Abstract
Aims
SAR247799 is a G‐protein‐biased sphingosine‐1 phosphate receptor‐1 (S1P1) agonist designed to activate endothelial S1P1 and provide endothelial‐protective properties, while limiting S1P1 desensitization and consequent lymphocyte‐count reduction associated with higher doses. The aim was to show whether S1P1 activation can promote endothelial effects in patients and, if so, select SAR247799 doses for further clinical investigation.
Methods
Type‐2 diabetes patients, enriched for endothelial dysfunction (flow‐mediated dilation, FMD <7%; n = 54), were randomized, in 2 sequential cohorts, to 28‐day once‐daily treatment with SAR247799 (1 or 5 mg in ascending cohorts), placebo or 50 mg sildenafil (positive control) in a 5:2:2 ratio per cohort. Endothelial function was assessed by brachial artery FMD. Renal function, biomarkers and lymphocytes were measured following 5‐week SAR247799 treatment (3 doses) to Zucker diabetic fatty rats and the data used to select the doses for human testing.
Results
The maximum FMD change from baseline vs placebo for all treatments was reached on day 35; mean differences vs placebo were 0.60% (95% confidence interval [CI] −0.34 to 1.53%; P = .203) for 1 mg SAR247799, 1.07% (95% CI 0.13 to 2.01%; P = .026) for 5 mg SAR247799 and 0.88% (95% CI −0.15 to 1.91%; P = .093) for 50 mg sildenafil. Both doses of SAR247799 were well tolerated, did not affect blood pressure, and were associated with minimal‐to‐no lymphocyte reduction and small‐to‐moderate heart rate decrease.
Conclusion
These data provide the first human evidence suggesting endothelial‐protective properties of S1P1 activation, with SAR247799 being as effective as the clinical benchmark, sildenafil. Further clinical testing of SAR247799, at sub‐lymphocyte‐reducing doses (≤5 mg), is warranted in vascular diseases associated with endothelial dysfunction.
Keywords: diabetes, endothelium, flow‐mediated dilation, SAR247799, sphingosine‐1 phosphate receptor‐1
1. What is already known about this subject
The potential endothelial pharmacology associated with sphingosine‐1 phosphate receptor‐1 (S1P1) activation has not been assessed in patients because previous molecules developed for multiple sclerosis are S1P1‐desensitizing and have endothelial‐damaging side‐effects.
SAR247799, a first‐in‐class S1P1 agonist that activates S1P1 without desensitization, protects endothelium in preclinical models without reducing lymphocytes.
What this study adds
The results characterize the first human pharmacological effect of S1P1 activation on the endothelium.
Repeated administration of SAR247799 improved endothelial function (flow‐mediated dilation) in type‐2 diabetes patients without reducing lymphocytes or blood pressure.
SAR247799 is a novel endothelial approach that warrants evaluation in populations with more advanced vascular disease.
1. INTRODUCTION
Endothelial dysfunction is believed to contribute to the progression of a variety of vascular diseases. 1 It manifests as impaired reactivity of the micro‐ and/or macro‐vasculature to pharmacological or physiological stimuli, as disruption of barrier integrity causing vascular leakage, and as changes in the levels of plasma and/or urinary biomarkers. 2 These changes are considered to play a major role in the development of atherosclerosis, acute coronary syndromes, stroke and renal damage, and are frequent complications in diabetes. 3 Diabetes is a predisposing factor for worsening cardiovascular outcomes, and indices of endothelial dysfunction can predict such risk. 4
Sphingosine‐1 phosphate (S1P) is a sphingolipid mediator present at high levels in the plasma of healthy individuals, where it is believed to play an important role in maintaining vascular health. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Endothelium and erythrocytes contribute, primarily, to the plasma pool of S1P, and the apoM‐component of high‐density lipoprotein (HDL) is the major carrier in plasma. 5 , 13 Not surprisingly, S1P and HDL‐bound S1P are reduced in pathologies or conditions associated with endothelial injury, including diabetes, 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 metabolic syndrome, 22 myocardial infarction, 20 , 21 sepsis, 23 , 24 in‐stent restenosis, 25 and in coronary and peripheral artery disease. 26 In patients with cardiovascular disease, higher S1P is associated with cardio‐protective settings such as preinfarction angina 27 and coronary collateral circulation. 28
Although a large body of genetic and epidemiological evidence implicates HDL as a cardioprotective agent, multiple outcome trials with HDL‐elevating drugs have provided disappointing results. 29 , 30 , 31 It has been proposed that this apparent discrepancy can be explained by the quality (or functional capacity) of HDL particles being the critical factor affecting cardiovascular outcomes rather than the absolute HDL level. 29 , 32 In this regard, the endothelial‐protective properties of HDL are mediated by its S1P cargo, acting via S1P receptor‐1 (S1P1). 33 , 34 , 35 , 36 , 37 Interestingly, oestrogen, another component of HDL, considered to be cardio‐protective, has endothelial‐protective properties mediated through S1P1 signalling. 38 , 39 , 40 Dysfunctional HDL from atherosclerosis and/or diabetes patients has reduced S1P, 26 and the endothelial barrier‐promoting properties of dysfunctional HDL can be restored by S1P1 activation. 41 In preclinical models, S1P1 activation maintains endothelial function whereas S1P1 inactivation causes endothelial damage. 42 , 43 Lymphocyte reduction is a clinically validated marker of S1P1 desensitization, because in patients all S1P1 agonists tested to date were designed as S1P1‐desensitizing agents to reduce peripheral blood lymphocytes and provide therapeutic benefit in MS. 44 , 45 Consequently, the effect of selective S1P1 activation, without desensitization, on endothelial function in patients has not been studied to date. Rather, S1P1‐desensitizing molecules, at doses characterized by marked S1P1 desensitization, demonstrate evidence of endothelial damage based on the incidence of macular oedema, renal dysfunction and respiratory dysfunction in various patient populations. 44 , 45 , 46 , 47 , 48 , 49
SAR247799 is a G‐protein‐biased S1P1 agonist (inhibiting adenylate cyclase preferentially over activation of β‐arrestin and internalization pathways) with endothelial‐protective properties in rats and pigs at doses that do not desensitize S1P1, namely non‐lymphocyte‐reducing doses. 50 , 51 In healthy subjects, SAR247799 has an attractive safety, tolerability and pharmacokinetics (PK) profile and the dose–response relationship for lymphocyte pharmacodynamics (PD) has been characterized. 52 Although preclinical studies with SAR247799 showed protective effects in the renal and coronary vasculature in a preventative setting using single doses administered prior to an insult of ischaemia/reperfusion‐induced endothelial injury, the effect of chronic administration of SAR247799 on the endothelium is currently unknown. Furthermore, as previous molecules were S1P1−desensitizing, the effect of S1P1 activation on endothelial properties in patients has not yet been studied. In preclinical models, SAR247799 is a biphasic molecule that can activate S1P1 with endothelial‐protective properties at low doses, while causing lymphocyte reduction, like other S1P1‐desensitizing molecules, at higher doses. 50 , 51 Biological activities often follow the 4‐parameter logistic model in which Emax is related to dose (i.e. the bigger the dose, the larger the effect), 53 and with this assumption, many proof‐of‐concept trials test a single dose, at‐or‐close‐to the maximum tolerated dose. However, given that S1P1 desensitization has been associated with endothelial‐damaging effects, a U‐shaped dose response of SAR247799 for endothelial‐protective effects was considered pharmacologically plausible. Therefore, to reduce the likelihood of missing the clinical efficacy of the compound in patients, there is a need to understand the dose–response relationship of SAR247799 for endothelial‐protective effects, under repeated‐dosing conditions, and put this in the context of the level of S1P1 desensitization.
The aim of the current study was to determine whether 4‐week therapy with SAR247799 improves reactivity of the dysfunction endothelium in type‐2 diabetes patients using flow‐mediated dilation (FMD). FMD is a technique that is regarded as a gold standard, 54 predicts cardiovascular events 55 and can reveal pharmacological improvements in diabetes patients. 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 We also compared the level of response to sildenafil; a positive control with established efficacy on FMD in this population. 56 , 58 , 59 As SAR247799 is a biphasic molecule, we payed close attention to choosing doses that would be S1P1‐activating, while limiting S1P1‐desensitization, and performed a 5‐week repeated‐dosing study in a rat model of type‐2 diabetes to facilitate the selection of appropriate doses for human testing. Zucker diabetic fatty (ZDF) rats 65 have a defect in the leptin receptor gene and were used because they closely match the pathological characteristics of type‐2 diabetes mellitus including hyperglycaemia, insulin resistance, obesity and dyslipidaemia. 66 We first used the rat model to address whether chronic administration of SAR247799 has endothelial‐protective properties and the level of S1P1 desensitization associated with such effects. The concentration–effect relationships of SAR247799 in the rat model were used to guide selection of doses for evaluation on endothelial function in type‐2 diabetes patients.
2. METHODS
2.1. Animal studies
All animal studies were performed in accordance with the European Community standard on the care and use of laboratory animals and approved by the IACUC of Sanofi R&D.
2.1.1. Animals
Five‐week‐old (~125–150 g) male ZDF Fa/Fa rats and Zucker lean Fa/+ rats (Lean; Charles River, France) were housed in groups of 2 in 1500 UTemp cages (Tecniplast, France) in 29/12 Plus sawdust (Souralit) enriched with aspen bricks and play tunnels (Datesand). They were kept in a climate‐controlled environment (21–22°C, 55% humidity) with a 12:12‐hour light–dark cycle and provided with diet and water (0.2‐μm filtered tap water) ad libitum throughout the duration of the study. Control diet A04 (SAFE) was used from the arrival of the animals up to age 9 weeks.
2.1.2. Experimental design
At age 9 weeks, ZDF rats were randomized by body weight to a chow formulation: control chow (SSNIFF Spezialdiäten GmbH) or chow formulated with 1 of 3 dose strengths of SAR247799: low, intermediate and high corresponding to 0.002, 0.007 and 0.0245% (w/w), respectively. Twelve ZDF rats were included in each of the control, low and high dose groups. A slightly higher number of ZDF rats (14) were included in the intermediate dose group, as it was predicted to be the most effective dose. Eight lean rats, fed with control chow, were included in the study to confirm the pathological status of ZDF rats (and the lower number was based on the principle of reduction of 3Rs). All analyses were performed by a blinded experimenter.
2.1.3. Urinary evaluation
Eighteen‐hour urine samples were collected at baseline and after 5 weeks of treatment using metabolic cages (Tecniplast, France). Urinary protein, creatinine, chloride and sodium concentration were measured by ABX PENTRA 400 (Horiba). Creatinine clearance was calculated as urinary creatinine X urine volume/plasma creatinine and expressed as microliters per minute.
2.1.4. Plasma assessments
Blood samples were collected in K2‐EDTA tubes from the caudal vein under 1.5–2% isoflurane anaesthesia at baseline and after 5 weeks of treatment. Blood lymphocyte count was measured by MS9–5 (Melet Schloesing) in freshly collected blood, then blood samples were immediately centrifuged at 4°C/10 min/10 000 g and the plasma was stored at −80°C. Plasma creatinine and fructosamine were measured by ABX PENTRA 400 (Horiba). SAR247799 concentrations were measured from 5‐week plasma samples using liquid chromatography tandem mass spectrometry.
Plasma biomarkers were measured by ELISA following 5 weeks of treatment according to manufacturers' instructions. Soluble ICAM‐1, sE‐selectin and von Willebrand Factor (vWF) were measured using MILLIPLEX MAP Rat Vascular Injury Magnetic Bead Panel 2 (Merck), sVCAM‐1 from VCAM‐1 Rat ProcartaPlex Simplex Kit (ThermoFisher Scientific), CRP from CRP Rat ProcartaPlex Simplex Kit (ThermoFisher Scientific) and sPECAM‐1 from Rat PECAM‐1 Elisa kit (MyoBioSource). Nitrate/nitrite concentration was measured by nitrate/nitrite fluorometric assay kit (Cayman Chemical) in plasma samples previously filtered with Microcon‐10 kDa Centrifugal Filter Unit with Ultracel‐10 membrane (Merck).
2.1.5. Heart rate
Heart rate was measured at the end of the study under anaesthesia (1.5–2% isoflurane) after all other assessments were completed. Heart rate was measured from consecutive R‐R intervals in the electrocardiogram (ECG) signals recorded from suitable electrodes connected to Vivid E9 (GE).
2.1.6. Statistical analysis
SAS version 9.2 for Windows 7 was used for statistical analysis. Single comparison was performed by 2‐tailed unpaired Student t‐test or Wilcoxon test, when normality was not met. Multiple comparisons were analysed by 1‐way ANOVA, following by Dunnett's posthoc test in normally distributed and homogeneous parameters, otherwise a Kruskal–Wallis test followed by Wilcoxon 2‐tailed comparison test was performed. A P‐value of ≤.05 was considered significant. Data are expressed as mean ± standard error of the mean for normally distributed data, otherwise as median ± interquartile range.
2.2. Clinical study
The clinical study was conducted as a multicentre study by Profil (2 centres in Germany: Profil Institut für Stoffwechselforschung GmbH, Neuss and Profil Mainz GmbH & Co. KG, Mainz) and was approved by the competent authority as well as 2 independent ethics committees (Local Ethical Committee of Düsseldorf and Local Ethical Committee of Mainz, Germany). The study was performed in accordance with the clinical trial protocol and with ethical principles having their origin in the Declaration of Helsinki. All patients provided written informed consent before participating. The trial was prospectively registered with Unique identifier NCT0346201 on 12/03/2018 (www.clinicaltrials.gov). The study dosing scheme is summarized in Figure 3A.
FIGURE 3.
Design and patient (pt) progress through randomized, double‐blinded clinical trial to evaluate SAR247799 on flow‐mediated dilation in type‐2 diabetes patients. (A) Study design. (B) Flow chart of patient progress through trial. of the 122 patients not meeting inclusion criteria, 14 were because flow‐mediated dilation (FMD) was >7%. # 1 patient excluded for unusable FMD data and 1 patient withdrawn. DSM, dose selection meeting; PK, pharmacokinetics; B, baseline; EOS, end of study; BAD, brachial artery diameter; HR, heart rate; BP, blood pressure; PK, pharmacokinetics
2.2.1. Patients
The study population included male and female type‐2 diabetes patients, with >6 months from diagnosis of diabetes at the time of screening, and otherwise healthy as assessed by clinical and laboratory assessments and detailed medical history. Main inclusion criteria included age 18–64, body mass index 18–35 kg/m2, haemoglobin A1c <8.5%, flow‐mediated dilation ≤7%, estimated glomerular filtration rate >60 mL/min/1.73 m2 and vital signs after 10 minutes rest in supine position in the following ranges: systolic blood pressure 95–160 mmHg, diastolic blood pressure 45–100 mmHg, heart rate 60–100 beats/min. In addition, clinical laboratory evaluations and ECG recording were to be normal or without any clinically relevant findings.
Patients with reported pathologies (apart from stable type‐2 diabetes mellitus status), which, as judged by the investigator, may affect the patient's health, participation in, or the outcome of the study, were excluded. Particularly, any history of clinically relevant cardiovascular disease (including established atherosclerosis or noncontrolled hypertension) or pulmonary, renal, hepatic, ophthalmological or infectious diseases led to exclusion. A few comorbidities such as controlled dyslipidaemia, controlled hypertension, stable and treated hypothyroidism were accepted.
Any medication which had a potential to interfere with safety, PK or PD of SAR247799 and sildenafil or study measurements was prohibited; this included nitrates, all calcium channel blockers, phosphodiesterase type 5 (PDE5) inhibitors, guanylate cyclase stimulators, β‐blockers, glucagon‐like peptide‐1 agonists, insulins (all types), drugs that decrease heart rate, anticoagulants and antithrombotics (except aspirin), antiarhythmics (such as digoxin), recent (≤3 months) use of systemic immunosuppressive or corticosteroid therapy, any inactivated vaccination during study treatment, any attenuated vaccination within 2 months before inclusion, and any biologics (antibody or its derivatives) given within 4 months before inclusion. The following medications were authorized if patients were on stable therapy: oral antidiabetics other than glucagon‐like peptide‐1 agonists; angiotensin converting enzyme inhibitors; angiotensin II receptor blockers; statins; aspirin as antithrombotic therapy; diuretics; hormonal contraception; menopausal hormone replacement therapy; and thyroid hormone replacement therapy. Only patients smoking fewer than 5 cigarettes or equivalent per week were enrolled. Smoking and tobacco were not allowed during institutionalization periods.
A patient could be withdrawn from the study at any time for any of the following reasons: refusal to comply with the parameters of the study; desire to withdraw; medical condition that the investigator considered to contraindicate the continuation of the study; or the occurrence of an adverse event (AE).
The above patient characteristics for the trial were modelled from previously performed FMD studies. 46 , 47 , 48 , 49 , 50 , 51
2.2.2. Clinical study design
The selected design was a double‐blinded, double‐dummy, randomized, placebo and active‐controlled study. Such design was selected to allow comparison of SAR247799 to placebo as well as the positive control, sildenafil, which has been shown to improve FMD in a variety of populations including patients with type‐2 diabetes. 46 , 48 , 49 The study was performed with 27 patients per cohort (6 placebo, 6 sildenafil and 15 on a given dose of SAR247799) who received the treatments for 28 days in a once‐a‐day regimen. Two cohorts with escalating doses of SAR247799 (1 and 5 mg) were conducted and the protocol had the option for a third cohort, which was not performed. Capsules, blisters and boxes of investigational medicinal product (IMP; SAR247799, sildenafil [50 mg] or placebo) were indistinguishable to preserve the double‐blind.
A decision to proceed from 1 dose to the next was made jointly by the sponsor and the investigator on the basis of reviewing the preliminary safety and PK exposure data of the previous dose.
The study comprised of a screening period of up to 26 days during which an FMD threshold of <7% was used to enrich for patients with dysfunctional endothelium. SAR247799, sildenafil and placebo capsules were taken under fasted conditions each morning from day 1 to day 28 (inclusive) and there was a 14‐day follow‐up period up to the end of study visit on day 42. There was a 4‐day in‐house period (day −2 to day 3) followed by ambulatory conditions with various in‐house periods for FMD assessments (day 13–14, day 20–21, day 27–28, day 34–35 and day 41–42).
Patients were randomized on day 1 to receive SAR247799, placebo or sildenafil in 5:2:2 ratio if they met all inclusion/exclusion criteria (at day −1 and/or day −2). Sanofi controlled the double‐blind key for the study. The IMP was administered under medical supervision during in‐house periods and patients were instructed to take the IMP under fasted conditions each morning during ambulatory periods for which compliance was checked by counting the number of returned capsules at the end of the study.
2.2.3. Endothelial function assessments by flow‐mediated dilation
Endothelial function was assessed by measuring FMD of the brachial artery following reactive hyperaemia. FMD examinations were performed exclusively by research physicians (4 at the Mainz site and 2 at the Neuss site) and were analysed centrally by 3 qualified FMD analysts. As the FMD methodology is known to be operator dependent, both the research physicians and analysts had to perform qualification training prior to the start of the clinical trial and achieve the following targets: each physician performed 25–30 FMD examinations twice in 1 subject and also evaluated the data to be certified as expert by an external expert; 20 of these assessments had to have a standard deviation (SD) <1%. Each analyst had to evaluate the same 40 measurements compared to the evaluation of a qualified expert and at least 20 of them had to have a SD of <1% compared to the reference. In practice all physicians and analysts produced SD of <0.5%.
During the clinical trial measurements were made during screening, at baseline (day −1) and on day 14, 21, 28, 35 and 42 (Figure 1) and the same arm (right side when possible) was used throughout. Patients were institutionalized overnight prior to each assessment, during which smoking and tobacco were prohibited, and measurements made in the morning under fasted conditions. Patients were examined in a quiet temperature‐controlled room and asked to avoid caffeinated beverages for 12 hours prior to each assessment. The brachial artery diameter (BAD) was assessed by high‐resolution ultrasound imaging above the elbow, initially after 15 minutes rest in a supine position, and then following reactive hyperaemia, which was induced by 5 minutes inflation of a pneumatic tourniquet inflated to a pressure of 200 mmHg around the forearm and sudden cuff‐deflation. The focus zone was set to the depth of the anterior vessel wall and depth and gain settings were optimized to identify the lumen vessel wall interface. The maximum brachial artery diameter was determined by continuously imaging from prior to cuff inflation until 2 minutes after cuff release. The ultrasound images were recorded directly onto the hard disk of the ultrasound machine and image a;alysis performed using an automated edge‐detection software (Vascular Research Tools 5, Medical Imaging Applications LLC, Coralville, Iowa, USA) as previously described. 67 FMD was expressed as the percentage change in BAD following reactive hyperaemia.
FIGURE 1.
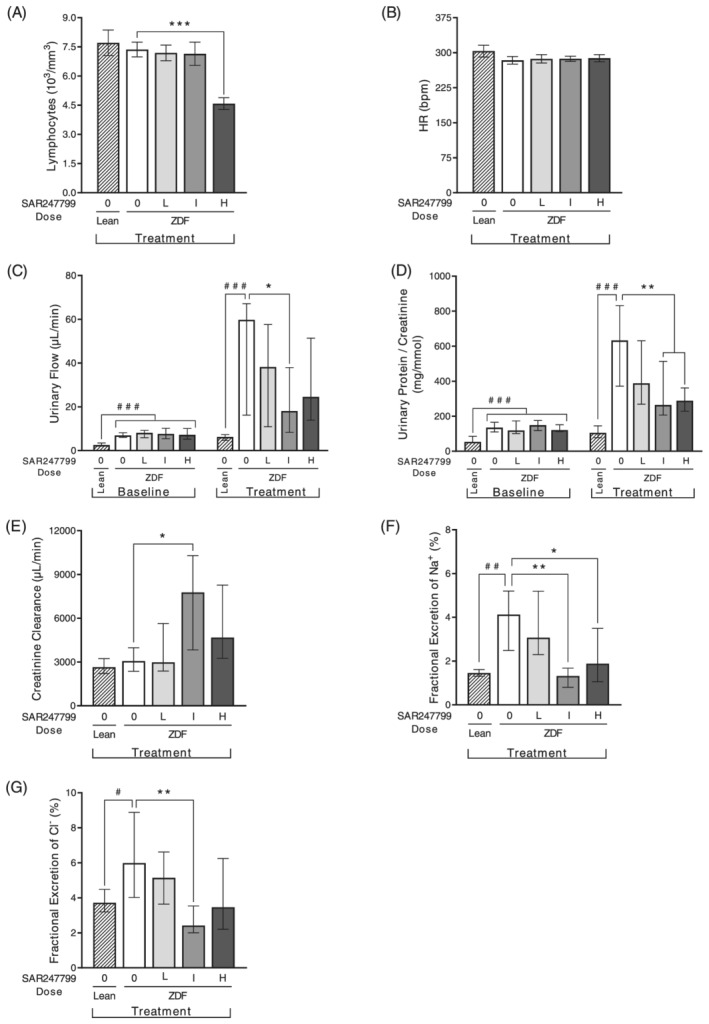
Effects of SAR247799 on lymphocytes, heart rate and renal parameters in diabetic rats. Zucker diabetic fatty (ZDF) rats treated for 5 weeks with chow formulated with control (0), low (L; 0.002% w/w), intermediate (I; 0.007% w/w) or high (H; 0.0245% w/w) doses of SAR247799. Age‐matched lean rats treated with control chow (0) for comparison. n = 8, 12, 14, 12 rats in lean, ZDF 0, ZDF L, ZDF I, ZDF H groups, respectively. Bars are mean ± standard error of the mean when Shapiro–Wilks test did not reject normality hypothesis (A, B) and median ± interquartile range otherwise (C–G). # P < .05, ## P < .01, ### P < .001 for lean vs ZDF control group; A and B by Student t test; E, F, G by Wilcoxon test; C and D baseline comparisons of lean vs the 4 ZDF groups by Kruskal–Wallis followed by Wilcoxon posthoc test; C and D treatment comparisons of lean vs ZDF control group by Wilcoxon test. * P < .05, ** P < .01, *** P < .001 for treated groups vs ZDF control group; A and B by 1‐way ANOVA followed by Dunnett's test; E, F, G and C & D (treatment) by Kruskal–Wallis test followed by Wilcoxon posthoc test
2.2.4. Other assessments
Lymphocytes and heart rate were assessed at timepoints as shown in Figure 1. Other safety assessments were performed throughout the study period and included AE recording, physical examinations, vital signs, laboratory tests (haematology, urinalysis, biochemistry including high‐sensitivity c‐reactive protein) and continuous ECG telemetry.
2.2.5. PK
SAR247799 concentrations were measured from plasma samples at the following time points: 4 and 12 hours postdose on day 1; 0 hours (predose) on days 2, 3 and 7; and at 3 hours postdose on days 14, 21 and 28. A validated liquid chromatography–tandem mass spectrometry method with a lower limit of quantification of 5 ng/mL was used. The following PK parameters were calculated with a Bayesian approach using a population PK model developed for SAR247799 in healthy subjects following single and repeated administration: maximum concentration observed (Cmax), time to reach Cmax, area under plasma concentration vs time curve calculated using a trapezoid method over 24 hours (AUC0–24) and accumulation ratio (Rac [Cmax, AUC0–24]).
2.2.6. Sample size considerations
Sample size calculations were based on previous FMD studies in type‐2 diabetes patients where sildenafil produced at least a 3% FMD increase from baseline compared to placebo, 46 , 48 , 49 and a SD of 2.5% was assumed (corresponding to a statistical effect size of at least 1.2).
The sample size was calculated by assuming that 3 doses of SAR247799 would be evaluated over 3 cohorts. To control the global type I error rate, a 1‐sided α = 1.67% (5% divided by 3 doses) was used. A sample size of 13 evaluable patients per group would have had 80% power to detect an increase of 3% of the change in FMD from baseline of SAR247799 vs placebo (assuming a common SD of 2.5%) with a 1‐sided 1.67% significance level. Considering that 10% of patients may not have evaluable baseline and postbaseline FMD, 15 included patients per group was considered necessary.
As the third cohort was optional, to permit the analysis at the end of 2 cohorts with 1‐sided α = 2.5% and at least 80% power, the sample size for sildenafil and placebo was increased by 1 patient per cohort (from 5 to 6), resulting in the following distribution of patients randomized across the first 2 cohorts: 15 patients on 1 mg SAR247799; 15 patients on 5 mg SAR247799; 12 patients on placebo; and 12 patients on sildenafil.
2.2.7. Statistical analysis
SAS version 9.4 for Windows 7 was used for statistical analysis. Demographic data were summarized in descriptive statistics by treatment group. All biomarkers were also summarized in descriptive statistics by treatment group and time.
Count and percentage of patients experiencing an AE were provided for each treatment by Medical Dictionary for Regulatory Activities (MedDRA version 21.1) primary system organ classes and preferred terms.
FMD change from baseline on days 14, 21, 28, 35 and 42 was analysed within a repeated‐measures model with fixed terms for treatment, time, treatment‐by‐time interaction and with sex and baseline FMD value as covariate and with subject as a random term with an unstructured variance–covariance matrix. Mean difference between treatments and their corresponding 95% confidence interval were estimated within the model framework at each time.
Similarly, heart rate (change from baseline at 4 h) and lymphocytes (% change from baseline at 6.5 h) on days 1, 7, 14, 21 and 28 were analysed within the same repeated measures model as for FMD with mean difference between treatments and their corresponding 95% confidence interval estimated within the model framework at each time.
2.3. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 68 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18. 69
3. RESULTS
3.1. PK, lymphocyte and heart rate effects of SAR247799 in rats
To assess the ability of different dose strengths of SAR247799 to desensitize S1P1, peripheral blood lymphocyte counts were assessed following 5 weeks of oral administration to ZDF rats, alongside an assessment of the plasma concentrations of SAR247799.
The low and intermediate dose strengths of SAR247799 (0.002 and 0.007% [w/w] chow) did not reduce lymphocytes (Figure 1A). The high dose strength (0.0245% [w/w] chow) caused a 35% decrease in peripheral blood lymphocytes (Figure 1A), indicating a partial desensitization of S1P1 at this dose. None of the doses of SAR247799 altered heart rate (Figure 1B).
Plasma concentrations of SAR247799 increased consistent with dose proportionality from low to intermediate dose strength, with mean AUC values of 2.27 and 10.2 μg h/mL, respectively (Table 1). At the high dose strength there was a 10.5‐fold increase in plasma concentration (106 μg h/mL) for a 3.5‐fold increase in dose from the intermediate dose.
TABLE 1.
Descriptive statistics of plasma pharmacokinetics parameters in diabetic rats
| SAR247799 dose % (w/w) in chow | Parameter | Mean | Median | SD | CV % | Min | Max |
|---|---|---|---|---|---|---|---|
| 0.002% (low) | C (μg/mL) | 0.0944 | 0.0917 | 0.0188 | 19.9 | 0.0697 | 0.124 |
| AUC0–24 (μg h/mL) | 2.27 | 2.20 | 0.451 | 1.67 | 2.98 | ||
| 0.007% (intermediate) | C (μg/mL) | 0.423 | 0.432 | 0.172 | 40.6 | 0.154 | 0.768 |
| AUC0–24 (μg h/mL) | 10.2 | 10.4 | 4.23 | 3.70 | 18.4 | ||
| 0.0245% (high) | C (μg/mL) | 4.43 | 4.60 | 2.21 | 49.9 | 0.506 | 8.05 |
| AUC0–24 (μg h/mL) | 106 | 110 | 53.0 | 12.1 | 193 |
C refers to concentration at 5 weeks postdosing
AUC0–24 calculated by assuming C is the average concentration over the dosing interval (AUC0–24 = C × 24)
3.2. SAR247799 improves renal function in diabetic rats independent of lymphocyte reduction
Compared to lean control rats, ZDF rats had higher body weight and higher plasma fructosamine levels (consistent with their diabetic status; Figure S1). Five‐week treatment with SAR247799 (at any dose) did not alter fructosamine levels (Figure S1B), consistent with SAR247799 not being a classical antidiabetic drug controlling glucose levels. To determine the efficacy of SAR247799 following repeated administration, a variety of renal parameters were measured following 5‐week SAR247799 treatment.
Urinary output was 10‐fold higher in vehicle‐treated ZDF rats compared to lean rats at the end of the 5‐week treatment period (Figure 1C). SAR247799 treatment significantly blunted the increase in urinary output at the intermediate dose (Figure 1C).
The urinary protein:creatinine ratio was 6‐fold higher in diabetic rats compared to lean controls (Figure 1D). SAR247799 caused a dose‐dependent reduction in urinary protein: creatinine ratio, which was statistically significant at the intermediate and high doses. The lowest dose showed a partial effect and the top 2 doses caused similar effects, suggesting that a maximal PD effect on this parameter had been reached at the intermediate dose (Figure 1D).
Creatinine clearance was similar in ZDF and lean rats (Figure 1E). Five‐week treatment with the intermediate dose of SAR247799 caused a 2.5‐fold increase in creatinine clearance compared to vehicle‐treated ZDF rats. There was no increase at the low dose, and a lesser (nonsignificant) increase at the high dose.
The fractional excretion of electrolytes (Na+ and Cl−) was increased in ZDF rats compared to lean (Figure 1F, 1G). These increases were most effectively reduced by the intermediate dose of SAR247799, with levels similar to lean control rats, and to a lesser extent at the high dose.
Overall, the dose–response characterization on renal parameters (Figure 1C‐G) demonstrated that SAR247799 had maximal pharmacological effects on all parameters at the intermediate dose; a dose that did not reduce lymphocytes (Figure 1A) or heart rate (Figure 1B). There was a partial or lesser effect on most parameters at the low and high doses (Figure 1C–G).
3.3. SAR247799 improves endothelial biomarkers in diabetic rats independent of lymphocyte reduction
To assess the ability of repeated administration of SAR247799 to alter biomarkers of endothelial function the level of the following factors was assessed following 5‐week treatment: vWF (coagulation), sICAM‐1, sVCAM‐1 and sE‐selectin (adhesion molecule shedding), sPECAM‐1 (capillary integrity), nitrite/nitrate and CRP (inflammation). All markers showed an increase in diabetic compared to lean rats and these achieved statistical significance except for vWF and nitrite/nitrate (Figure 2A‐G). SAR247799 treatment caused a dose‐dependent reduction in all of these markers; the reductions were statistically significant for at least 1 dose for all biomarkers except sPECAM‐1. Overall, SAR247799 showed the most effective reduction of endothelial biomarkers at the intermediate and high doses, with little difference between them.
FIGURE 2.
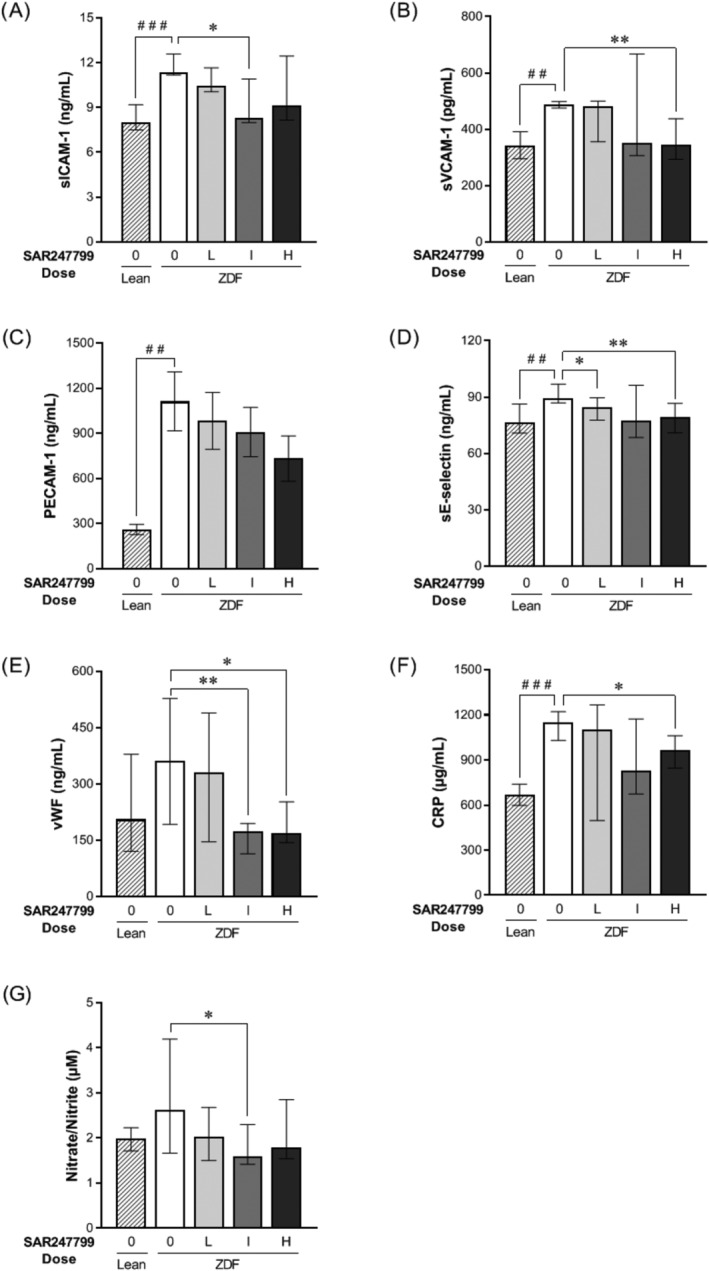
Effects of SAR247799 on plasma biomarkers in diabetic rats. Zucker diabetic fatty (ZDF) rats following 5‐week treatment with chow formulated with control (0), low (L; 0.002% w/w), intermediate (I; 0.007% w/w), or high (H; 0.0245% w/w) doses of SAR247799. Age‐matched lean rats treated with control chow (0) for comparison. n = 8, 12, 14, 12 rats in lean, ZDF 0, ZDF L, ZDF I, ZDF H groups, respectively. Bars represent mean ± standard error of the mean when Shapiro–Wilks test did not reject normality hypothesis (C) and median ± interquartile range otherwise (A, B, D, E, F, G). ## P < .01, ### P < .001 for lean vs ZDF control group; Student t‐test for C, or Wilcoxon test for A, B, D, E, F, G. * P < .05, ** P < .01, for treated groups vs ZDF control group; Kruskal–Wallis test followed by Wilcoxon posthoc test for A, B, D, E, F, G
Considering the combined renal function and plasma biomarker data (Figure 1 and 2), the intermediate dose of SAR247799 provides the best overall efficacy profile. The lesser effects at the low dose could reflect suboptimal exposure, and at the high dose could reflect partial S1P1 desensitization (35% reduction in lymphocytes) causing some loss of protective effect.
3.4. Clinical study population and safety assessments
We performed a randomized, double‐blind, multicentre, active‐ and placebo‐controlled clinical trial in type‐2 diabetes patients to evaluate the activity of SAR247799 on endothelial function using flow‐mediated dilation (FMD; Figure 3A). The baseline clinical characteristics are shown in Table 2. Patients progressed through the clinical trial as shown in Figure 3B. Safety analysis included all 54 enrolled patients and is summarized in Table 3. There were no deaths, no serious or severe treatment‐emergent AEs (TEAEs) and no AEs of special interest during the study. Of the total 54 patients enrolled in the study, 53 completed their assigned IMP for 28 days as planned. One patient, assigned to the sildenafil group, discontinued treatment on day 3 due to the occurrence of ventricular extrasystoles (grade 1 intensity according to NCI‐CTCAE v4.03). Overall 19 of 54 patients experienced at least 1 TEAE during the study (Table 3), with the number of patients with TEAEs in the SAR247799 and sildenafil treatment groups being similar and slightly higher than in the placebo group. The most commonly reported TEAEs were headache and nasopharyngitis, with 5 and 4 patients reporting these TEAEs, respectively. The TEAEs were typically reported as mild, and all of them resolved with time.
TABLE 2.
Demographic and clinical variables at baseline
| SAR247799 | |||||
|---|---|---|---|---|---|
| Variables | Placebo (n = 12) | 1 mg (n = 15) | 5 mg (n = 15) | Sildenafil (n = 12) | |
| Age (y) | Mean (SD) | 56.3 (7.9) | 54.7 (7.0) | 56.4 (6.0) | 58.6 (3.8) |
| Sex | n females (%) | 3 (25) | 2 (13.3) | 6 (40) | 4 (33.3) |
| Race | n (%) | ||||
| • White | 11 (91.7%) | 14 (93.3%) | 14 (93.3%) | 12 (100%) | |
| • Black or African American | 0 | 1 (6.7%) | 1 (6.7%) | 0 | |
| • Multiple | 1 (8.3%) | 0 | 0 | 0 | |
| Ethnicity | n not Hispanic or Latino (%) | 12 (100%) | 15 (100%) | 15 (100%) | 12 (100%) |
| Weight (kg) | Mean (SD) | 89.7 (13.1) | 87.9 (13.9) | 84.9 (13.1) | 88.3 (14.4) |
| BMI (kg/m2) | Mean (SD) | 29.9 (3.5) | 28.6 (3.0) | 29.1 (3.0) | 28.6 (3.7) |
| BMI | n < 30 kg/m2 (%) | 5 (41.7%) | 9 (60.0%) | 10 (66.7%) | 7 (58.3%) |
| Haemoglobin A1c (%) | Mean (SD) | 7.02 (0.62) | 7.64 (0.68) | 6.84 (0.74) | 7.3 (0.63) |
| SBP (mmHg) | Mean (SD) | 133 (14) | 131 (12) | 123 (14) | (129 (12) |
| Heart rate (beats/min) | Mean (SD) | 64 (7) | 69 (8) | 64 (6) | 68 (8) |
| FMD (%) | Mean (SD) | 3.88 (1.25) | 4.32 (1.67) | 3.87 (1.67) | 4.20 (1.52) |
| Concurrent therapy | n | ||||
| • No treatment | 3 | 0 | 1 | 1 | |
| • Oral antidiabetic treatment | 8 | 13 | 14 | 11 | |
| • ACEi/ARB | 4 | 4 | 3 | 7 | |
| • Statins | 3 | 2 | 1 | 1 | |
| • Thyroid replacement therapy | 1 | 1 | 4 | 0 | |
| • Nonsteroidal anti‐inflammatory | 1 | 1 | 1 | 1 | |
| • Diuretic | 0 | 0 | 0 | 0 | |
| • Other | 0 | 3 | 1 | 1 | |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; FMD, flow‐mediated dilation; SBP, systolic blood pressure; SD, standard deviation
TABLE 3.
Number (%) of patients with TEAE(s) by primary system organ class and preferred term
| Primary system organ class preferred term, n (%) | SAR247799 | Sildenafil | ||
|---|---|---|---|---|
| Placebo (N = 12) | 1 mg (N = 15) | 5 mg (N = 15) | 50 mg (N = 12) | |
| Any class | 3 (25.0%) | 5 (33.3%) | 6 (40.0%) | 5 (41.7%) |
| Severe AE | 0 | 0 | 0 | 0 |
| Leading to treatment discontinuation | 0 | 0 | 0 | 1 (8.3%) |
| Infections and infestations | 1 (8.3%) | 1 (6.7%) | 2 (13.3%) | 1 (8.3%) |
| Nasopharyngitis | 0 | 1 (6.7%) | 2 (13.3%) | 1 (8.3%) |
| Otitis externa | 1 (8.3%) | 0 | 0 | 0 |
| Nervous system disorders | 1 (8.3%) | 1 (6.7%) | 2 (13.3%) | 2 (16.7%) |
| Headache | 1 (8.3%) | 1 (6.7%) | 1 (6.7%) | 2 (16.7%) |
| Orthostatic intolerance | 0 | 0 | 1 (6.7%) | 0 |
| Cardiac disorders | 0 | 0 | 1 (6.7%) | 1 (8.3%) |
| Ventricular extrasystoles | 0 | 0 | 0 | 1 (8.3%) |
| Palpitations | 0 | 0 | 1 (6.7%) | 0 |
| Respiratory, thoracic and mediastinal disorders | 0 | 2 (13.3%) | 0 | 1 (8.3%) |
| Oropharyngeal pain | 0 | 0 | 0 | 1 (8.3%) |
| Cough | 0 | 1 (6.7%) | 0 | 0 |
| Epistaxis | 0 | 1 (6.7%) | 0 | 0 |
| Obstructive airways disorder | 0 | 1 (6.7%) | 0 | 0 |
| Gastrointestinal disorders | 0 | 2 (13.3%) | 1 (6.7%) | 3 (25.0%) |
| Diarrhoea | 0 | 0 | 0 | 2 (16.7%) |
| Dyspepsia | 0 | 0 | 0 | 1 (8.3%) |
| Gastroesophageal reflux disease | 0 | 1 (6.7%) | 0 | 1 (8.3%) |
| Nausea | 0 | 1 (6.7%) | 1 (6.7%) | 0 |
| Vomiting | 0 | 0 | 1 (6.7%) | 0 |
| Skin and subcutaneous tissue disorders | 0 | 1 (6.7%) | 0 | 2 (16.7%) |
| Erythema | 0 | 1 (6.7%) | 0 | 2 (16.7%) |
| Pruritus | 0 | 1 (6.7%) | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 3 (20.0%) | 1 (8.3%) |
| Back pain | 0 | 0 | 1 (6.7%) | 1 (8.3%) |
| Musculoskeletal pain | 0 | 0 | 1 (6.7%) | 0 |
| Neck pain | 0 | 0 | 1 (6.7%) | 0 |
| General disorders and administration site conditions | 0 | 0 | 1 (6.7%) | 0 |
| Vessel puncture site haematoma | 0 | 0 | 1 (6.7%) | 0 |
| Injury, poisoning and procedural complications | 1 (8.3%) | 0 | 0 | 0 |
| Arthropod sting | 1 (8.3%) | 0 | 0 | 0 |
| Contusion | 1 (8.3%) | 0 | 0 | 0 |
| Skin abrasion | 1 (8.3%) | 0 | 0 | 0 |
| Traumatic haematoma | 1 (8.3%) | 0 | 0 | 0 |
AE, adverse event; TEAE, treatment‐emergent AE (MedDRA version 21.1); N = number of patients treated within each group; n (%) = number and % of patients with at least 1 TEAE in each category.
Note: An adverse event was considered as treatment‐emergent if it occurred from the time of the first IMP administration up to 7 days (included) after the last IMP administration.
On day 1, SAR247799 caused a dose‐dependent reduction in heart rate (mean reductions of 2 and 4 beats/min at 1 and 5 mg, respectively) compared to no change in the placebo or sildenafil groups (Figure 4A). As the study progressed (day 7–28), there was a mean 5–6 beats/min heart rate increase in the placebo group, a similar (3–6 beats/min) increase in the sildenafil group, a smaller (0–4 beats/min) increase in the 5 mg group and no increase in the 1 mg SAR247799 group. Relative to the placebo group, the maximum heart rate decrease was observed with 5 mg SAR247799 on day 14 (mean difference vs placebo −6.4 beats/min; 95% confidence interval [CI] −2.1 to −10.7 beats/min; P = .004). Relative to the placebo group, in the 1 mg SAR247799 group a lower heart rate was sustained over the 28‐day treatment period, but in the 5 mg SAR247799 group the heart rate effects diminished slightly on day 21 and day 28 (suggesting the onset of S1P1 desensitization).
FIGURE 4.
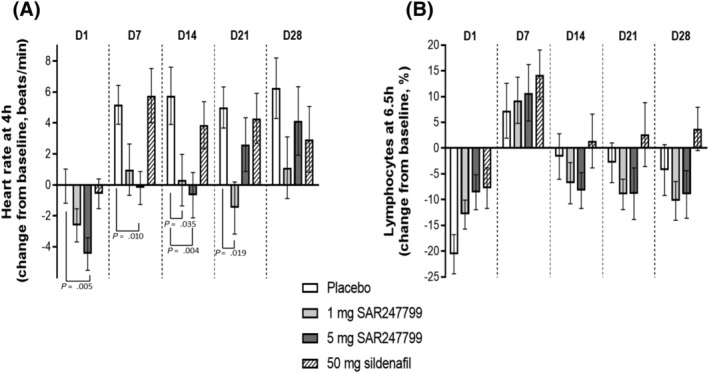
Effect of SAR247799 (1 and 5 mg), sildenafil (50 mg), or placebo on heart rate (A) and lymphocytes (B) in type‐2 diabetes patients. Heart rate and lymphocytes expressed on each day (D1–D28) at time points associated with maximal pharmacodynamic effects (4 and 6.5 h, respectively). Mean ± standard error of the mean; P values shown when < .05 (repeated‐measure model as described under Methods)
Systolic and diastolic blood pressure were slightly reduced over the treatment period, but without significant differences between placebo and treatment groups (Figure S2).
3.5. PK and lymphocyte‐reducing effects of SAR247799 in patients
To assess the ability of the 2 doses of SAR247799 to desensitize S1P1, peripheral blood lymphocyte counts were assessed over the 28 days of oral treatment to type‐2 diabetes patients. The 2 doses of SAR247799 showed lymphocyte changes which were indistinguishable from placebo (Figure 4B; mean reductions vs placebo <8% at all timepoints).
Plasma concentrations of SAR247799 increased from day 1 to day 14 with an accumulation ratio of 2.5–2.9 and were consistent with dose proportionality between 1 and 5 mg/d (Table 4). The steady state AUC0–24h of SAR247799 were similar between the 1‐mg/d human dose compared to the low dose in rats (2.97 vs 2.27 μg/mL, respectively; Tables 1 and 4). Similarly, the steady state AUC0–24h were similar between the 5‐mg/d human dose and the intermediate dose in rat (14.9 vs 10.2 μg/mL, respectively; Tables 1 and 4). Both human doses (and the low and intermediate rat doses) did not significantly reduce lymphocytes (Figure 1A and 4B). The AUC0–24h at the high dose in rats, inducing partial S1P1 desensitization (Figure 1A), was 7.5‐fold higher than the 5‐mg/d human dose (106 vs 14.9 μg h/mL, respectively; Table 1 and Table 4).
TABLE 4.
Descriptive statistics of plasma pharmacokinetic parameters in type‐2 diabetes patients following 1.0 and 5.0 mg SAR247799 repeated once daily oral administration by dose level on days 1 and 14
| Day | Parameter | Dose | n | Mean | Median | SD | CV% | Min | Max |
|---|---|---|---|---|---|---|---|---|---|
| 1 | T max | 1 | 15 | 2.68 | 2.50 | 0.320 | 11.9 | 2.25 | 3.25 |
| (h) | 5 | 15 | 2.42 | 2.50 | 0.122 | 5.05 | 2.25 | 2.5 | |
| C max | 1 | 15 | 0.0574 | 0.0538 | 0.0131 | 22.8 | 0.0403 | 0.0907 | |
| (μg/mL) | 5 | 15 | 0.310 | 0.315 | 0.0635 | 20.5 | 0.207 | 0.414 | |
| AUC 0–24 | 1 | 15 | 1.03 | 0.983 | 0.232 | 22.5 | 0.750 | 1.69 | |
| (μg h/mL) | 5 | 15 | 5.31 | 5.31 | 0.955 | 18.0 | 3.75 | 6.87 | |
| 14 | T max | 1 | 15 | 2.24 | 2.25 | 0.0969 | 4.32 | 2.00 | 2.50 |
| (h) | 5 | 15 | 2.15 | 2.25 | 0.116 | 5.40 | 2.00 | 2.25 | |
| C max | 1 | 15 | 0.152 | 0.147 | 0.0419 | 27.6 | 0.0998 | 0.271 | |
| (μg/mL) | 5 | 15 | 0.779 | 0.760 | 0.186 | 23.9 | 0.500 | 1.13 | |
| AUC 0–24 | 1 | 15 | 2.97 | 2.78 | 0.902 | 30.4 | 1.84 | 5.46 | |
| (μg h/mL) | 5 | 15 | 14.9 | 14.8 | 4.03 | 27.0 | 8.74 | 22.3 | |
| Rac_AUC 0–24 | 1 | 15 | 2.86 | 2.70 | 0.432 | 15.1 | 2.10 | 3.56 | |
| 5 | 15 | 2.79 | 2.73 | 0.492 | 17.6 | 2.10 | 3.64 | ||
| Rac_C max | 1 | 15 | 2.66 | 2.50 | 0.443 | 16.7 | 1.89 | 3.41 | |
| 5 | 15 | 2.54 | 2.46 | 0.468 | 18.4 | 1.86 | 3.49 |
AUC0–24, area under plasma concentration vs time curve over 24 hours; Cmax, maximum concentration observed; Rac, accumulation ratio; Tmax, time to reach Cmax
3.6. SAR247799 improves flow‐mediated dilation in type‐2 diabetes patients
Of the 54 patients enrolled in the trial, 52 had FMD data that were analysed (Figure 3B). One patient in the sildenafil group was discontinued from treatment on day 3, as previously mentioned, and 1 further patient (also in the sildenafil group) was excluded from the FMD analysis because the FMD curves during hyperaemia did not show the typical shape of the brachial diameter curve. At baseline, the mean FMD was well below the 7% threshold for inclusion into the study and ranged from 3.8 to 4.3% in the 4 treatment groups (Table 2). The prehyperaemia BAD remained unchanged in all groups over the 42‐day study, indicating that the arterial vasomotor tone was not affected and did not interfere with the FMD measurements (Figure 2C).
Variations in FMD were first assessed by comparing the mean placebo values over the 42‐day study period. The placebo group showed mean increases compared to baseline of 0.7, 0.6 and 0.7% on days 14, 21 and 28 respectively, and minimal change at days 35 and 42 (end of study; Figure 5A). Given this variation in the placebo group, FMD changes at each time‐point were expressed as placebo‐corrected values (Figure 5B).
FIGURE 5.
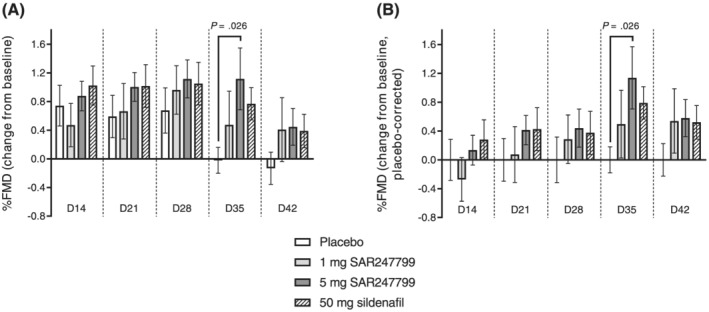
Effect of SAR247799 (1 and 5 mg), sildenafil (50 mg), or placebo on % flow‐mediated dilation (FMD) in type‐2 diabetes patients. FMD data expressed as change from baseline (A), or change from baseline, placebo corrected (B). Bars are mean ± standard error of the mean. Variation in the placebo groups in B (placebo‐corrected FMD change) illustrated by retaining error bars with mean zero (first group at each time point). Precise P‐values for difference vs placebo shown when P < .05; repeated‐measure model as described under Methods. Number of patients with FMD data in each histogram was as follows for placebo/1 mg SAR247799/5 mg SAR247799/50 mg sildenafil, respectively; 12/15/15/10 (D14, D21, D28 and D42) and 12/15/13/10 (D35)
The mean FMD change from baseline with 50 mg sildenafil (positive control) and 5 mg SAR247799 was higher than placebo at all time points up to D42 (Figure 5, Tables S1 and S2). For all 3 treatment groups (1 and 5 mg SAR247799 and 50 mg sildenafil) the maximum FMD change from baseline vs placebo occurred at 1 week after the end of treatment (day 35), indicating that an effect had persisted after compound wash‐out. On day 35, mean differences vs placebo were 0.60% (95% CI −0.34 to 1.53%; P = .203) for 1 mg SAR247799, 1.07% (95% CI 0.13 to 2.01%; P = .026) for 5 mg SAR247799 and 0.88% (95% CI −0.15 to 1.91%; P = .093) for 50 mg sildenafil. Overall SAR247799 showed a dose‐dependent improvement in FMD: 5 mg > 1 mg > placebo at days 21, 28, 35 and 42 (but not at day 14). Both doses of SAR247799 also showed a time‐dependent improvement in FMD (day 14 < day 21 < day 28 < day 35).
4. DISCUSSION
The study evaluated, for the first time, the pharmacological effect of S1P1 activation on endothelial function in patients. The experiments expand on a wealth of preclinical studies implicating S1P1 in regulating endothelial function and provide the first relevance of the S1P1 mechanism‐of‐action to patients with endothelial dysfunction. SAR247799 treatment progressively improved endothelial function (FMD) over 4 weeks in type‐2 diabetes patients to levels that were in a similar range to sildenafil, and the vascular effects of SAR247799 were independent of lymphocyte reduction (unlike S1P1 functional antagonists used for multiple sclerosis treatment) or blood pressure‐lowering.
SAR247799 showed a dose‐dependent effect (5 mg > 1 mg > placebo) at most of the time‐points evaluated, and the 5‐mg dose was at least as effective as 50 mg sildenafil. As SAR247799 has an elimination half‐life of 32 hours, 52 day 35 corresponds to >5 half‐lives after the last dose on day 28 (or >30‐fold reduction in compound exposure). Therefore, an important finding of our study was that the effect of SAR247799 persisted after the end of treatment and compound wash‐out, suggesting a potential disease‐modifying effect to the endothelium. Although it may appear surprising that the maximal FMD change was observed after the end of the treatment period, repair of dysfunctional endothelium is considered to be a gradual process taking weeks and includes restoring endothelial junctions, barrier integrity and overall endothelial regeneration. 70 As the effect appeared to be both time‐ and dose‐dependent, it is difficult to conclude whether the maximal possible effect of SAR247799 on FMD was reached. However, as SAR247799 was at least as effective as sildenafil under the conditions evaluated, a level of clinical relevance of the S1P1 mechanism has been established that warrants further evaluation in patients, including settings with longer treatment duration and/or with more relevant clinical endpoints.
Our results are consistent with a vascular function study performed in renal transplant recipients with 2.5 mg fingolimod. 49 The 2.5‐mg dose was 5‐fold higher than the approved dose in multiple sclerosis patients (0.5 mg), caused marked S1P1 desensitization (70–80% lymphocyte reduction), and was associated with creatinine clearance impairment in renal transplant recipients. 48 , 49 The study, performed 1.5 years post‐transplant, demonstrated that FMD improved following 3‐month fingolimod discontinuation. Although the investigators did not measure FMD prior to initiating fingolimod treatment, we hypothesize that the 2.5 mg dose (which was associated with marked S1P1‐desensitization) caused endothelial damage, and the FMD improvement seen over the 3‐month fingolimod‐withdrawal period reflects a restoration of endothelial function. In combination with the findings from the current study, S1P1 activation and desensitization appear to improve and impair FMD, respectively.
PDE5 inhibitors decrease forearm vascular resistance by direct vascular smooth muscle effects (e.g. through reduced breakdown of cGMP) as well as endothelium‐dependent effects (release of vasodilatory factors including nitric oxide). High‐dose (100 mg) sildenafil failed to improve FMD in type‐2 diabetes patients, but caused marked vasodilation as evidenced by blood pressure‐lowering and dilation of the BAD. 71 Two studies with lower doses of sildenafil (50 mg daily and 25 mg twice a day) showed 5–6% increases from baseline FMD values in the 7–9% range, and with no evidence of vasodilation. 56 , 58 Thus, we selected 50 mg sildenafil daily for the current study, confirmed no vasodilatory effect (i.e. no blood pressure‐lowering or BAD increase) and showed an increase in FMD from 4% at baseline to about 5% with treatment. While it is not immediately apparent why sildenafil showed a smaller effect on FMD in our study, it is possible that the enrichment of patients for marked endothelial dysfunction in our study (lower baseline FMD) could have influenced the absolute effect of sildenafil. Nevertheless, the inclusion of sildenafil as positive control allowed us to appropriately benchmark the effect of SAR247799 to a clinical calibrator and show that this new S1P1 mechanism‐of‐action can produce effects on FMD at least as effective as PDE5 inhibition.
The improvement in FMD demonstrated with SAR247799 may have clinical implications for cardiovascular (CV) risk reduction. FMD, in addition to an index of endothelial dysfunction, predicts the rate of progression of carotid intima media thickness with superiority to the Framingham score. 72 Furthermore, drugs that improve FMD have proved beneficial on CV outcomes such as statins and antihypertensive drugs (including angiotensin converting enzyme‐inhibitors, angiotensin I receptor blockers and calcium channel blockers) with average effects for these drug classes of 1–1.5% increase in FMD. 62 , 63 , 64 , 73 , 74 Over a broad range of populations, including patients with CV disease, a 1% improvement of FMD is associated with a 12% reduction in CV events. 55 As noted above, since FMD improvement with sildenafil varied between studies, it is difficult to extrapolate quantitatively the benefit of SAR247799 on CV risk reduction based on the existing data alone. SAR247799 is also expected to improve the microvasculature (as shown in animal models) and indices of microvascular function predict CV risk independent of FMD. 55 , 75 The potential CV risk reduction with SAR247799 could be underestimated by relying on the FMD (macrovascular) contribution alone, and further studies to assess microvascular function are needed.
The effects of SAR247799 in the current study were measured on top of current therapy, which included, for example, antidiabetic medicines, statins and antihypertensives. These concurrent therapies are documented to have small effects on FMD 62 , 63 , 64 , 73 and therefore could have reduced the overall effect of the study treatments. However, as they are a mainstay of patient use, it was important in the current, as well as in future studies, to evaluate the benefit of SAR247799 on top of existing treatments.
All previous S1P1 functional antagonists tested in humans cause first‐dose heart rate reduction, which is time‐dependently lost as S1P1 desensitization sets in. In our clinical study, 1 mg SAR247799 caused a small and sustained level of heart rate reduction over 28 days compared to placebo, whereas the 5‐mg dose showed some evidence for desensitization on days 21 and 28. While heart rate reduction and endothelial protection are both activities associated with S1P1 activation, they exhibited different kinetics: heart rate reduction displayed a rapid onset‐of‐action and was subject to rapid desensitization, whereas endothelial vasoreactivity (FMD) displayed a progressive time‐dependent effect with propensity for desensitization that is not yet characterized. As SAR247799 distributes with higher concentrations in plasma compared to heart, 52 the demonstration of sustained cardiac pharmacology over 28 days with 1 mg SAR247799 suggests that doses ≤1 mg may warrant evaluation for endothelial effects in studies of longer duration.
Although the long‐term CV safety profile of SAR247799 remains to be established, it has been suggested that FMD trials play an important role in de‐risking new CV agents. For example, the first cholesterylester transfer protein inhibitor, torcetrapib, unexpectedly demonstrated an increase in CV events in the >15 000 patient ILLUMINATE trial, despite a substantial increase in HDL cholesterol, and this adverse signal was subsequently associated with a pharmacological decrease in FMD. 76 , 77 In light of a subsequent cholesterylester transfer protein inhibitor, dalcetrapib, being neutral on both FMD and CV events, 30 , 78 , 79 it has been proposed that demonstrating a lack of adverse vascular effects or even vascular benefit through increasing FMD, as demonstrated here with SAR247799, increases confidence in the use of new treatments in large‐scale clinical trials over years. 80 The point is particularly noteworthy given that HDL is the main carrier of S1P in plasma, and there is correspondingly an elusive link between HDL‐raising agents and S1P1 agonists.
Our experiments were translational in nature as we used 2 complementary experimental systems (human and rat) to study the endothelial properties of SAR247799 in the setting of diabetes. Since SAR247799 is a biphasic molecule, it was necessary to study doses providing sufficient S1P1 activation to cause endothelial‐protective effects, while avoiding higher endothelial‐damaging doses by not exceeding a certain threshold of S1P1 desensitization. The precise threshold of S1P1 desensitization (based on lymphocyte reduction) associated with unwanted endothelial properties is currently unknown. The preclinical dose‐ranging provided the foundation for selecting 2 doses of SAR247799 that provided partial and maximal efficacy (based on renal function and endothelial biomarkers in rats), while avoiding a higher dose that was less efficacious and associated with a certain level of S1P1 desensitization (35% lymphocyte reduction). In diabetes patients, we observed that the 2 corresponding doses (1 and 5 mg, which yielded exposures that approximated the lower 2 animal doses), produced a similar dose‐dependent endothelial effect (FMD change at 5 mg > 1 mg > placebo), while also avoiding lymphocyte reduction. An even higher dose (corresponding to exposure at the highest rat dose which exhibited protective effects but with 35% lymphocyte reduction) may not have been warranted in patients, because the 5‐mg dose produced moderate change in heart rate and 5–10% lymphocyte reduction, and the objective was to avoid further S1P1 desensitization. As there was a time‐dependent FMD increase with SAR247799 over 35 days, it would be important in subsequent studies to consider whether low doses (1 mg or less) might produce more profound effects with longer treatment duration. However, as lower/higher doses or longer treatment times were not evaluated in patients, we cannot conclude whether the maximal endothelial effect for this mechanism of action had been reached.
Although sildenafil is reported to exert endothelial effects in various diabetic rat models, 81 , 82 , 83 , 84 , 85 a direct comparison to SAR247799 was not performed in ZDF rats as the objective of our preclinical studies was simply to aid in selecting doses of SAR247799 for human testing. The preclinical studies complement the clinical study with respect to the mechanism of action of SAR247799. The preclinical results suggest that SAR247799 could also improve renal function in patients (based on improvements in protein:creatinine ratio, creatinine clearance and fractional excretion of electrolytes) and are particularly relevant given the matching concentration‐effect relationships between clinical (on FMD) and preclinical studies (on renal function and biomarkers). The creatinine clearance improvement demonstrated with S1P1‐activating doses of SAR247799 in rats are consistent with a creatinine clearance decline with S1P1‐desensitizing doses of fingolimod in renal transplant patients. 48 Given the endothelial effects of SAR247799 demonstrated herein in humans, SAR247799 is an attractive drug candidate for evaluation in renal, cardiac and other manifestations of endothelial dysfunction.
The study had several limitations. Firstly, the FMD study could have benefited from a slightly larger sample size, especially as the number of patients in the placebo group ended up being imbalanced due to the optional third cohort not being conducted (for strategic reasons), and the P‐value at day 35 with 5 mg SAR247799 (0.026) slightly exceeded our significance threshold that considered multiple‐arm adjustment (α = .025). Furthermore, with 2 drop‐outs in the sildenafil group (n = 10 usable FMD data) it is not surprising that sildenafil did not achieve statistical significance. Nevertheless, the inclusion of a positive control (sildenafil) that had demonstrated improvements in FMD in type‐2 diabetes patients, facilitated interpreting the effects of SAR247799 in relation to a clinical benchmark. Secondly, given the finding of a time‐dependent effect of SAR247799 on FMD, in hindsight, 4‐week treatment duration may not have been optimal to characterize the maximal effects of this new mechanism of action on endothelial properties. Thirdly, we did not assess the direct vascular smooth muscle effects of the compounds by performing an endothelium‐independent vasodilation test using sublingual nitro‐glycerine administration (due to its contraindication with sildenafil). However, in none of the treatment groups was blood pressure or BAD (prehyperaemia) altered compared to placebo; findings consistent with an absence of blood pressure effect with SAR247799 in healthy subjects. 52 Although S1P modulates blood pressure by acting on vascular smooth muscle (S1P2/3) and endothelium (S1P1/3), a lack of functional S1P1 receptors on vascular smooth muscle, 81 , 82 , 83 lends further support that our FMD results with SAR247799 are unlikely to be influenced by a direct action on vascular smooth muscle.
Overall, these preclinical and clinical pharmacology findings in experimental models of type‐2 diabetes indicate consistent endothelial properties of 4–5‐week SAR247799 treatment. Endothelial effects were observed without lymphocyte reduction and at similar compound exposures between the human and animal systems. Given that SAR247799 improved FMD with similar effects to sildenafil under the conditions evaluated, and that SAR247799 did not lower blood pressure compared to placebo, the results support further evaluation of S1P1 agonists in diseases associated with endothelial dysfunction.
COMPETING INTERESTS
At the time of conduct of the studies, L.B., M.F.E., B.P., A.T., O.V., F.H., R.B., X.B., D.R., P.J., A.J.M., L.H., S.K., P.D. and A.A.P. were employees of Sanofi; G.A., L.P., M.E., T.H., J.A. and H.C. were employees of Profil Institute; R.S. and C.H. received personal fees from Profil Institute. Authors affiliated with Sanofi may have equity interest in Sanofi. A.A.P., B.P. and P.J. are inventors of US patent number 9 782 411. C.H. received grants from Deutsche Forschungsgemeinschaft, Mitsubishi Cleansui, Wild Blueberry Association of North America, University of Surrey, Philips, National Processed Raspberry Council, Cranberry Institute and Rheacell, and personal fees from Bayer and Novo Nordisk, all outside of the submitted work.
CONTRIBUTORS
Conceptualization: L.B., G.A., L.P., B.P., R.S., C.H., D.R., P.J., A.J.M., S.K., P.D., A.A.P. Data curation: M.F.E., B.P., R.B., X.B. Formal analysis: L.B., M.F.E., B.P., O.V., F.H., R.B., X.B., D.R., L.H., A.A.P. Funding acquisition: B.P., P.J., A.J.M., P.D., A.A.P. Investigation: G.A., L.P., M.F.E., T.H. Methodology: L.B., G.A., L.P., M.F.E., B.P., T.H., J.A., R.S., C.H., O.V., F.H., D.R., A.J.M., L.H., S.K., P.D., A.A.P. Project administration: A.T., J.A. Resources: G.A., L.P., A.T., J.A. Supervision: L.B., G.A., L.P., B.P., P.J., A.J.M., L.H., S.K., P.D., A.A.P. Validation: G.A., L.P., B.P., L.B., A.A.P. Visualization: M.F.E., B.P., X.B., A.A.P. Writing—original draft: L.B., M.F.E., B.P., A.A.P. Writing—review and editing: G.A., L.P., A.T., M.E., T.H., J.A., H.C., R.S., C.H., O.V., F.H., R.B., X.B., D.R., P.J., A.J.M., L.H., S.K., P.D. Accountable for accuracy, integrity and approval of final version: all authors.
Supporting information
FIGURE S1 Body weight and fructosamine changes in diabetic rats.
FIGURE S2. Effect of SAR247799 (1 and 5 mg), sildenafil (50 mg), or placebo on systolic (A) and diastolic (B) blood pressure and prehyperaemia brachial artery diameter (C) in type‐2 diabetes patients.
TABLE S1 Descriptive statistics of FMD (%) following 1 and 5 mg SAR247799, 50 mg sildenafil, and placebo measured at screening, baseline, Days 14, 21, 28, 35, and 42 (EOS); raw data and change from baseline.
TABLE S2 Mean estimate of FMD change vs baseline at individual time‐points (D14, D21, D28, D35, D42) and across repeated measurements (D14–42).
ACKNOWLEDGEMENTS
The authors thank the study participants; the clinical research staff at the 2 sites, Charlene Buffat and Thomas Rizard for trial management support; Ariane‐Saskia Ries and Katharina Iwaniuk for study organization at Mainz and Neuss sites, respectively; Carol Damon for data programming; Anne Charlier, Clementine Chopin and Mélanie Mellerin for compound supply: Delphine Marin and Mélodie Hermier for data management; Michel Scemama for pharmacovigilance support; Helene Joyeux, Christine Maestrini and Tadashi Sugihara for regulatory support; Didier Simard and Brigitte Molinier for biomarker evaluation; Catherine Cadrouvele for in vivo study support; and Dorothee Tamarelle for certifying preclinical statistics. This work was funded by Sanofi.
Bergougnan L, Andersen G, Plum‐Mörschel L, et al. Endothelial‐protective effects of a G‐protein‐biased sphingosine‐1 phosphate receptor‐1 agonist, SAR247799, in type‐2 diabetes rats and a randomized placebo‐controlled patient trial. Br J Clin Pharmacol. 2021;87:2303–2320. 10.1111/bcp.14632
The authors confirm that the Principal Investigators for the clinical study were Grit Anderson and Leona Plum‐Mörschel and that they had direct clinical responsibility for patients at the Neuss and Mainz sites, respectively. In addition, Grit Andersen was coordinating principal investigator.
Funding information Sanofi, Grant/Award Number: N/A
DATA AVAILABILITY STATEMENT
All relevant preclinical and clinical data are provided within the paper and its supporting information files. Qualified researchers may request access to patient level data and related study documents including the clinical study report, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at www.clinicalstudydatarequest.com.
REFERENCES
- 1. Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72(21):2625‐2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzilli M, Merz CN, Boden WE, et al. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol. 2012;60(11):951‐956. [DOI] [PubMed] [Google Scholar]
- 4. van Sloten TT, Henry RM, Dekker JM, et al. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: the Hoorn study. Hypertension. 2014;64(6):1299‐1305. [DOI] [PubMed] [Google Scholar]
- 5. Thuy AV, Reimann CM, Hemdan NY, Gräler MH. Sphingosine 1‐phosphate in blood: function, metabolism, and fate. Cell Physiol Biochem. 2014;34(1):158‐171. [DOI] [PubMed] [Google Scholar]
- 6. Jozefczuk E, Guzik TJ, Siedlinski M. Significance of sphingosine‐1‐phosphate in cardiovascular physiology and pathology. Pharmacol Res. 2020;156:104793. 10.1016/j.phrs.2020.104793 [DOI] [PubMed] [Google Scholar]
- 7. Cartier A, Hla T. Sphingosine 1‐phosphate: lipid signaling in pathology and therapy. Science. 2019;366:eaar5551. 10.1126/science.aar5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guitton J, Bandet CL, Mariko ML, et al. Sphingosine‐1‐phosphate metabolism in the regulation of obesity/type 2 diabetes. Cell. 2020;9:1682. 10.3390/cells9071682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ng ML, Wadham C, Sukocheva OA. The role of sphingolipid signalling in diabetes‐associated pathologies. Int J Mol Med. 2017;39(2):243‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raza Z, Saleem U, Naureen Z. Sphingosine 1‐phosphate signaling in ischemia and reperfusion injury. Prostaglandins Other Lipid Mediat. 2020;149:106436. 10.1016/j.prostaglandins.2020.106436 [DOI] [PubMed] [Google Scholar]
- 11. Intapad S. Sphingosine‐1‐phosphate signaling in blood pressure regulation. Am J Physiol Renal Physiol. 2019;317(3):F638‐F640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cantalupo A, di Lorenzo A. S1P signaling and De novo biosynthesis in blood pressure homeostasis. J Pharmacol Exp Ther. 2016;358(2):359‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1‐phosphate: is this an atherogenic mediator or an anti‐atherogenic mediator? Biochim Biophys Acta. 2002;1582(1‐3):132‐137. [DOI] [PubMed] [Google Scholar]
- 14. Frej C, Mendez AJ, Ruiz M, et al. A shift in ApoM/S1P between HDL‐particles in women with type 1 diabetes mellitus is associated with impaired anti‐inflammatory effects of the ApoM/S1P complex. Arterioscler Thromb Vasc Biol. 2017;37(6):1194‐1205. [DOI] [PubMed] [Google Scholar]
- 15. Diarte‐Añazco EMG, Méndez‐Lara KA, Pérez A, Alonso N, Blanco‐Vaca F, Julve J. Novel insights into the role of HDL‐associated Sphingosine‐1‐phosphate in Cardiometabolic diseases. Int J Mol Sci. 2019;20:6273. 10.3390/ijms20246273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaisar T, Couzens E, Hwang A, et al. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS One. 2018. Mar 15:e0192616. 10.1371/journal.pone.0192616 eCollection 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brinck JW, Thomas A, Lauer E, et al. Associated with reduced high‐density lipoprotein Sphingosine‐1‐phosphate content and impaired high‐density lipoprotein cardiac cell protection. Arterioscler Thromb Vasc Biol. 2016;36:817‐824. [DOI] [PubMed] [Google Scholar]
- 18. Sattler KJE, Elbasan Ş, Keul P, et al. Sphingosine 1‐phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821‐832. [DOI] [PubMed] [Google Scholar]
- 19. Argraves K, Sethi A, Gazzolo P, et al. S1P, dihydro‐S1P and C24:1‐ceramide levels in the HDL‐containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 2011. May 9;10(1):1‐12. 10.1186/1476-511X-10-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knapp M, Baranowski M, Czarnowski D, et al. Plasma sphingosine‐1‐phosphate concentration is reduced in patients with myocardial infarction. Med Sci Monit. 2009;15(9):CR490‐CR493. [PubMed] [Google Scholar]
- 21. Knapp M, Lisowska A, Zabielski P, Musiał W, Baranowski M. Sustained decrease in plasma sphingosine‐1‐phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat. 2013;106:53‐61. [DOI] [PubMed] [Google Scholar]
- 22. Denimal D, Monier S, Brindisi MC, et al. Impairment of the ability of HDL from patients with metabolic syndrome but without diabetes mellitus to activate eNOS: correction by S1P enrichment. Arterioscler Thromb Vasc Biol. 2017;37:804‐811. [DOI] [PubMed] [Google Scholar]
- 23. Coldewey SM, Benetti E, Collino M, et al. Elevation of serum sphingosine‐1‐phosphate attenuates impaired cardiac function in experimental sepsis. Sci Rep. 2016;6:27594. 10.1038/srep27594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winkler MS, Märtz KB, Nierhaus A, et al. Loss of sphingosine 1‐phosphate (S1P) in septic shock is predominantly caused by decreased levels of high‐density lipoproteins (HDL). J Intensive Care. 2019;7(1):1‐9. 10.1186/s40560-019-0376-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jing XD, Wei XM, Deng SB, Du JL, Liu YJ, She Q. The relationship between the high‐density lipoprotein (HDL)‐associated sphingosine‐1‐phosphate (S1P) and coronary in‐stent restenosis. Clin Chim Acta. 2015;446:248‐252. [DOI] [PubMed] [Google Scholar]
- 26. Soltau I, Mudersbach E, Geissen M, et al. Serum‐Sphingosine‐1‐phosphate concentrations are inversely associated with atherosclerotic diseases in humans. PLoS One. 2016. Dec 14;e0168302. 10.1371/journal.pone.0168302 eCollection 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kiziltunc E, Abaci A, Ozkan S, et al. The relationship between pre‐infarction angina and serum Sphingosine‐1‐phosphate levels. Acta Cardiol Sin. 2014;30(6):546‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kızıltunç E, Gök M, Topçuoğlu C, et al. Serum sphingosine 1 phosphate levels in patients with and without coronary collateral circulation. Acta Cardiol Sin. 2018;34(5):379‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenson RS, Brewer HB Jr, Ansell BJ, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13(1):48‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armitage J, Holmes MV, Preiss D. Cholesteryl Ester transfer protein inhibition for preventing cardiovascular events: JACC review topic of the week. J Am Coll Cardiol. 2019;73(4):477‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255‐2267. [DOI] [PubMed] [Google Scholar]
- 32. Galvani S, Hla T. Quality versus quantity: making HDL great again. Arterioscler Thromb Vasc Biol. 2017;37:1018‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egom EE, Mamas MA, Soran H. HDL quality or cholesterol cargo: what really matters – spotlight on sphingosine‐1‐phophate‐rich HDL. Curr Op Lipidol. 2013;24(4):351‐356. [DOI] [PubMed] [Google Scholar]
- 34. Kurano M, Yatomi Y. Sphingosine‐1‐phosphate and atherosclerosis. J Atheroscler Thromb. 2018;25(1):16‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Argraves KM, Gazzolo PJ, Groh EM, et al. High density lipoprotein‐associated sphingosine‐1 phosphate promotes endothelial barrier function. J Biol Chem. 2008;283(36):25074‐25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Potì F, Simoni M, Nofer J‐R. Atheroprotective role of high‐density lipoprotein (HDL)‐associated sphingosine‐1‐phosphate (S1P). Cardiovasc Res. 2014;103(3):395‐404. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz M, Frej C, Holmér A, Guo LJ, Tran S, Dahlbäck B. High‐density lipoprotein‐associated Apolipoprotein M limits endothelial inflammation by delivering Sphingosine‐1‐phosphate to the Sphingosine‐1‐phosphate receptor 1. Arterioscler Thromb Vasc Biol. 2017;37:118‐129. [DOI] [PubMed] [Google Scholar]
- 38. Gong M, Wilson M, Kelly T, et al. HDL‐associated estradiol stimulates endothelial NO synthase and vasodilation in an SR‐BI‐dependent manner. J Clin Invest. 2003;111(1587):1579‐1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sukocheva O, Wadham C, Gamble J, Xia P. Sphingosine‐1‐phosphate receptor 1 transmits estrogens' effects in endothelial cells. Steroids. 2015;104:237‐245. [DOI] [PubMed] [Google Scholar]
- 40. Guo S, Yu Y, Zhang N, et al. Higher level of plasma bioactive molecule sphingosine 1‐phosphate in women is associated with estrogen. Biochim Biophys Acta. 2014. Jun;1841(6):836‐846. [DOI] [PubMed] [Google Scholar]
- 41. Sattler JKE. Defects of high‐density lipoproteins in coronary artery disease caused by low Sphingosine‐1‐phosphate content. Correction by Sphingosine‐1‐phosphate loading. J Am Coll Cardiol. 2015;66:1470‐1485. [DOI] [PubMed] [Google Scholar]
- 42. Christensen PM, Liu CH, Swendeman SL, et al. Impaired endothelial barrier function in apolipoprotein M–deficient mice is dependent on sphingosine‐1‐phosphate receptor 1. FASEB J. 2016;30(6):2351‐2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanchez T. Sphingosine‐1‐phosphate signaling in endothelial disorders. Curr Atheroscler Rep. 2016. 18:31;18(6):1‐13. 10.1007/s11883-016-0586-1 [DOI] [PubMed] [Google Scholar]
- 44. Kappos L, Radue EW, O'Connor P, et al. FREEDOMS study group. A placebo‐controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387‐401. [DOI] [PubMed] [Google Scholar]
- 45. Kappos L, Bar‐Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double‐blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263‐1273. [DOI] [PubMed] [Google Scholar]
- 46. Hoch M, D'Ambrosio D, Wilbraham D, Brossard P, Dingemanse J. Clinical pharmacology of ponesimod, a selective S1P1 receptor modulator, after uptitration to supratherapeutic doses in healthy subjects. Eur J Pharm Sci. 2014;63:147‐153. [DOI] [PubMed] [Google Scholar]
- 47. Jain N, Bhatti T. Fingolimod‐associated macular edema: incidence, detection and management. Neurology. 2012;78:672‐680. [DOI] [PubMed] [Google Scholar]
- 48. Salvadori ML, Budde K, Charpentier B, et al. FTY720 0124 study group. FTY720 versus MMF with cyclosporine in de novo renal transplantation: a 1‐year, randomized controlled trial in Europe and Australasia. Am J Transplant. 2006;6:2912‐2921. [DOI] [PubMed] [Google Scholar]
- 49. Westhoff TH, Schmidt S, Glander P, et al. The impact of FTY720 (fingolimod) on vasodilatory function and arterial elasticity in renal transplant patients. Nephrol Dial Transplant. 2007;22:2354‐2358. [DOI] [PubMed] [Google Scholar]
- 50. Poirier B, Briand V, Kadereit D, et al. A G‐protein‐biased S1P1 agonist, SAR247799, protects endothelial cells without affecting lymphocyte numbers. Sci Sign. 2020;13:eaax8050. [DOI] [PubMed] [Google Scholar]
- 51. Graihle P, Boutarfa‐Madec A, Beauverger P, Janiak P, Parkar AA. A label‐free impedance assay in endothelial cells distinguishes between the activation and desensitization properties of clinical sphingosine‐1 phosphate receptor‐1 agonists. FEBS Open Bio. 2020:2010‐2020. 10.1002/2211-5463.12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bergougnan L, Armani S, Golor G, et al. First‐in‐human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple oral doses of SAR247799, a selective G‐protein‐biased Sphingosine‐1 phosphate receptor‐1 agonist for endothelial protection. Br J Clin Pharm. 2020:1‐14. 10.1111/bcp.14422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas N, Sweeney K, Somayaji V. Meta‐analysis of clinical dose‐response in a large drug development portfolio. Stat Biopharmaceutical Res. 2014;6:302‐317. [Google Scholar]
- 54. Clarkson P, Celermajer DS, Donald AE, et al. Impaired vascular reactivity in insulin dependent diabetes mellitus is related to disease duration and low‐density lipoprotein cholesterol levels. J Am Coll Cardiol. 1996;28(3):573‐579. [DOI] [PubMed] [Google Scholar]
- 55. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta‐analysis. J Am Heart Assoc. 2015:4. 10.1161/JAHA.115.002270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aversa A, Vitale C, Volterrani M, et al. Chronic administration of sildenafil improves markers of endothelial function in men with type 2 diabetes. Diabet Med. 2008;25(1):37‐44. [DOI] [PubMed] [Google Scholar]
- 57. Balzer J, Rassaf T, Heiss C, et al. Sustained benefits in vascular function through Flavanol‐containing cocoa in medicated diabetic patients. A double‐masked, randomized, controlled trial. J Am Coll Cardiol. 2008;51(22):2141‐2149. [DOI] [PubMed] [Google Scholar]
- 58. Desouza C, Parulkar A, Lumpkin D, Akers D, Fonseca V. Acute and prolonged effects of sildenafil on brachial artery flow‐mediated dilatation in type 2 diabetes. Diabetes Care. 2002;25(8):1336‐1339. [DOI] [PubMed] [Google Scholar]
- 59. Deyoung L, Chung E, Kovac JR, Romano W, Brock GB. Daily use of sildenafil improves endothelial function in men with type 2 diabetes. J Androl. 2012;33(2):176‐180. [DOI] [PubMed] [Google Scholar]
- 60. Halcox JP, Nour KR, Zalos G, et al. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40(7):1232‐1240. [DOI] [PubMed] [Google Scholar]
- 61. Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with cardiovascular risk. Eur Urol. 2005;47:214‐222. [DOI] [PubMed] [Google Scholar]
- 62. Shahin Y, Khan JA, Samuel N, Cheeler I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta‐analysis of randomised controlled trials. Atherosclerosis. 2011;216(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 63. Zhang L, Gong D, Li S, Zhou X. Meta‐analysis of the effects of statin therapy on endothelial function in patients with diabetes mellitus. Atherosclerosis. 2012;223(1):78‐85. [DOI] [PubMed] [Google Scholar]
- 64. Li S, Wu Y, Yu G, Xia Q, Xu Y. Angiotensin II receptor blockers improve peripheral endothelial function: a meta‐analysis of randomized controlled trials. PloS One. 2014. Mar 3;9(3):e90217. 10.1371/journal.pone.0090217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker fatty rat. Proc Soc Exp Biol Med. 1983;173(1):68‐75. [DOI] [PubMed] [Google Scholar]
- 66. Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000;49(5):684‐688. [DOI] [PubMed] [Google Scholar]
- 67. Ratcliffe B, Pawlak R, Morales F, Harrison C, Gurovich AN. Internal validation of an automated system for brachial and femoral flow mediated dilation. Clin Hypertens. 2017;23:17. 10.1186/s40885-017-0073-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Southan C, Sharman JL, Benson HE, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016;44(D1):D1054‐D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alexander SP, Christopoulos A, Davenport AP, et al. The concise guide to pharmacology 2019/20: G protein‐coupled receptors; 2019:S21‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Evans CE, Iruela‐Arispe ME, Zhao Y‐Y. Mechanisms of endothelial regeneration and vascular repair and their application to regenerative medicine. Am J Pathology. 2020. 10.1016/j.ajpath.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stirban A, Laude D, Elghozi JL, et al. Acute effects of sildenafil on flow mediated dilatation and cardiovascular autonomic nerve function in type 2 diabetic patients. Diabetes Metab Res Rev. 2009;25(2):136‐143. [DOI] [PubMed] [Google Scholar]
- 72. Halcox JP, Donald AE, Ellins E, et al. Endothelial function predicts progression of carotid intima‐media thickness. Circulation. 2009;119(7):1005‐1012. [DOI] [PubMed] [Google Scholar]
- 73. Ceconi C, Fox KM, Remme WJ, et al. ACE inhibition with perindopril and endothelial function. Results of a substudy of the EUROPA study: PERTINENT. Cardiovasc Res. 2007;73(1):237‐246. [DOI] [PubMed] [Google Scholar]
- 74. Taddei S, Virdis A, Ghiadoni L, et al. Effect of calcium antagonist or beta blockade treatment on nitric oxide‐dependent vasodilation and oxidative stress in essential hypertensive patients. J Hypertens. 2001;19(8):1379‐1386. [DOI] [PubMed] [Google Scholar]
- 75. Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142‐1148. [DOI] [PubMed] [Google Scholar]
- 76. Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109‐2122. [DOI] [PubMed] [Google Scholar]
- 77. Simic B, Hermann M, Shaw SG, et al. Torcetrapib impairs endothelial function in hypertension. Eur Heart J. 2012;33(13):1615‐1624. [DOI] [PubMed] [Google Scholar]
- 78. Lüscher TF, Taddei S, Kaski J‐C, et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal‐VESSEL randomized clinical trial. Eur Heart J. 2012;33(7):857‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schwartz GG, Olsson AG, Abt M, et al. Dal‐OUTCOMES investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089‐2099. [DOI] [PubMed] [Google Scholar]
- 80. Charakida M, Masi S, Lüscher TF, Kastelein JJ, Deanfield JE. Assessment of atherosclerosis: the role of flow‐mediated dilatation. Eur Heart J. 2010;31:2854‐2861. [DOI] [PubMed] [Google Scholar]
- 81. Kuno Y, Iyoda M, Shibata T, Hirai Y, Akizawa T. Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non‐insulin‐dependent Otsuka long‐Evans Tokushima fatty rats. Br J Pharmacol. 2011;162(6):1389‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin‐induced diabetic rats. Am J Nephrol. 2009;29(3):274‐282. [DOI] [PubMed] [Google Scholar]
- 83. Igarashi J, Michel T. Sphingosine‐1‐phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82(2):212‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Toblli JE, Cao G, Angerosa M, Rivero M. Long‐term phosphodiesterase 5 inhibitor administration reduces inflammatory markers and heat‐shock proteins in cavernous tissue of Zucker diabetic fatty rat (ZDF/fa/fa). Int J Impot Res. 2015;27(5):182‐190. [DOI] [PubMed] [Google Scholar]
- 85. El‐Mahdy NA, El‐Sayad ME, El‐Kadem AH. Combination of telmisartan with sildenafil ameliorate progression of diabetic nephropathy in streptozotocin‐induced diabetic model. Biomed Pharmacother. 2016;81:136‐144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Body weight and fructosamine changes in diabetic rats.
FIGURE S2. Effect of SAR247799 (1 and 5 mg), sildenafil (50 mg), or placebo on systolic (A) and diastolic (B) blood pressure and prehyperaemia brachial artery diameter (C) in type‐2 diabetes patients.
TABLE S1 Descriptive statistics of FMD (%) following 1 and 5 mg SAR247799, 50 mg sildenafil, and placebo measured at screening, baseline, Days 14, 21, 28, 35, and 42 (EOS); raw data and change from baseline.
TABLE S2 Mean estimate of FMD change vs baseline at individual time‐points (D14, D21, D28, D35, D42) and across repeated measurements (D14–42).
Data Availability Statement
All relevant preclinical and clinical data are provided within the paper and its supporting information files. Qualified researchers may request access to patient level data and related study documents including the clinical study report, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at www.clinicalstudydatarequest.com.


