Abstract
Objective
The best screening strategy for gestational diabetes mellitus (GDM) remains a topic of debate. Several organizations made a statement in favor of universal screening, but the volume of oral glucose tolerance tests (OGTT) required may burden healthcare systems. As a result, many countries still rely on selective screening using a checklist of risk factors, but reported diagnostic characteristics vary. Moreover, women's discomfort due to an OGTT is often neglected. Since 2017, obstetric healthcare professionals in a Dutch region assessed women's GDM risk with a prediction model and counseled those with an increased risk regarding an OGTT.
Methods
From 2017 to 2018, 865 women were recruited in a multicenter prospective cohort.
Results
In total, 385 women (48%) had an increased predicted GDM risk. Of all women, 78% reported that their healthcare professional discussed their GDM risk. Predicted GDM risks were positively correlated with conducting an OGTT.
Conclusion
Implementation of a GDM prediction model resulted in moderate rates of OGTTs performed in general, but high rates in high‐risk women. As 25% of women experienced discomfort from the OGTT, a selective screening strategy based on a prediction model with a high detection rate may be an interesting alternative to universal screening.
Study cohort registration
Netherlands Trial Register: NTR4143; http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4143.
Keywords: gestational diabetes mellitus, oral glucose tolerance test, prediction, pregnancy, screening
Synopsis
Implementing a gestational diabetes mellitus prediction model resulted in a majority of high‐risk women receiving an oral glucose tolerance test within the recommended gestational window.
1. INTRODUCTION
Gestational diabetes mellitus (GDM) is characterized by any new onset disturbances in blood glucose regulation during pregnancy. 1 Although it usually resolves after pregnancy, the condition is related to several complications for both mothers and their offspring, i.e. pre‐eclampsia, birth injury, cesarean section, large‐for‐gestational‐age infant, and neonatal hypoglycemia. 2
Diagnosis of GDM has been a topic of debate. Recent evidence indicates that risk of adverse perinatal outcomes increases at relatively mildly elevated blood glucose levels. 3 , 4 Moreover, risk of adverse outcomes falls with appropriate treatment in mild as well as severe hyperglycemia. 3 , 4 In concordance with these findings, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) proposed a one‐step approach to detect GDM by using a 75‐g oral glucose tolerance test (OGTT) with threshold values equaling or exceeding 5.1, 10.0, or 8.5 mmol/L for fasting, 1‐hour, and 2‐hour plasma glucose levels, respectively. 1
Universal screening, i.e. testing all pregnant women, can be expected to yield higher GDM detection rates than testing specific risk groups. Pregnant women, however, may experience the OGTT as burdensome as the procedure is time consuming and the concentrated glucose solution used can cause nausea and vomiting. 5 , 6 , 7 Furthermore, because of a lack of trial‐based cost‐effectiveness studies making use of the new diagnostic criteria, it is unclear which screening strategy is preferable. 8 Although several international guidelines promote universal screening, selective screening remains common practice in many developed countries including the Netherlands. 9 , 10 , 11
Women with an increased GDM risk can be identified either by a list comprising separate risk factors or by prediction models. Prediction models take into account the weighted risk of multiple factors and possible inter‐relations between them, allowing for a more personalized estimation of the absolute risk. 12 Furthermore, when using a prediction model any risk‐threshold can be selected, in contrast to current selective screening strategies. This makes it possible to adjust the tool to situation‐specific preferences, e.g. to enhance the detection rate or minimize the false positive rate.
Recently, healthcare professionals in the southeastern part of the Netherlands implemented an externally validated prediction model to assess women's risk of developing GDM during the first trimester of pregnancy. 13 The detection rate was set at 80%, leading to a false‐positive rate of 49%. After risk assessment by means of the model‐based prediction tool, women with an increased risk were offered an OGTT in a shared decision setting.
In this paper, we report on the degree of implementation of the prediction tool and determinants of OGTT use in a cohort of 805 women. We also assessed women's experiences with the OGTT procedure.
2. MATERIALS AND METHODS
In 2017, members of the Limburg Obstetric Consortium, located in the southeastern part of the Netherlands, adopted risk‐based care pathways. These pathways consist of basic prenatal care for low‐risk women and additional recommendations for women with an increased risk of one or more pregnancy‐related complications such as GDM. The ACCORD methodology followed in formulating these pathways and their content are reported elsewhere. 14 , 15
Women's GDM risk was assessed during the first prenatal visits by means of an online prediction tool. This tool embedded the externally validated model of van Leeuwen et al., 13 , 16 a prediction model based on maternal characteristics (age, body mass index, ethnicity, family history, and obstetric history). The consortium achieved consensus regarding a suitable risk‐threshold. A predicted risk of 3.5% or more was used as the cut‐off value to identify women with an increased GDM risk (sensitivity 80%, specificity 51%). 13
For women with an increased GDM risk estimation, additional risk‐based care included the recommendation of a 75‐g 2‐h OGTT within the gestational window of 24–28 weeks, in a shared decisional approach. The results of the OGTT were evaluated in accordance with the IADPSG criteria. 1
Data were collected as part of the Expect Study II, a multicenter prospective cohort study among pregnant women in the Netherlands. A detailed description of the study design has been published elsewhere. 14 In short, from 2017 to 2018, women were recruited during the first prenatal visit (<16 weeks of pregnancy) if their healthcare professional used the prediction tool. All women aged 18 years and older with a singleton pregnancy were eligible for inclusion. Study information was provided in Dutch only. For this paper, women diagnosed with overt diabetes mellitus, i.e. diabetes mellitus diagnosed before pregnancy regardless of the subtype, were excluded from the analyses. Additionally, we excluded women who were unable to have an OGTT because of bariatric surgery and women giving birth before 29+0 weeks of pregnancy.
Participating women were invited to complete four web‐based surveys: at enrollment, at 24 weeks of pregnancy, at 34 weeks of pregnancy, and postpartum (6 weeks after the due date). In case of no response, two automatic reminders were sent with 3‐day intervals and eventually women were contacted by phone. Women reporting preterm birth in survey two or three were automatically redirected to the postpartum survey. Besides the surveys, data of the prediction tool were logged and we retrieved the medical records of all enrolled women.
In the first survey, women were asked whether their healthcare professional had discussed their GDM risk. Furthermore, they were asked whether the option of OGTT was discussed during any of the first prenatal visits. Postpartum, women were asked whether they had undergone an OGTT. If so, they received additional questions: the gestational age at which the OGTT was performed, the amount of discomfort they experienced from the OGTT on a 10‐point Likert scale (1‐not unpleasant at all, to 10‐ extremely unpleasant), and whether they had any remarks regarding the OGTT procedure.
To estimate the level of concern regarding GDM and related complications, women were asked during the first survey how often they worried about GDM and macrosomia. Women could choose from not at all, sometimes, regularly, and often. Answers were transformed to a four‐point scale (0‐ not at all to 3‐ often).
If women did not complete the postpartum survey, or when they did not recall the gestational age at the time of the OGTT, OGTT dates were retrieved from their medical records. Adherence to GDM risk‐based care was defined as an OGTT performed before 29+0 completed weeks of pregnancy in women with an increased predicted GDM risk (≥3.5%; estimated by the prediction tool). As OGTTs performed after the recommended gestational window were more likely to be motivated by symptoms arising during the pregnancy (e.g. suspected macrosomia, raised amniotic fluid) rather than the predicted risk at the first prenatal visit, these cases were classified as non‐adherent to GDM risk‐based care.
The Medical Ethics Committee of the Maastricht University Medical Center evaluated the study protocol and concluded that the Expect Study does not fall under the Medical Research Involving Human Subjects Act (METC‐17‐4‐057). Online informed consent was obtained from all participants.
With respect to the predicted risk (low risk/increased risk) we calculated proportions of women who were reported to have communicated with their obstetric healthcare provider about their GDM risk and about an OGTT. Proportions of women who received an OGTT were also calculated. Furthermore, we plotted the association of the predicted risk with the proportion who communicated risk, communicated the option of an OGTT, and underwent an OGTT using nonparametric local weighted regression (Loess regression).
By multiple logistic regression, determinants of performing an OGTT within the recommended gestational window were analyzed. This analysis was restricted to women with an increased GDM risk, who reported that their healthcare professional discussed the option of an OGTT with them, as only these women are able to make an informed decision. In the analysis, we corrected for the predicted GDM risk (as a continuous variable). Factors of interest for the primary model were type of healthcare professional during the first prenatal visits (midwife or gynecologist), tertiary educational level (yes/no), and medical history of polycystic ovarian syndrome (yes/no). Additionally, the level of women's concerns regarding a pregnancy complicated by GDM or macrosomia was considered (continuous), and we performed a multiple logistic regression in the subgroup of nulliparous women.
Using the likelihood‐ratio test we analyzed whether our primary model could be improved by separately adding the factors used to predict the GDM risk instead of the resulting GDM risk estimate itself: body mass index (continuous), Caucasian ethnicity (yes/no), family history of diabetes mellitus type 2 (yes/no), and obstetric history (nulliparous, previous GDM, no previous GDM). If the likelihood‐ratio test was significant, this would indicate that one or more predictors had an additional effect upon adherence rates besides the estimated GDM risk.
Statistical analyses were performed using R statistical software version 3.6.0. 17 We checked for collinearity using variance inflation factors and condition indices. A subgroup analysis was carried out in nulliparous women to see whether results were parity‐dependent.
3. RESULTS
A flowchart of study enrolment is shown in Figure 1. In total, 865 women gave informed consent. Sixty women (7%) were excluded, 12 (1.3%) because of an incomplete survey. As a result, 805 women (93%) were available for the analyses in this paper. Of these women, 385 (48%) were identified as having an increased GDM risk. The population characteristics of the Expect Study II cohort are displayed in Table 1.
FIGURE 1.
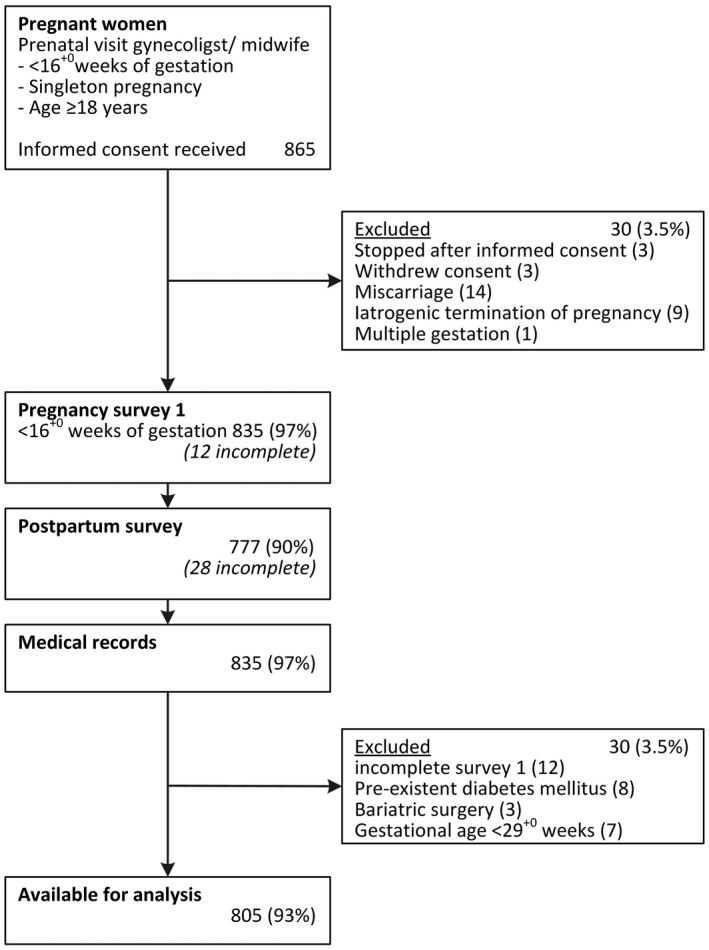
Flowchart of participant enrollment Expect Study II
TABLE 1.
Baseline characteristics of the Expect Study II cohort a
| Baseline characteristics <16 weeks of pregnancy | Expect II cohort (n = 805) |
|---|---|
| Age, years | 30.7 ± 4.1 |
| Ethnicity | |
| Caucasian | 788 (97.9) |
| Other | 17 (2.1) |
| Educational level | |
| Primary or secondary | 327 (40.6) |
| Tertiary level of education | 478 (59.4) |
| BMI | 24.7 ± 4.6 |
| Conception | |
| Natural | 732 (90.9) |
| Ovulation induction | 35 (4.3) |
| In vitro fertilization | 38 (4.7) |
| Obstetric history | |
| Nulliparous | 405 (50.3) |
| Prior GDM | 17 (2.1) |
| No prior | 383 (47.6) |
| Family history of diabetes mellitus | 146 (18.1) |
| Polycystic ovarian syndrome | 49 (6.1) |
| Predicted GDM risk, % | 3.4 (2–6) |
| Increased GDM risk | 385 (47.8) |
| Counseling of GDM risk | |
| By midwife | 602 (74.8) |
| By obstetrician | 203 (25.2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); GDM, gestational diabetes mellitus.
Values are presented as mean ± standard deviation, median (interquartile range), or as number (percentage).
Table 2 shows the numbers of women who reported having discussed their GDM risk with their obstetric care provider, having discussed the option of an OGTT, and having undergone an OGTT. In total, 627 women (78%) reported that their GDM risk had been discussed during the first prenatal visits. In case of an increased GDM risk (n = 385), 292 women (76%) discussed the option of an OGTT. Moreover, of women with an increased GDM risk, 226 (59%) underwent the OGTT before 29 completed weeks of pregnancy. Sixty‐six women (8.2%) were diagnosed with GDM, of whom 51 (77%) were identified with an increased GDM risk.
TABLE 2.
Adherence indicators for all women and for women with low and increased gestational diabetes mellitus risk a
| All women (n = 805) | GDM risk <3.5% (n = 420) | GDM risk ≥3.5% (n = 385) | |
|---|---|---|---|
| GDM risk discussed | |||
| Yes | 627 (77.9) | 291 (69.3) | 336 (87.3) |
| No | 151 (18.8) | 110 (26.2) | 41 (10.6) |
| Uncertain | 27 (3.4) | 19 (4.5) | 8 (2.1) |
| OGTT discussed | |||
| Yes | 389 (48.3) | 97 (23.1) | 292 (75.8) |
| No | 416 (51.7) | 323 (76.9) | 93 (24.2) |
| OGTT performed | |||
| No OGTT | 448 (55.8) | 328 (78.1) | 120 (31.2) |
| Any OGTT | 357 (44.3) | 92 (21.9) | 265 (68.8) |
| Per protocol | 283 (35.2) | 57 (13.6) | 226 (58.7) |
| GDM diagnosed | |||
| Yes | 66 (8.2) | 15 (3.6) | 51 (13.2) |
| No | 739 (91.8) | 405 (96.4) | 334 (86.8) |
Abbreviations: GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test.
Values are presented as number (percentage).
In total, 159 women (41%) with an increased GDM risk did not receive an OGTT before 29 weeks of pregnancy. Of these, 93 (58%) reported never having discussed an OGTT with their healthcare professional and 18 (11%) received the OGTT after the recommended gestational window.
Figure 2 shows the proportions of women discussing the GDM risk, the option of an OGTT, and conduction of an OGTT by predicted GDM risk. This graph points to a positive linear relationship between the estimated GDM risk and the likelihood of discussing the risk, discussing an OGTT, or performing an OGTT.
FIGURE 2.
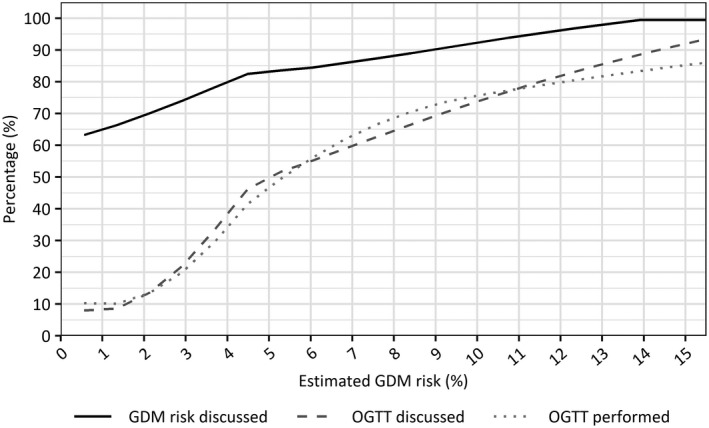
Discussion rates of gestational diabetes mellitus (GDM) risk and of an oral glucose tolerance test (OGTT), and proportion of women who have undergone an OGTT <29+0 weeks per predicted GDM risk
We performed a multiple logistic regression in the subgroup with an increased GDM risk who reported having discussed the option of an OGTT, to identify determinants of performing an OGTT within the recommended gestational window. The results indicate that, as expected from Figure 2, the predicted GDM risk is positively correlated with the adherence to OGTT recommendations (adjusted odds ratio [aOR] 1.10; 95% confidence interval [CI] 1.04–1.18) (Table 3). Furthermore, our results may be suggestive for an effect due to polycystic ovarian syndrome status (aOR 0.36; 95% CI 0.13–1.00). Adding the predictors contributing to the predicted risk separately did not result in a better model of adherence (χ2 = 3.42, df = 4, p = 0.49).
TABLE 3.
Multiple logistic regression of potential determinants of OGTT testing among women with an increased risk with whom an OGTT was discussed
| Determinants | No. of participants | No. with OGTT performed, n (%; 95% CI) | Unadjusted OR (95% CI) | Adjusted OR a (95% CI) |
|---|---|---|---|---|
| All | 292 | 226 (77; 72–82) | – | – |
| Predicted GDM risk (%) | 1.11 (1.05–1.20) | 1.10 (1.04–1.18) | ||
| Polycystic ovarian syndrome | ||||
| Yes | 24 | 15 (62; 43–79) | 0.45 (0.19–1.12) | 0.36 (0.13–1.00) |
| No | 268 | 211 (79; 73–83) | 1 (Reference) | 1 (Reference) |
| Educational level | ||||
| Primary or secondary | 132 | 108 (82; 74–87) | 1 (Reference) | 1 (Reference) |
| Tertiary | 160 | 118 (74; 66–80) | 0.62 (0.35–1.09) | 0.79 (0.43–1.43) |
| Concerns regarding GDM | 1.32 (0.98–1.80) | 1.21 (0.87–1.70) | ||
| Concerns regarding macrosomia | 0.97 (0.68–1.42) | 0.89 (0.60–1.35) | ||
| Counseling of GDM risk | ||||
| by midwife | 201 | 152 (76; 69–81) | 1 (Reference) | 1 (Reference) |
| by obstetrician | 91 | 74 (81; 72–88) | 1.40 (0.77–2.66) | 1.50 (0.77–3.08) |
Abbreviations: CI, confidence interval; GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test; OR odds ratio.
Odds ratios adjusted for variables listed in left column.
A subgroup analysis performed among nulliparous women did not result in essential differences except for women's concerns regarding a pregnancy complicated by GDM. In the subgroup analysis the level of concern was related with performing an OGTT (aOR 1.86; 95% CI 1.33–2.64).
Figure 3 displays the results on the experienced discomfort of the OGTT, as measured with a 10‐point Likert scale. With a median score of 3.5 (interquartile range 2–6) the experienced discomfort seems to be small for most women. On the other hand, 25% of women who had an OGTT experienced it as uncomfortable with scores ranging from 6 to 10. Moreover, 48 women left a remark regarding their experience. The OGTT was addressed by these women as very unpleasant because it was perceived as either time‐consuming, or invoked nausea or dizziness caused by fasting.
FIGURE 3.
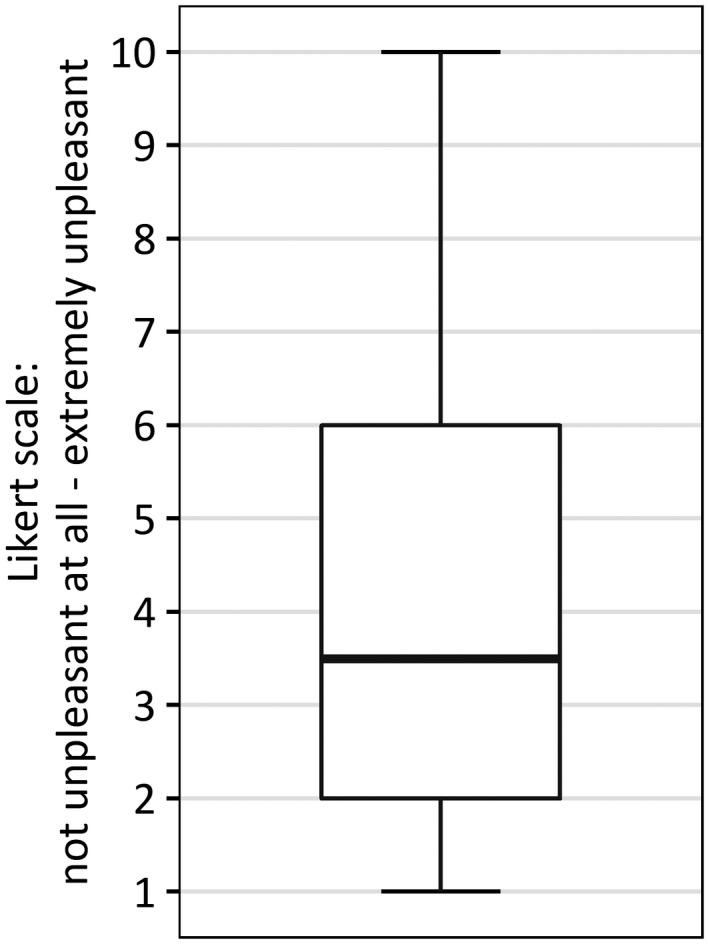
Boxplot of women's discomfort caused by the oral glucose tolerance test expressed at a 10‐point Likert scale
4. DISCUSSION
Healthcare professionals discussed the estimated GDM risk with 78% of the women. In case of an increased risk (n = 385), the OGTT was discussed with 76% of the women (n = 292). The OGTT was eventually performed in 59% (n = 226) of the women with an increased GDM risk. Conducting an OGTT within the recommended gestational window was positively related with predicted GDM risk (aOR 1.10; 95% CI 1.04–1.18).
Strengths of our study include its design, a prospective multicenter cohort, and the completeness of data. In the Netherlands, most women initially receive prenatal care from autonomous midwives. 18 For this reason, recruitment by multiple centers (hospitals as well as numerous midwifery clinics) was essential. The use of multiple web‐based questionnaires at intervals, combined with medical records and data from our prediction tool, resulted in high data quality with low numbers of missing data. Still, it could be possible that some women did not recall having discussed the GDM risk or the OGTT with their healthcare professional, despite having talked about it.
Since the Expect Study II is focused on analyzing the impact and results of risk‐based care, only women for whom the prediction tool was used were eligible for inclusion. Use of our prediction tool as inclusion method enabled us to link the healthcare services reported by women to their individual GDM risk estimate. All obstetric healthcare professionals of our region committed themselves to use the prediction tool. Nevertheless, this may have introduced a selection bias, as proactive healthcare professionals may be overrepresented among the professionals who use our prediction tool. The intensive usage of the prediction tool throughout the region and the multitude of collaborating centers diminishes the potential influence of selection bias.
To ensure a heterogeneous population, inclusion and exclusion criteria were kept as broad as possible. Still, women of Caucasian origin were overrepresented in our cohort. Furthermore, the majority of women had a tertiary educational level, which may imply an above‐average degree of health literacy. As impaired health literacy is correlated with non‐adherence, 19 adherence rates in our study may be somewhat overestimated.
In our study, 15 (3.6%) women with a low GDM risk were eventually diagnosed with GDM. These women constitute 23% of the detected GDM cases in our cohort. The GDM diagnosis in these women probably resulted from clinical symptoms arising during pregnancy (e.g. fetal macrosomia or polyhydramnios). As no universal screening was performed, it is possible that some GDM cases have remained undiagnosed. However, recent reported prevalence rates in Europe resulting from universal screening using the IADPSG criteria vary from 7.4% to 12.5%. 20 , 21 , 22 This suggests that the number of missed cases in our cohort, with a prevalence rate of 8.2%, was probably limited.
Although the general adherence rate of 59% is in line with previous studies reporting adherence rates regarding selective screening for GDM, 23 , 24 it remains suboptimal. On the other hand, GDM risk estimates were discussed with the vast majority of women and risk estimates were clearly related to the adherence rate. This may suggest that for lower risk estimates, healthcare professionals and pregnant women consciously chose not to perform an OGTT rather than that they forgot the OGTT.
Most women who did not have an OGTT within the gestational window reported never having discussed an OGTT with their healthcare professional. This may indicate that the healthcare professional has a key role in whether women receive an OGTT within the recommended gestational window. The discomfort of the OGTT experienced by women may be another potential barrier decreasing adherence rates. Future qualitative research, for example with the aid of focus groups among both healthcare professionals as well as pregnant women, may improve our insight and understanding regarding the barriers at play when implementing a GDM screening strategy.
Currently, there is a great variety of screening practices for GDM across Europe. 9 Although, several institutes made a statement in favor of universal screening using the IADPSG one‐step diagnostic criteria, many European countries still rely on selective screening strategies. 9 Arguments in favor of universal screening include a high detection rate and a timely diagnosis of GDM, which could eventually reduce both maternal and perinatal morbidity. 25 Furthermore, women with a history of GDM are at risk of developing type 2 diabetes. Identification of GDM is a reason for monitoring and (continued) lifestyle interventions after pregnancy. 26 However, the impact of universal screening on healthcare systems as well as the cost‐effectiveness and additional benefits regarding long‐term health outcomes remain a topic of debate. 8 , 27 , 28 , 29
Global increases in age and weight, as well as the correlated prevalence of GDM, have regularly been used as arguments emphasizing the need for universal screening in pregnant women. 10 , 25 Although a reasonable argument at population level, it is less logical for an individual woman. A pregnant woman in 2019 likely has the same GDM risk as a woman in 2000 with similar characteristics (e.g. age, weight, medical history). Thus, recommendation of an OGTT solely because of increased global incidence rates to this particular woman makes less sense. Another disadvantage of universal screening is that it bypasses women's feelings and thoughts regarding an OGTT, which is experienced as uncomfortable by a substantial proportion of women. Nevertheless, most statements advocating universal screening do not mention the OGTT itself as a potential burden. 10 , 29 , 30
On the other hand, selective screening strategies usually do not have a 100% detection rate and so will automatically result in false negatives and, consequently, missed GDM cases. For selective screening strategies, there will always be a trade‐off between a reduction of the false‐positive rate (unnecessary OGTTs) and the false‐negative rate (missed GDM cases). Selective screening by using a prediction tool has the advantage that the GDM risk can be taken into account. Calculating absolute GDM risks empowers pregnant women to make an informed decision, together with their healthcare professional, by enabling them to weigh the possible advantages and disadvantages for their individual situation.
In the present study, a prediction tool with a high detection rate was used for selective GDM screening. The majority of women (76%) reported that their healthcare professional discussed the outcomes of the prediction tool with them. On average, 59% of women with an increased GDM risk received an OGTT within the recommended gestational window. Timely receipt of an OGTT was strongly associated with predicted GDM risk resulting in high adherence rates among women with the highest risk estimates.
The OGTT was experienced as uncomfortable by 25% of the women. Using a prediction tool for selective GDM screening, allows women and healthcare professionals to make a well‐considered, informed decision. A selective screening strategy with a high detection rate and a sizeable proportion of test negatives reduces the amount of OGTTs performed in low‐risk women, who are least likely to benefit from an OGTT test. Future qualitative research is necessary to improve our insights regarding barriers and facilitators at play within GDM screening.
AUTHOR CONTRIBUTIONS
The Expect Study was designed by LS, HS, and MS. PvM elaborated and carried out the Expect Study II under the supervision of LS and HS. PvM conducted the analyses, interpreted the data, and drafted the manuscript. LM, LS, and HS contributed to the interpretation of the outcomes and critically reviewed draft versions. LW contributed to the statistical analysis of the data and critically reviewed draft versions. IvD, EW, MZ, IZ, and MS collaborated in data collection and critically reviewed draft versions. All authors gave approval of the final version of the manuscript.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Funding information
Financial support was provided entirely by a grant from ZonMw (The Netherlands Organization for Health Research and Development; federal funding). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the report.
REFERENCES
- 1. International Association of Diabetes and Pregnancy Study Groups . Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. NEJM. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 3. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. NEJM. 2005;352(24):2477–2486. [DOI] [PubMed] [Google Scholar]
- 4. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. NEJM. 2009;361(14):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nielsen KK, de Courten M, Kapur A. The urgent need for universally applicable simple screening procedures and diagnostic criteria for gestational diabetes mellitus–lessons from projects funded by the World Diabetes Foundation. Glob Health Action. 2012;5:17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanna F, Peters J. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19(5):351–358. [DOI] [PubMed] [Google Scholar]
- 7. Görig T, Schneider S, Bock C, et al. Screening for gestational diabetes mellitus in Germany: a qualitative study on pregnant women׳ s attitudes, experiences, and suggestions. Midwifery. 2015;31(11):1026–1031. [DOI] [PubMed] [Google Scholar]
- 8. Tieu J, McPhee AJ, Crowther CA, et al. Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health. Cochr Database Syst Rev. 2017;8:Cd007222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benhalima K, Mathieu C, Van Assche A, et al. Survey by the European Board and College of Obstetrics and Gynaecology on screening for gestational diabetes in Europe. Eur J Obstet Gynecol Reprod Biol. 2016;201:197–202. [DOI] [PubMed] [Google Scholar]
- 10. Hod M, Pretty M, Mahmood T. Joint position statement on universal screening for GDM in Europe by FIGO, EBCOG and EAPM. Eur J Obstet Gynecol Reprod Biol. 2018;228:329–330. [DOI] [PubMed] [Google Scholar]
- 11. NVOG. [Dutch association for obstetrics and gynecology] : Guideline Diabetes Mellitus and pregnancy (3.0). 2018.
- 12. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meertens LJE, Scheepers HCJ, van Kuijk SMJ, et al. External validation and clinical utility of prognostic prediction models for gestational diabetes mellitus: a prospective cohort study. Acta Obstet Gynecol Scand. 2020;99(7):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Montfort P, Willemse PPMJ, Dirksen DC, et al. Implementation and effects of risk‐dependent obstetric care in the Netherlands (Expect Study II): protocol for an impact study. JMIR Res Protoc. 2018;7(5):e10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemmens SM. Agreement conform current operational rules and directives (ACCORD): a novel tool to reach multidisciplinary consensus. J Womens Health Gyn. 2019;5:1–11. [Google Scholar]
- 16. Van Leeuwen M, Opmeer B, Zweers E, et al. Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history. BJOG. 2010;117(1):69–75. [DOI] [PubMed] [Google Scholar]
- 17. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2019. [Google Scholar]
- 18. Visser GHA. Obstetric care in the Netherlands: relic or example? JOGC. 2012;34(10):971–975. [DOI] [PubMed] [Google Scholar]
- 19. Lupattelli A, Spigset O, Nordeng H. Adherence to medication for chronic disorders during pregnancy: results from a multinational study. Int J Clin Pharm. 2014;36(1):145–153. [DOI] [PubMed] [Google Scholar]
- 20. Helseth R, Salvesen O, Stafne SN, et al. Gestational diabetes mellitus among Nordic Caucasian women: prevalence and risk factors according to WHO and simplified IADPSG criteria. Scand J Clin Lab Invest. 2014;74(7):620–628. [DOI] [PubMed] [Google Scholar]
- 21. O'Sullivan EP, Avalos G, O'Reilly M, et al. Atlantic DIP: the prevalence and consequences of gestational diabetes in Ireland. Ir Med J. 2012;105(5 Suppl):13–15. [PubMed] [Google Scholar]
- 22. Benhalima K, Van Crombrugge P, Moyson C, et al. Risk factor screening for gestational diabetes mellitus based on the 2013 WHO criteria. Eur J Endocrinol. 2019;180(6):353–363. [DOI] [PubMed] [Google Scholar]
- 23. Murphy NM, McCarthy FP, Khashan AS, et al. Compliance with National Institute of Health and Care Excellence risk‐based screening for Gestational Diabetes Mellitus in nulliparous women. Eur J Obstet Gynecol Reprod Biol. 2016;199:60–65. [DOI] [PubMed] [Google Scholar]
- 24. Persson M, Winkvist A, Mogren I. Surprisingly low compliance to local guidelines for risk factor based screening for gestational diabetes mellitus ‐ a population‐based study. BMC Pregnancy Childbirth. 2009;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapur A, Mahmood T, Hod M. The unmet need for universal testing for hyperglycaemia in pregnancy and the FIGO guideline. BJOG. 2018;125(5):529–531. [DOI] [PubMed] [Google Scholar]
- 26. Gabbe SG, Landon M, Warren‐Boulton E, et al. Promoting health after gestational diabetes: a National Diabetes Education Program call to action. Obstet Gynecol. 2012;119(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ. 2014;348:g1567. [DOI] [PubMed] [Google Scholar]
- 28. Farrar D, Simmonds M, Griffin S, et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta‐analyses and an economic evaluation. Health Technology Assess. 2016;20(86):1–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moyer VA. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(6):414–420. [DOI] [PubMed] [Google Scholar]
- 30. Committee on Practice Bulletins—Obstetrics . ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–e64. [DOI] [PubMed] [Google Scholar]


