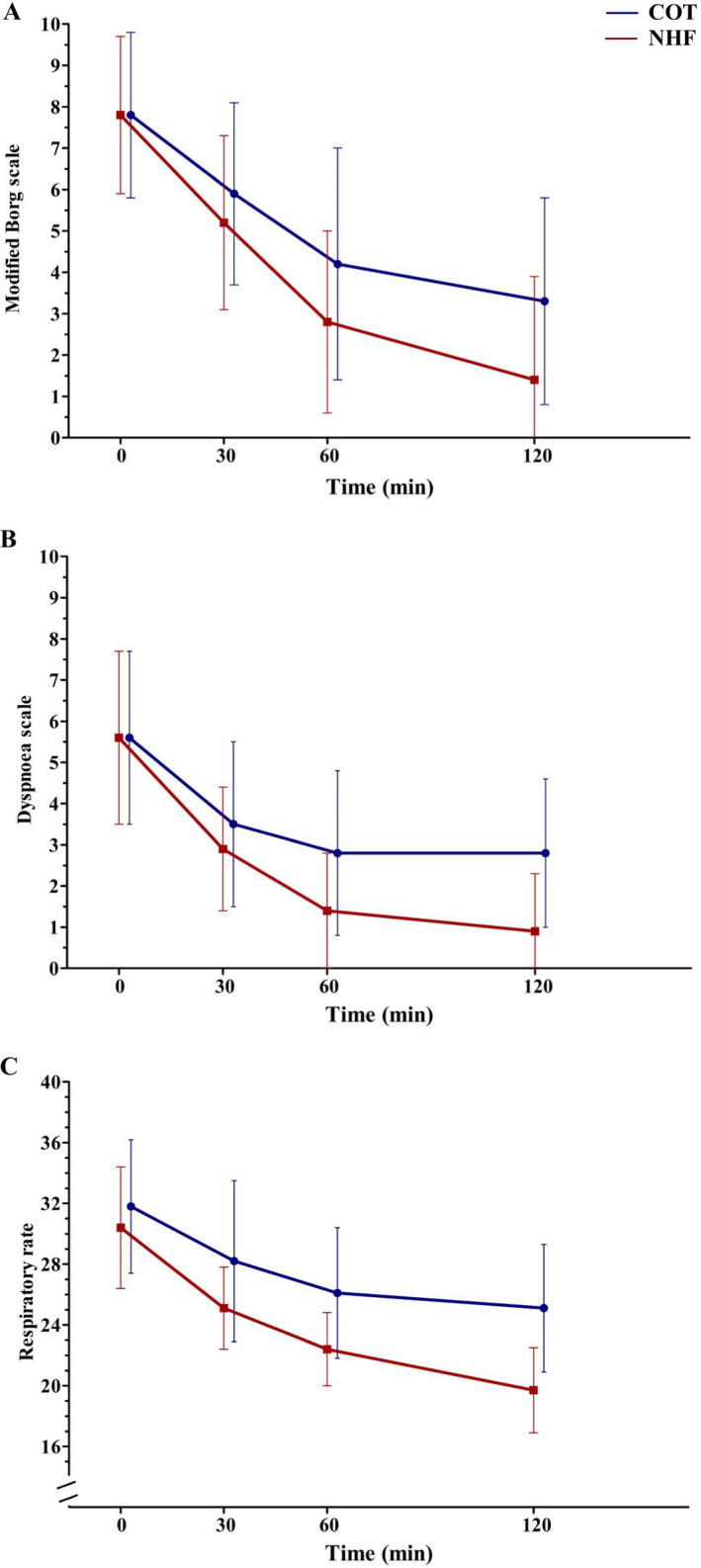
Changes in the primary and secondary outcomes over time. Note: respiratory rate in breaths/min. COT = conventional oxygen therapy; NHF = nasal high flow.
Secondary Outcomes
Similar results to the mean MBS were seen in the mean NRS. The dyspnea scale results also had a similar trend to both the patient‐reported dyspnea scale scores (Figure 2B). The comfort scale was significantly higher in patients treated with NHF compared with those treated with COT (mean ± SD = 9.1 ± 1.3 vs. 6.1 ± 1.6, mean difference = 3 [95% CI = 2 to 4], p < 0.0001). The mean (±SD) respiratory rate at the end of the study was also significantly decreased during NHF compared with COT (19.7 [±2.9] vs. 25.1 [±4.2], mean difference = 4.7 [95% CI = 1.5 to 7.8], p = 0.001). However, it was not significantly decreased at 30 and 60 minutes after initiating NHF treatment (Figure 2C). Moreover, no between‐group differences were found in the mean SpO2, heart rate, and mean arterial blood pressure (Table 2). Per‐protocol analyses of the secondary outcomes showed that NRS, respiratory rate, and dyspnea scale were also significantly decreased at 60 minutes (Data Supplement S1, Table S1).
Arterial blood gas was analyzed in 12 and 18 patients receiving COT and NHF, respectively. One patient in the COT group only had pretreatment gas analyzed but did not have posttreatment gas analyzed. Pretreatment results were comparable despite a significantly higher PaO2/FiO2 ratio in the COT group and slightly lower pH and higher PaCO2 levels in the NHF group. After the intervention, both unadjusted and adjusted gas results were not significantly different between patients treated with COT and NHF. Within‐group pre‐ and post‐treatment differences were also not seen in both groups (Table 3). Sensitivity analyses including only complete cases and excluding patients with chronic lung diseases (COT [n = 10], NHF [n = 17]) showed results similar to those of the primary analyses (Data Supplement S1, Table S2).
Table 3.
Arterial Blood Gas Results
| Before Intervention | After Intervention (at 120 Minutes) | Within‐group Difference‡ (95% CI); p‐value | ||||||
|---|---|---|---|---|---|---|---|---|
| COT (n = 12) | NHF (n = 18) | Difference* (95% CI); p‐value | COT (n = 11) | NHF (n = 18) | Difference† (95% CI); p‐value |
COT (n = 12) |
NHF (n = 18) |
|
| pH | 7.39 ± 0.07 | 7.37 ± 0.05 | 0.02 (–0.02 to 0.07); p = 0.35 | 7.40 ± 0.04 | 7.37 ± 0.05 | 0.03 (–0.002 to 0.05); p = 0.06 | –0.01 (–0.04 to 0.03); p = 0.62 | 0.002 (–0.02 to 0.02); p = 0.84 |
| pHadj | 7.39 (0.01) | 7.37 (0.01) | 0.02 (–0.01 to 0.05); p = 0.12 | |||||
| PaCO2 | 37.4 ± 13.4 | 39.4 ± 7.6 | –2.0 (–11.1 to 7.1); p = 0.65 | 33.3 ± 7.4 | 37.9 ± 8.7 | –4.6 (–11.2 to 1.9); p = 0.16 | 2.8 (–2.7 to 8.3); p = 0.29 | 1.5 (–0.5 to 3.5); p = 0.13 |
| PaCO2adj | 34.6 (1.5) | 37.7 (1.2) | –2.4 (–6.3 to 1.5); p = 0.22 | |||||
| PaO2 | 161.4 ± 72.4 | 134.0 ± 48.4 | 27.4 (–17.6 to 72.4); p = 0.22 | 139.4 ± 48.9 | 119.2 ± 37.0 | 20.2 (–12.6 to 53.0); p = 0.22 | 31.8 (–5.8 to 69.4); p = 0.09 | 14.8 (–6.1 to 35.8); p = 0.11 |
| PaO2 adj | 129.8 (11.0) | 125.0 (8.5) | 4.7 (–24.6 to 34.0); p = 0.74 | |||||
| P/F ratio | 334.5 ± 67.3 | 272.2 ± 59.3 | 62.3 (14.5 to 110.0); p = 0.01 | 337.3 ± 162.2 | 306.9 ± 108.9 | 30.4 (–72.6 to 133.4); p = 0.55 | 4.3 (–93.0 to 101.5); p = 0.92 | –34.7 (–90.3 to 20.9); p = 0.21 |
| P/F ratioadj | 306.9 (41.6) | 325.4 (31.5) | –18.4 (–132.3 to 95.4); p = 0.63 | |||||
| HCO3 | 24.1 ± 4.3 | 24.4 ± 6.0 | –0.2 (–4.4 to 3.9); p = 0.91 | 22.4 ± 4.0 | 21.9 ± 4.2 | 0.4 (–2.8 to 3.7); p = 0.78 | 1.3 (–0.02 to 2.6); p = 0.05 | 2.5 (–0.6 to 5.5); p = 0.11 |
| HCO3adj | 22.5 (1.1) | 21.8 (0.9) | 0.7 (–2.2 to 3.6); p = 0.63 | |||||
Data are presented as mean ± SD or adjusted mean (SE).
COT = conventional oxygen therapy; HCO3 = bicarbonate; NHF = nasal high flow; PaCO2 = arterial partial pressure of carbon dioxide, PaO2 = arterial partial pressure of oxygen; P/F = PaO2/fraction of inspired oxygen.
Independent‐sample t‐test.
Independent‐sample t‐test and analysis of covariance adjusted for baseline values of that outcome variable.
Before–after paired‐sample t‐test.
There were no complications in the NHF group. Patients tolerated the equipment well with a median duration of 3.8 hours on the device. Moreover, slightly more patients receiving NHF were able to be discharged from the ED with shorter ED and hospital length of stay although these did not reach significance (Table 4). No patients died at hospital discharge.
Table 4.
Cointerventions and Disposition of Participants
| Variables |
COT (n = 18) |
NHF (n = 19) |
p‐value |
|---|---|---|---|
| Total dosage of bronchodilators used in the ED | 6 (5–9) | 8 (7–9) | 0.08 |
| Co‐intervention | |||
| Magnesium sulfate | 9 (50.0) | 11 (57.9) | 0.41 |
| Penicillin or cephalosporins | 3 (16.7) | 2 (10.5) | 0.59 |
| Macrolide | 8 (44.4) | 6 (31.6) | 0.25 |
| Oseltamivir | 7 (38.9) | 6 (31.6) | 0.64 |
| Intubation in 24 hours | 1 (5.6) | 1 (5.6) | 0.97 |
| ED disposition | |||
| Discharge | 6 (33.3) | 8 (42.1) | 0.35 |
| Admit to general ward | 4 (22.2) | 4 (21.1) | |
| Admit to respiratory intensive care unit | — | 2 (10.5) | |
| Observation unit | 6 (33.3) | 2 (10.5) | |
| Refer | 2 (11.1) | 3 (15.8) | |
| ED length of stay (hour) | 6.8 (4.9–11.6) | 4.4 (3.2–10.1) | 0.26 |
| Hospital length of stay (hour) | 12.0 (4.8–69.6) | 9.6 (4.5–67.2) | 0.87 |
Data are presented as n (%) or median (range).
COT = conventional oxygen therapy; NHF = nasal high flow.
DISCUSSION
In this pilot randomized controlled trial of patients with acute severe asthma and hypoxemia, NHF could significantly decrease the degree of dyspnea compared with COT assessed primarily by MBS along with NRS, dyspnea scale, and respiratory rate at 120 minutes after initiating treatment. However, there was no evidence of differences in pH and PaCO2 levels from arterial blood gas after using the two interventions.
Nasal high‐flow therapy is a relatively new form of respiratory support. 7 , 8 , 9 , 10 Although analyzed in patients with acute hypoxemic respiratory failure of various etiologies and acute hypercapnic respiratory failure primarily due to COPD, a limited number of studies have validated the benefit of NHF treatment in adult patients with acute severe asthma. Retrospective observational studies in pediatric patients with severe acute asthma exacerbation have reported an improvement in physiologic variables and blood gas results using NHF 23 and a decrease in dyspnea evaluated by pulmonary score, which is comparable with NIV. 24 One pilot randomized controlled trial in children visiting the ED with asthma exacerbation found that NHF could decrease the pulmonary score better than standard oxygen. 22 Initially the trial investigators hypothesized that, from a physiologic perspective, NHF may also be a favorable treatment measure for adult patients with acute severe asthma. A recent pilot randomized trial comparing NHF with COT in adult patients with asthma complicated with respiratory failure, which was published after the present trial was commenced and completed, reported similar clinical improvement and effectiveness between the two interventions. However, it was not conducted exclusively in an ED setting and may have represented patients with different characteristics. 32 Thus, this study is only the first randomized controlled trial of NHF versus COT in patients with acute severe asthma and hypoxemia in the ED.
The primary outcome of the present study was similar to a previous randomized trial in adult asthma patients, which demonstrated an improvement in the MBS score of 3 to 4 points at 60 minutes postintervention with NIV. 27 In this study, NHF could also decrease the MBS by about 5 points after 60 minutes of treatment. This more rapid improvement could have been because the participants in this study were more severe with a higher baseline MBS. In addition, outcomes of mean scores for the NRS and MBS were similar, which suggested favorable convergent validity of the patient‐reported dyspnea ratings. Results of the dyspnea scale assessed by the trial investigators were also in agreement with patient‐reported outcomes. The comfort scale after using NHF was also significantly higher, which is concordant with the results from previous studies. 12 , 14 , 16 The results of this study therefore support the hypothesis that NHF can relieve dyspnea and improve subjective comfort of adult patients with acute severe asthma.
In this study, the respiratory rate was significantly improved in participants receiving NHF therapy compared with COT. These findings were similar to those of a previous trial by Gupta et al. 33 that studied the use of NIV in acute asthma patients of the same level of severity to this study as well as a trial that included patients with a milder degree of exacerbation of this condition. 34 This study therefore supports the hypothesis that NHF can decrease the degree of dyspnea in acute severe asthma patients. Also, beneficial effects of NHF are seen early, which is similar to results in previous studies in ED patients with other conditions. 15 , 16 , 17 Consequently it is suggested that NHF is a highly favorable therapy to use within emergency settings.
However, the trial investigators did not detect an improvement in blood gas results, which was similar to results of Gupta et al., who reported comparable initial pH and PaCO2 levels. 33 Unlike in adult patients, there was an increase in pH and a decrease in PaCO2 in pediatric patients, but most predominantly in those with acute respiratory acidosis at the initial analysis. 23 A pilot study in adult patients with baseline PaCO2 of approximately 50 mm Hg also reported significant improvement in PaCO2. 32 There may have been multiple reasons for the insignificant improvement in blood gas results in this study. First, there were insufficient data and the results may have been disproportionate. Second, the initial mean pH and PaCO2 levels of the patients in this study were mainly within the normal ranges similar to those in the study by Gupta et al.; therefore, it may not have been possible to detect significant CO2 clearance, unlike in other hypercapnic conditions. Finally, patients with acute severe asthma and acute respiratory acidosis may be too severe to be treated using noninvasive airway equipment and need mechanical ventilation. There were three patients in this study with initial PaCO2 over 50 mm Hg; one had associated allergic bronchopulmonary aspergillosis and chronic respiratory acidosis while the other two were intubated early after initiation of the trial.
In addition, the mean age of participants in this study was much higher than that in previous studies. 27 , 33 , 34 This may have been because of the hospital setting. Siriraj Hospital is one of the largest tertiary university hospitals in Thailand, situated at the city center of Bangkok. Thus, this hospital usually provides care for patients with advanced and complicated diseases who are referred from other hospitals. Younger patients with pure asthma may have been treated at smaller or rural hospitals in Thailand, so they tend not to present at our institution. With advancing age, patients’ pulmonary function might have partially progressed to irreversible obstructive pattern and might have not represented the population with pure asthma. Nevertheless, our study results are still very promising even for these patients with presumed higher severity of disease.
LIMITATIONS
There were several limitations of this pilot study. First, the trial was a single‐center pilot study with a small sample size. The study may have lacked the power to make any conclusions regarding the treatment effect, especially for blood gas analysis which was not undertaken in some of the study participants. Early termination of the trial may have also resulted in a larger between‐group differences than might have been observed had the study continue to completion. Secondly, the participants in this current study were relatively older than adult patients with asthma in previous studies, 27 , 33 , 34 which may have been because of the tertiary setting of the hospital. With advancing age, patients’ pulmonary function might have partially progressed to irreversible obstructive pattern and might have not represented the population with pure asthma. Third, we initially aimed to compare the efficacy of oxygen therapy in acute severe asthma. Therefore, we had to exclude a significant number of patients because they did not have hypoxia, thereby not requiring oxygen therapy. Consequently, only patients with moderate to severe symptoms participated, which was the same as in Gupta et al. This could limit the generalizability of the study findings. Further trials may include these nonhypoxemic patients since hypoxemia is not a predominant problem in acute severe asthma and the beneficial effects of heated and humidified air can still be delivered. Fourth, the trial investigators did not detect change in management, intubation, and mortality rate. Moreover, we did not use lung function as an outcome measure. This was because the primary outcome focused on each patient’s subjective level of dyspnea relief. Although the severity of asthma exacerbation using both dyspnea scale and other physiologic parameters was assessed, more reliable objective parameters such as lung function tests should be included. Further randomized controlled trials with larger sample sizes, longer duration of NHF examining other long‐term objective outcomes, and spirometry findings should be conducted. Furthermore, the lack of any blinding to the devices used may have affected the outcomes measured.
CONCLUSION
This study showed that nasal high‐flow therapy may decrease the degree of dyspnea more than the use of conventional oxygen therapy in patients with acute severe asthma and hypoxemia in the ED setting. This was demonstrated by the significant improvements in the mean modified Borg scale, mean numeric rating scale, mean dyspnea scale score, and the mean respiratory rate. Therefore, nasal high flow may be beneficial to respiratory support and oxygenation in patients with acute severe asthma and hypoxemia in the ED.
Supporting information
Data Supplement S1. Supplemental material.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Chulaluk Komoltri, PhD, for assistance with the statistical analysis.
Fisher & Paykel Healthcare provided the NHF devices, which were Optiflow cannula and AIRVOTM 2 machines. The company played no role in the trial design, data collection, data analysis, or manuscript preparation.
The authors have no relevant financial information or potential conflicts to disclose.
Author contribution: OR conceived the study, designed the trial, and supervised the conduct of the trial and data collection; OR, CL, NP, AP, and TC recruited the patients and managed the data; OR analyzed the data; OR drafted the article; and OR takes responsibility for the paper as a whole.
REFERENCES
- 1. Global Initiative for Asthma . 2019 GINA Report, Global Strategy for Asthma Management and Prevention. Available at: https://ginasthma.org/gina‐reports/. Accessed Apr 4, 2020.
- 2. Vichyanond P, Jirapongsananuruk O, Visitsuntorn N, Tuchinda M. Prevalence of asthma, rhinitis and eczema in children from the Bangkok area using the International Study for Asthma and Allergy in Children (ISAAC) questionnaires. J Med Assoc Thai 1998;81:175–84. [PubMed] [Google Scholar]
- 3. Teeratakulpisarn J, Pairojkul S, Heng S. Survey of the prevalence of asthma, allergic rhinitis and eczema in schoolchildren from Khon Kaen, north‐east Thailand, an ISAAC study. Asian Pac J Allergy Immunol 2000;18:187–94. [PubMed] [Google Scholar]
- 4. Boonsawat W, Charoenphan P, Kiatboonsri S, et al. Survey of asthma control in Thailand. Respirology 2004;9:373–8. [DOI] [PubMed] [Google Scholar]
- 5. Boonsawat W, Thompson PJ, Zaeoui U, et al. Survey of asthma management in Thailand – the asthma insight and management study. Asian Pac J Allergy Immunol 2015;33:14–20. [DOI] [PubMed] [Google Scholar]
- 6. Lim WJ, Mohammed Akram R, Carson KV, et al. Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2012;12:CD004360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kernick J, Magarey J. What is the evidence for the use of high‐flow nasal cannula oxygen in adult participants admitted to critical care units? A systematic review. Aust Crit Care 2010;23:53–70. [DOI] [PubMed] [Google Scholar]
- 8. Roca O, Riera J, Torres F, Masclans JR. High‐flow oxygen therapy in acute respiratory failure. Respir Care 2010; 55:408–13. [PubMed] [Google Scholar]
- 9. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high‐flow therapy: mechanisms of action. Respir Med 2009;103:1400–5. [DOI] [PubMed] [Google Scholar]
- 10. Gotera C, Díaz S, Lobato PT, Winck JC. Clinical evidence on high flow oxygen therapy and active humidification in adults. Rev Port Pneumol 2013;19:217–27. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura M. High‐flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care 2016;61:529–41. [DOI] [PubMed] [Google Scholar]
- 12. Roca O, Hernández G, Díaz‐Lobato S, Carratala JM, Gutierrez RM, Masclans JR. Current evidence for the effectiveness of heated and humidified high‐flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care 2016;20:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frat JP, Thille AW, Mercat A, et al. High‐flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185–96. [DOI] [PubMed] [Google Scholar]
- 14. Helviz Y, Einav S. A systematic review of the high‐flow nasal cannula for adult patients. Crit Care 2018;22:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rittayamai N, Tscheikuna J, Praphruetkit N, Kijpinyochai S. Use of high‐flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care 2015;60:1377–82. [DOI] [PubMed] [Google Scholar]
- 16. Makdee O, Monsomboon A, Surabenjawong U, et al. High‐flow nasal cannula versus conventional oxygen therapy in emergency department patients with cardiogenic pulmonary edema: a randomized controlled trial. Ann Emerg Med 2017;70:465–72. [DOI] [PubMed] [Google Scholar]
- 17. Ruangsomboon O, Dorongthom T, Chakorn T, et al. High‐flow nasal cannula versus conventional oxygen therapy in relieving dyspnea in emergency palliative patients with do‐not‐intubate status: a randomized cross‐over study. Ann Emerg Med 2019;75:612–26. [DOI] [PubMed] [Google Scholar]
- 18. Lee HW, Choi SM, Lee J, et al. Reduction of PaCO2 by high‐flow nasal cannula in acute hypercapnic respiratory failure patients receiving conventional oxygen therapy. Acute Crit Care 2019;34:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pisani L, Betti S, Biglia C, et al. Effects of high‐flow nasal cannula in patients with persistent hypercapnia after an acute COPD exacerbation: a prospective pilot study. BMC Pulm Med 2020;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rittayamai N, Phuangchoei P, Tscheikuna J, Praphruetkit N, Brochard L. Effects of high‐flow nasal cannula and non‐invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: a preliminary study. Ann Intensive Care 2019;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berk JL, Lenner KA, McFadden ER Jr. Cold‐induced bronchoconstriction: role of cutaneous reflexes vs. direct airway effects. J Appl Physiol 1987;63:659–64. [DOI] [PubMed] [Google Scholar]
- 22. Ballestero Y, De Pedro J, Portillo N, Martinez‐Mugica O, Arana‐Arri E, Benito J. Pilot clinical trial of high‐flow oxygen therapy in children with asthma in the emergency service. J Pediatr 2018;194:204–10. [DOI] [PubMed] [Google Scholar]
- 23. Baudin F, Buisson A, Vanel B, Massenavette B, Pouyau R, Javouhey E. Nasal high flow in management of children with status asthmaticus: a retrospective observational study. Ann Intensive Care 2017;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. David MM, Gomes EL, Cavassin CL, Luiz JG, Andrade MC, Costa D. Clinical impact of the high‐flow nasal cannula in the treatment of asthma crisis in children and adolescents: a pilot study. Eur Respir J 2019;54(Suppl 63):PA1228. [Google Scholar]
- 25. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. [PubMed] [Google Scholar]
- 26. Moy ML, Lantin ML, Harver A, Schwartzstein RM. Language of dyspnea in assessment of patients with acute asthma treated with nebulized albuterol. Am J Respir Crit Care Med 1998;158:749–53. [DOI] [PubMed] [Google Scholar]
- 27. Soma T, Hino M, Kida K, Kudoh S. A prospective and randomized study for improvement of acute asthma by non‐invasive positive pressure ventilation (NPPV). Intern Med 2008;47:493–501. [DOI] [PubMed] [Google Scholar]
- 28. Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care 1998;7:200–4. [PubMed] [Google Scholar]
- 29. Bell ML, Whitehead AL, Julious SA. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clin Epidemiol 2018;18:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65. [DOI] [PubMed] [Google Scholar]
- 31. Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117:549–56. [DOI] [PubMed] [Google Scholar]
- 32. Geng W, Batu W, You S, Tong Z, He H. High‐flow nasal cannula: a promising oxygen therapy for patients with severe bronchial asthma complicated with respiratory failure. Can Respir J 2020;2020:2301712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care 2010;55:536–43. [PubMed] [Google Scholar]
- 34. Brandão DC, Lima VM, Filho VG, et al. Reversal of bronchial obstruction with bi‐level positive airway pressure and nebulization in patients with acute asthma. J Asthma 2009;46:356–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Supplemental material.


