Abstract
Introduction
We investigate dementia risk in older adults with different disease patterns and explore the role of inflammation and apolipoprotein E (APOE) genotype.
Methods
A total of 2,478 dementia‐free participants with two or more chronic diseases (ie, multimorbidity) part of the Swedish National study on Aging and Care in Kungsholmen (SNAC‐K) were grouped according to their multimorbidity patterns and followed to detect clinical dementia. The potential modifier effect of C‐reactive protein (CRP) and apolipoprotein E (APOE) genotype was tested through stratified analyses.
Results
People with neuropsychiatric, cardiovascular, and sensory impairment/cancer multimorbidity had increased hazards for dementia compared to the unspecific (Hazard ration (HR) 1.66, 95% confidence interval [CI] 1.13‐2.42; 1.61, 95% CI 1.17‐2.29; 1.32, 95% CI 1.10‐1.71, respectively). Despite the lack of statistically significant interaction, high CRP increased dementia risk within these patterns, and being APOE ε4 carriers heightened dementia risk for neuropsychiatric and cardiovascular multimorbidity.
Discussion
Individuals with neuropsychiatric, cardiovascular, and sensory impairment/cancer patterns are at increased risk for dementia and APOE ε4, and inflammation may further increase the risk. Identifying such high‐risk groups might allow tailored interventions for dementia prevention.
Keywords: dementia, genetics, inflammation, multimorbidity patterns
1. INTRODUCTION
As a consequence of the worldwide aging of the population, dementia will increasingly become a challenge faced by individuals and societies. 1 Owing to the current lack of curative drugs, dementia prevention has become an especially relevant strategy. 2 As such, the identification of individuals at higher risk of dementia is a clinical and public health priority. 3
Several longitudinal studies have identified specific diseases (eg, diabetes, atrial fibrillation, depression) as at‐risk conditions for increased dementia incidence. 4 , 5 However, it is relatively uncommon for older adults to be affected by only one chronic disease. In fact, up to 90% of adults 60 years of age or older adults have two or more chronic diseases, 6 a condition referred to as multimorbidity. Multimorbidity has been associated with incident mild cognitive impairment 7 and greater brain pathology burden. 8 However, there is scanty and contrasting evidence of how clusters of diseases in individuals with multimorbidity are differentially associated with the occurrence of dementia. 9 Some diseases tend to aggregate in the same person due to similar pathophysiological mechanisms and/or shared risk factors, 10 which leads to multimorbidity patterns characterized by chronic disorders that systematically cluster together beyond chance. The identification of such patterns has provided useful clinical insight, 11 suggesting a potentially differential impact of specific multimorbidity patterns on dementia development.
One of the factors plausibly implicated in the association between specific multimorbidity patterns and dementia is inflammation. Systemic inflammation may accelerate the progression of neurodegeneration and vascular pathology in the brain, 12 and an imbalance between pro‐ and anti‐inflammatory responses is described in older individuals. 13 Likewise, as the role of the apolipoprotein E gene variant (APOE ε4) on dementia risk is well known, it is plausible to hypothesize that the combination of specific multimorbidity patterns and APOE ε4 allele puts individuals at even greater risk of dementia development.
In this study, we aimed to quantify the impact of specific patterns of multimorbidity on dementia risk, and to explore the role played by inflammation and APOE genotype in such an association over 12 years.
RESEARCH IN CONTEXT
Systematic review. After conducting a systematic review of the literature (PubMed and Embase) we found a limited number of studies investigating either cross‐sectional associations or the effect of the number of co‐existing diseases on dementia. No studies have been conducted to date on the impact of specific multimorbidity patterns on incident dementia.
Interpretation. Over a 12‐year period, people with neuropsychiatric, cardiovascular, and sensory impairment/cancer multimorbidity showed the highest hazards of dementia, and the presence of inflammation and APOE ε4 allele further increased this association. Identifying multimorbidity patterns in the presence of inflammation, and among APOE ε4 carriers, might allow for more tailored clinical interventions aimed at dementia prevention.
Future directions. Future studies specifically designed to evaluate the interplay between aging‐related inflammatory processes, genetic predisposition, multimorbidity patterns, and dementia are needed to explore the biological mechanisms behind these associations.
2. METHODS
2.1. Study population
Data were gathered from the Swedish National study on Aging and Care in Kungsholmen (SNAC‐K), an ongoing population‐based study. 14 SNAC‐K includes a random sample of community‐dwelling and institutionalized adults aged 60 years and older, living in the Kungsholmen district of Stockholm, Sweden. At baseline (2001 to 2004), a random sample of 5111 people from 11 age cohorts were invited to participate in the study. Of the 4590 eligible individuals, 3363 were examined (participation rate: 73%). Younger participants (age <78 years) were followed up every 6 years, and the older (age ≥78 years) every 3 years. In the present study, we identified a dementia‐free cohort of 2622 persons affected by multimorbidity (two or more chronic diseases) at baseline (Figure S1).
The Regional Ethical Review Board in Stockholm, Sweden approved the protocols of SNAC‐K. All participants, or next of kin for cognitively impaired individuals, provided written informed consent.
The results of this study are reported following the strengthening the reporting of observational studies in epidemiology (STROBE) recommendations.
2.2. Data collection
At each study wave, SNAC‐K participants underwent a comprehensive clinical and functional assessment by trained physicians, nurses, and psychologists, following standard procedures. Home visits were carried out for those who agreed to participate but were unable to travel to the research center.
2.2.1. Covariates
Participants’ sociodemographic information (ie, age, sex, education, and civil status) were collected during nurses’ interviews. Educational attainment was categorized as elementary, high school, and university or higher, indicating the highest level of education attained. Civil status was categorized as unmarried, married, divorced, and widowed. Body mass index (BMI) was obtained by dividing participants’ weight by their squared height (kg/m2). A BMI <18.5 kg/m2 was considered as a proxy of malnutrition. 15 The Mini‐Mental State Examination (MMSE) score was used as a measure of global cognition.
2.2.2. Potential modifiers
Serum C‐reactive protein (CRP) was measured at Karolinska Hospital, Stockholm, through an assay with a lower detection limit of 5 mg/L (ie, low‐sensitivity test). Consequently, such cut‐off was used to define low versus high CRP concentrations. 16 DNA was extracted from peripheral blood samples and APOE alleles were genotyped. Based on the APOE alleles, participants were categorized as ε4 carriers (at least one ε4 allele) versus non‐carriers. Serum CRP levels and APOE genotype were considered at baseline.
2.2.3. Chronic disease assessment
Details on definition and classifications of diseases are reported elsewhere. 6 Briefly, physicians collected information on diagnoses via physical examination, medical history, self‐reported information, and/or proxy interviews. Clinical parameters, lab tests, medication, and inpatient and outpatient care data were also used to identify specific conditions. All diagnoses were coded according to the International Classification of Diseases, Tenth Revision (ICD‐10) and classified into 60 chronic disease categories in accordance with a clinically driven methodology. Drugs were classified and coded in accordance with the Anatomical Therapeutic Chemical (ATC) classification. Chronic diseases were considered at baseline.
2.2.4. Dementia diagnosis
The diagnosis of clinical dementia was made in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria, following a three‐step procedure. First, a preliminary diagnosis was made by the examining physician, followed by a second preliminary diagnosis by a reviewing physician also involved in data collection. This process was blinded to the diagnosis of the first physician. In cases of disagreement between the first and the second diagnoses, the final diagnosis was made by a neurologist who was external to the data collection process (LF, GG). For those people who died between the follow‐up assessments, we gathered additional clinical information for the diagnosis of dementia from the clinical charts and medical records, the discharge diagnoses of the National Patient Register, and the Swedish National Cause of Death Register.
2.3. Statistical analysis
2.3.1. Patterns identification
Multimorbidity patterns at baseline were identified using a fuzzy c‐means cluster analysis algorithm, which allows for the clustering individuals based on their underlying combinations of chronic diseases. 10 Thirty‐five of 60 individual chronic disease categories were included in the cluster analysis; those with a prevalence of <2% were excluded to avoid statistical noise, and therefore, spurious findings in the models (Table S1). To further characterize the multimorbidity patterns, observed/expected ratios were calculated by dividing the prevalence of a given disease within a cluster by its prevalence in the overall population. Disease exclusivity, defined as the fraction of participants with the disease included in the cluster over the total number of participants with the disease, was also calculated. Diseases with both observed/expected ratio ≥2 and exclusivity ≥25% were considered as characterizing a given cluster. 17 , 18 Such criteria were used to name the multimorbidity patterns based on the diseases that characterized them. The fuzzy c‐means algorithm assigns a probability of cluster membership for all individuals within each cluster; however, participants were finally allocated a single cluster based on their highest membership probability. Details on the methodology are reported in the Supplementary Text and Table S3. The association between multimorbidity patterns and dementia was tested through Cox regression models, and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were obtained. The proportional hazard assumption was assessed by regressing the scaled Schoenfeld residuals against survival time. No deviation from the proportional hazard assumption was detected. Follow‐up time was estimated as the time from study entry until dementia diagnosis, death, or last examination, using whichever occurred first. Interactions between APOE genotype, CRP levels, and multimorbidity patterns were tested and stratified analyses were conducted.
2.3.2. Sensitivity analyses
To test the strength and consistency of our results, we repeated the Cox regression models, excluding those people who developed dementia within the first 6 years of follow‐up. In addition, to investigate the association of multimorbidity clusters with dementia in comparison with non‐multimorbid participants, we repeated the survival analysis including multimorbidity‐free individuals (N = 420) as reference group.
The proportion of individuals with missing data was 5.5% for serum CRP, 8.6% for APOE genotype, and 1% for civil status and education. A complete case data analysis was carried out based on 88.0% of the cohort. Those with missing data in these variables were more likely to be older, female, and less educated than those with no missing data at baseline.
A two‐tailed P‐value < 0.05 was considered statistically significant in all analyses. All analyses were performed using Stata version 15 (StataCorp, Texas, USA).
3. RESULTS
3.1. Patterns identification
At baseline, the following multimorbidity patterns were identified: neuropsychiatric (7.6%), cardiovascular (9.5%), sensory impairment/cancer (14.8%), and respiratory/metabolic/musculoskeletal multimorbidity (20.8%). The remaining 47.3% of the population was grouped in a cluster containing diseases that were not overexpressed in relation to the overall population prevalence, and thus was named “unspecific multimorbidity.” This pattern was considered as reference group because it includes individuals with the lowest mean number of chronic diseases and CRP level. In addition, in previous studies by our group, we showed that individuals in the unspecific pattern were also less disabled that those in the other patterns 10 and presented the lowest 6‐ and 12‐year crude mortality. 19 (Table S2 reports the list of chronic diseases included in each pattern.)
During the 12‐year follow‐up (mean 8.4 years ± 3.9) of the 2622 participants, 1003 died and 466 dropped out (Figure S1). Those who dropped out were more likely to be younger (difference: −1.49 years; 95% CI −2.35, −0.46), and with a lower burden of chronic diseases (difference: −0.34; 95% CI −1.54, −0.14), than those who participated in at least two assessments. In addition, they were more likely to belong to the unspecific multimorbidity pattern (50% vs 46%).
Of the 2478 participants with complete data, 506 (20%) developed dementia; among them, 383 were identified during SNAC‐K assessments and 123 based on clinical charts and the Cause of Death Register. Participants who developed dementia were older and more frequently women, with lower educational level, a greater number of chronic conditions, lower MMSE score, and more likely to be APOE ε4 allele carriers (P < 0.001 for all).
3.1.1. Baseline characteristics
At study entry, the mean (SD) age of the population was 75.0 years (10.4); 64.3% were women; 83.2% had high school or university level education; and 56.0% were unmarried, divorced, or widowed. Baseline sample characteristics by multimorbidity pattern are shown in Table 1.
TABLE 1.
Baseline characteristics of study sample by multimorbidity patterns
| Total sample (N = 2,478) | Neuro‐ psychiatric (n = 183) | Cardiovascular (n = 243) | Sensory impairment/cancer (n = 384) | Respiratory/metabolic/musculoskeletal (n = 503) | Unspecific (n = 1,165) | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 75.0 (10.4) | 72.5 (10.0) | 82.4 (8.5) | 83.4 (8.8) | 71.6 (8.9) | 72.6 (9.5) |
| Female, % | 64.3 | 70.5 | 60.0 | 67.2 | 59.6 | 65.3 |
| Education, % | ||||||
| Elementary | 17.0 | 13.7 | 26.0 | 21.5 | 18.3 | 13.4 |
| High school | 51.6 | 48.6 | 55.4 | 53.9 | 51.8 | 50.6 |
| University | 31.4 | 37.7 | 18.6 | 24.6 | 29.9 | 36.0 |
| Civil status, | ||||||
| Partnered, % | 44.2 | 39.6 | 40.0 | 30.3 | 47.7 | 49.7 |
| APOE ε4 allele, % | 29.2 | 27.3 | 23.9 | 24.8 | 27.7 | 32.6 |
| Serum CRP > 5 mg/L, % | 21.9 | 26.0 | 30.6 | 25.1 | 28.8 | 15.6 |
| Malnutrition*, % | 3.2 | 3.3 | 5.8 | 5.7 | 1.4 | 2.6 |
| Number of diseases, mean (SD) | 4.4 (2.2) | 5.3 (2.1) | 7.7 (2.4) | 5.8 (1.9) | 4.5 (1.8) | 3.2 (1.2) |
| MMSE score, mean (SD) | 28.5 (1.9) | 28.7 (1.6) | 27.9 (2.3) | 27.6 (2.3) | 28.9 (1.4) | 28.8 (1.6) |
Abbreviations: APOE ε4, apolipoprotein epsilon 4 (at least 1 allele); CRP, C‐reactive protein; MMSE, Mini Mental State Examination.
The distribution of all variables was significantly different across multimorbidity patterns (P < 0.05).
Defined as body mass index <18.5 kg/m2.
Missing data: 8 in educational level; 6 in civil status; 189 in APOE genotype, 136 in CRP, 5 in MMSE.
3.1.2. Multimorbidity patterns and dementia risk
Table 2 shows the association between the multimorbidity patterns and dementia risk. In the fully adjusted model (by age, sex, education, civil status, malnutrition, baseline MMSE score, APOE genotype, and CRP level), individuals with neuropsychiatric multimorbidity presented with a 66% higher hazard of dementia (HR 1.66; 95% CI 1.13, 2.42) as compared to those with unspecific multimorbidity. Similarly, participants with cardiovascular multimorbidity showed a 61% higher hazard of dementia (HR 1.61; 95% CI 1.17, 2.29), as compared to the reference group (ie, unspecific multimorbidity). Finally, the sensory impairment/cancer pattern was associated with a 32% increased hazard of dementia compared with the unspecific cluster (HR 1.32; 95% CI 1.10, 1.71). No statistically significant association was found between the respiratory/metabolic/musculoskeletal pattern and dementia development. Table 2 also shows the incidence rate (IR) of dementia per 100 person‐years by multimorbidity pattern.
TABLE 2.
Incidence rates (IRs) per 100 person‐years and adjusted hazard ratios (aHRs) of dementia with 95% confidence intervals (CIs) by baseline multimorbidity patterns (N = 2478)
| Incident dementia | |||
|---|---|---|---|
| Multimorbidity patterns | Cases | IR (95% CI) | aHR (95% CI) |
| Unspecific (N = 1165) | 208 | 1.9 (1.7‐2.2) | 1.00 (Ref.) |
| Neuropsychiatric (N = 183) | 38 | 2.5 (1.8‐3.5) | 1.66 (1.13‐2.42) |
| Cardiovascular (N = 243) | 65 | 4.7 (3.7‐6.0) | 1.61 (1.17‐2.29) |
| Sensory impairment/cancer (N = 384) | 122 | 4.7 (4.0‐5.7) | 1.32 (1.10‐1.71) |
| Respiratory/metabolic/musculoskeletal (N = 503) | 75 | 1.6 (1.7‐2.2) | 0.97 (0.73‐1.29) |
Cox models are adjusted for age, sex, education, civil status, malnutrition, C‐reactive protein levels, APOE genotype, and baseline Mini‐Mental State Examination score.
Consistent, but slightly attenuated, results were obtained when we excluded participants who developed dementia within the first 6 years of follow‐up (HR1.67; 95% CI 1.07, 2.62 for the neuropsychiatric pattern; HR 1.53; 95% CI 1.04, 2.26 for the cardiovascular pattern; HR 1.31; 95% CI 0.98, 1.78 for the sensory impairment/cancer pattern; HR 1.05; 95% CI 0.78,1.45 for the respiratory/metabolic/musculoskeletal pattern). Similar findings have been obtained when we excluded those people who developed dementia during the follow‐up also from the cluster analysis (data not shown).
Consistent results were obtained when we included the multimorbidity‐free individuals as reference group in the survival analysis (Table S4).
3.1.3. Role of CRP and APOE genotype
No statistically significant interaction was detected between APOE genotype, CRP levels, and multimorbidity patterns. Figure 1 reports stratified analyses by CRP level (Panel A) and APOE ε4 allele (Panel B). The association between the neuropsychiatric, cardiovascular, and sensory impairment/cancer multimorbidity and dementia was even stronger in the group with high levels of CRP (HR 2.69; 95% CI 1.19, 6.09; HR 2.34; 95% CI 1.08, 5.09; HR 1.92; 95% CI 1.02, 3.63, respectively) (Figure 1). No statistically significant association was detected between the respiratory/metabolic/musculoskeletal pattern and dementia development in either group.
FIGURE 1.
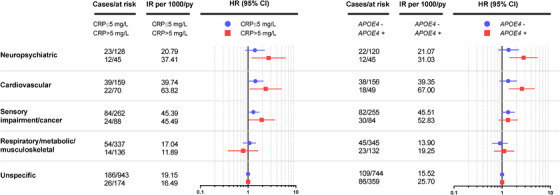
Incidence rates (IRs) per 100 person‐years and hazard ratios (HRs) of dementia with 95% confidence intervals (CIs) by baseline multimorbidity pattern, according to serum C‐reactive protein (CRP) levels and apolipoprotein E (APOE) genotype. HRs are plotted on a logarithmic scale. P for interaction: CRP: Neuropsychiatric MM#CRP: P = 0.151. Cardiovascular MM#CRP: P = 0.147. Sensory impairment/cancer MM#CRP: P = 0.833. Respiratory/metabolic/MSK MM#CRP: P = 0.419. APOE: Neuropsychiatric MM#APOE: P = 0.227. Cardiovascular MM#APOE: P = 0.895. Sensory impairment/cancer MM#APOE: P = 0.241. Respiratory/metabolic/MSK MM#APOE: P = 0.983. Models are adjusted for: Panel A: age, sex, education, civil status, malnutrition, and baseline Mini‐Mental State Examination score, and APOE ε4 allele. Panel B: age, sex, education, civil status, malnutrition, and baseline Mini‐Mental State Examination score, and CRP levels.
In the analyses stratified by APOE genotype, we detected a stronger association between the neuropsychiatric (HR 2.79; 95% CI 1.43, 5.43) and cardiovascular (HR 2.58; 95% CI 1.39, 4.76) multimorbidity patterns and dementia development in ε4 carriers with respect to non‐carriers. No statistically significant association with dementia development was detected for either the sensory impairment/cancer or the respiratory/metabolic/musculoskeletal pattern in APOE ε4 carriers.
Further adjustments (for use of drugs with anticholinergic action, corticosteroids, and polypharmacy) did not modify the results, as well as when we use age, instead of calendar time, as time scale (data not shown).
4. DISCUSSION
Older adults with neuropsychiatric and cardiovascular multimorbidity experienced the highest hazard of dementia throughout 12 years, followed by those with sensory impairment and cancer. Of interest, even if the interaction was not statistically significant, high serum CRP levels seemed to amplify this risk in all three multimorbidity patterns, whereas the presence of APOE ε4 appeared to increase dementia risk only in the neuropsychiatric and cardiovascular patterns.
Although the role of specific chronic diseases as risk factors for dementia has been largely acknowledged, 4 , 5 studies addressing dementia risk in relation to multimorbidity are limited to few reports. Multimorbidity has been associated with different degrees and severity of cognitive dysfunction, such as steeper cognitive decline, 20 , 21 increased risk of mild cognitive impairment 7 and dementia, 22 and faster dementia progression. 23 However, the majority of these studies have operationalized multimorbidity in quantitative terms through disease count, which is regarded by clinicians as uninformative. 24 Indeed, given a group of individuals with the same number of chronic diseases, understanding of how and which diseases cluster together and increase dementia risk compared to others may help to gain insight into the pathophysiology of dementia.
To date, the evidence relating multimorbidity patterns and dementia is extremely scarce, 25 , 26 and to the best of our knowledge, no studies have been conducted between multimorbidity patterns and incident dementia. An observational study carried out in The Netherlands evaluated the effects of groups of diseases on cognitive performance in a healthy population, 25 finding that heart diseases and cancer were associated with lower verbal memory performance, whereas cerebrovascular diseases, heart diseases, cancer, and movement disorders were related to worse processing speed.
Multimorbidity has been found previously to be associated with biomarkers of neurodegeneration and amyloid deposition 8 ; however, specific multimorbidity patterns may increase dementia risk through different pathways. The detrimental role of cardiovascular diseases on the brain is well established, being people with heart and cerebrovascular diseases at high risk of developing dementia and with a poor prognosis. 4 , 27 Indeed, accumulating evidence points toward a close interplay between body and mind in dementia development, 2 , 28 and our study aligns with this literature. It is notable that we also add weight to such knowledge by reporting that multiple, co‐occurring cardiovascular diseases are linked to dementia. The existence of a heart‐brain connection is supported by the fact that those with cardiovascular diseases tend to be at higher dementia risk due to a number of different mechanisms, including but not limited to cerebral hypoperfusion and infarcts, cardio‐embolization, and chronic inflammation. 29
In our study, we also found increased dementia risk among persons with neuropsychiatric diseases, which can be interpreted both as a risk factor or a prodromal manifestation of dementia. Notably, neuropsychiatric symptoms are common features of dementia, and can be part of its clinical picture, even in the early stages. Still, longitudinal studies with long follow‐ups have shown that depressive episodes are linked with incident dementia, reinforcing the idea of depression as a risk factor for Alzheimer's disease. 30 Regardless of the direction of this association, depression seems to lead to an imbalance in stress hormones, reduced hippocampal volume, and increased systemic inflammation—in turn, further impairing cognitive function.
Of interest, in our sample, sensory impairment (eg, visual impairment and deafness) and cancer clustered together and were associated with a 30% increased hazard of dementia. Previous studies have independently investigated both sensory impairments and cancer with incident dementia, finding conflicting results. 31 , 32 , 33 , 34 Among others, hearing loss has been linked with an increased dementia risk, 35 but such an association calls for further clarification, as to whether it can be considered a risk factor, or rather a prodromal sign of the disease. Greater debate exists surrounding the cognitive impact of cancer, with some studies pointing toward an inverse association with dementia 33 and others finding higher dementia risk in persons with neoplasms. 36 Previous studies have reported greater cancer risk in people with visual impairment 37 and, likewise, that age‐related macular degeneration occurs more frequently among smokers, which in turn increases the risk of different cancer types. 38 , 39 Furthermore, many cancer treatment regimens combine multiple ototoxic medications. 40 Finally, we can hypothesize that these two conditions might share common risk factors and pathophysiological mechanisms, and that they tend to co‐occur as an expression of independent biological degenerative changes occurring with age. This is also witnessed by the fact that, in our study, the cluster sensory impairment/cancer includes the oldest, more likely to be women, malnourished and with lower levels of cognitive performance. This profile identifies a frail proportion of our study population, and a number of studies have recently pointed out that cognitive impairment and frailty frequently co‐occur and that frail older adults present a higher risk of dementia. 41 Notably, the risk of dementia associated with this pattern was even higher in the presence of high CRP levels, but was not modified by the APOE ε4 allele. As such, dementia onset in this group of participants might be linked to systemic health deterioration, as a consequence of underlying complex diseases or drug regimens.
Of interest, we were not able to detect any association between the respiratory/metabolic/musculoskeletal cluster and dementia, although sleep disorders and diabetes (two known risk factors for dementia) 42 , 43 are part of this cluster. However, in the current study, we are not investigating the effect of individual diseases on dementia risk but the risk conveyed by belonging to a cluster of individuals displaying a given disease combination. Moreover, the comparison with the unspecific cluster—still composed by individuals with multimorbidity—may have diluted the effect.
A similar increased dementia risk was found in individuals with neuropsychiatric and cardiovascular multimorbidity and high levels of systemic inflammation. Although these results should be interpreted cautiously due to a lack of statistically significant interaction and therefore wide, overlapping confidence intervals in the stratified analyses, they indicate that inflammation might play a role in dementia development in those with neuropsychiatric and cardiovascular chronic conditions. Despite that the link between chronic conditions, inflammation, and dementia is yet far from being entirely understood, several hypotheses can be made. It is recognized that a low‐grade chronic proinflammatory state characterized by high levels of serum cytokines is very frequent in older individuals 44 and that older persons with high inflammatory markers have a higher number of chronic diseases as well as a steeper increase in multimorbidity over time. 45 At the same time, it is under debate if neuroinflammation is a driving force in the development of dementia 46 or an epiphenomenon of brain dysfunction and neuronal loss. Furthermore, the imbalance between inflammatory and anti‐inflammatory agents (a frequent finding in older individuals, termed ``inflammaging’’) 47 due to several chronic diseases can lead to a systemic and chronic proinflammatory state, which ultimately may affect the brain. The exact chain of events linking chronic diseases/inflammation/dementia remains a matter of debate, but based on the findings from our study we can speculate that the presence of inflammation further increases the risk of dementia within specific multimorbidity patterns. The possible role as mediator of inflammation has not been tested in the present study, since multimorbidity and CRP were assessed at the same time point, thus preventing us from having a proper chain of events as required in mediation analyses.
Because APOE ε4 is the strongest genetic risk factor for dementia, it is unsurprising that we found stronger associations with dementia among APOE ε4 carriers. It is notable that APOE has been linked to accelerated atherosclerosis and has been implicated in the modulation of proinflammatory and anti‐inflammatory cytokines. 48 , 49 It is worth noting that despite a lack of statistically significant interaction, we detected such an increased risk in the cardiovascular and neuropsychiatric multimorbidity patterns, but not in the sensory impairment/cancer pattern. Again, it is plausible to hypothesize that dementia development differs when related to the presence of sensory impairment/cancer versus cardiovascular and neuropsychiatric conditions, and might even follow different pathways. This hypothesis is in agreement with the greater dementia risk observed in the participants who had high systemic inflammation.
5. CLINICAL AND PUBLIC HEALTH IMPLICATIONS
The timely identification of neuropsychiatric, cardiovascular, and sensory impairment/cancer multimorbidity in older adults might have important implications in terms of prevention and clinical practice.
Our study adds weight to the evidence that cardiovascular diseases play a role in the development of adverse health‐related outcomes not only individually, but also as co‐occurring entities, and confirms the importance of assessing them holistically. 50 Dementia prevention may be optimized by controlling cardiovascular risk factors and enhancing the treatment of concurrent cardiovascular diseases (eg, improving medication adherence). It is worth mentioning that half of the study participants were part of the unspecific multimorbidity cluster, characterized by a high proportion of cardiovascular risk factors such as hypertension and dyslipidemia. In addition, early dementia diagnosis could be achieved by the close monitoring of persons affected by neuropsychiatric multimorbidity. Finally, the demographic and clinical characteristics of those belonging to the sensory impairment/cancer pattern calls for a close follow‐up of frail individuals. The identification of those patterns of multimorbidity in the presence of inflammation and among APOE ε4 carriers might help tailor clinical interventions aimed at dementia prevention, even to a greater extent.
6. STRENGTHS AND LIMITATIONS
To our knowledge, this is the first prospective observational study assessing dementia risk across different patterns of multimorbidity. These findings are derived from a large, well‐characterized population‐based cohort, with a long follow‐up and an extensive individual‐level clinical evaluation of diseases, including dementia. Of note, we were able to identify dementia cases, even among those individuals who died during follow‐up, by performing a comprehensive review of clinical records and death registers. This comprehensive identification of cases minimized the competing influence of death on dementia incidence. Notwithstanding, we acknowledge that some mild cases might not have been captured, resulting in a possible underestimation of the effect. Finally, even if the presence of residual confounding cannot be ruled out completely, our findings appeared robust to various sensitivity analyses and accounted for several relevant confounders.
Some limitations must be mentioned. First, the stratified analyses should be interpreted with caution due to the non‐statistically significant interaction, which is also reflected in the wide and overlapping confidence intervals. Notwithstanding, effect modification can be present also in the absence of a statistical interaction, 51 as witnessed by our findings that indicate that an interplay might exist and thus that inflammation and genetic predisposition might play a role. Second, despite the 12‐year follow‐up period, we cannot completely rule out the possibility of subclinical dementia. This has prevented us from making a conclusion on whether the neuropsychiatric multimorbidity pattern is more likely to be an at‐risk condition or a clinical manifestation of the disease. However, the results from the sensitivity analysis, excluding participants who developed dementia 6 years after baseline, were only slightly attenuated. Third, SNAC‐K is a population‐based study where the diagnosis of dementia is clinical, lacking biological markers for the characterization of the subtypes. Thus future studies with biological data are needed to confirm our results and move a step forward in the understanding of the mechanisms behind the associations we observed. Fourth, serum CRP was measured in a single time point, which does not capture chronic inflammation. Further evaluations on persistent levels of inflammation during follow‐up time are needed to confirm our findings. In addition, more fine markers of systemic inflammation exist (including tumor necrosis factor α [TNFα] and some interleukins), which were not available in the data set. Future studies are needed to better evaluate the role of inflammation in the studied association using more sensitive measures. Finally, being that our study was observational, we cannot draw any causal relationship between exposures and outcome.
7. CONCLUSIONS
Older adults with neuropsychiatric, cardiovascular, and sensory impairment/cancer multimorbidity are at high risk of dementia; this risk is further elevated by concurrent inflammation and genetic predisposition. Future studies, specifically designed to evaluate the interplay between aging‐related inflammatory processes, genetic predisposition, multimorbidity patterns, and dementia are needed to explore such mechanisms. Identifying older adults affected by specific multimorbidity patterns might be helpful in tailoring interventions for dementia prevention and targeting individuals who would benefit most from such interventions.
CONFLICT OF INTEREST
No conflict of interest to declare.
AUTHORS CONTRIBUTION
AM, GG, DLV, LF, and ACL contributed to the conception and design of the study. GG, ARL, and ACL conducted the statistical analyses. GG and AM conducted the literature search. All the authors contributed to interpretation of the results. GG and AM drafted the first version of the manuscript. All the authors critically revised the manuscript for important intellectual content. All the authors made a significant contribution to the research and the development of the manuscript and approved the final version for publication. SNAC‐K personnel collected the data for the study.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The authors are grateful to all the participants in the SNAC‐K project as well as staff in the SNAC‐K Study Group for their collaboration in data collection and management. This work was supported by the funders of the Swedish National study on Aging and Care (SNAC): the Ministry of Health and Social Affairs, Sweden; the participating county councils and municipalities and the Swedish Research Council. Specific grants were obtained from the Swedish Research Council (2016‐00981) and the Swedish Research Council for Health, Working Life and Welfare (2017‐01764). The Italian Ministry of Health (PE‐2016‐02364885). The financial sponsors played no role in the design, execution, analysis and interpretation of data, or writing of the study
Grande G, Marengoni A, Vetrano DL, et al. Multimorbidity burden and dementia risk in older adults: The role of inflammation and genetics. Alzheimer's Dement. 2021;17:768–776. 10.1002/alz.12237
REFERENCES
- 1. Shah H, Albanese E, Duggan C, et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016;15:1285‐1294. [DOI] [PubMed] [Google Scholar]
- 2. Grande G, Qiu C, Fratiglioni L. Prevention of dementia in an ageing world: evidence and biological rationale. Ageing Res Rev. 2020:101045. [DOI] [PubMed] [Google Scholar]
- 3. Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455‐532. [DOI] [PubMed] [Google Scholar]
- 4. Shang Y, Fratiglioni L, Marseglia A, et al. Association of diabetes with stroke and post‐stroke dementia: a population‐based cohort study. Alzheimers Dement. 2020. [DOI] [PubMed] [Google Scholar]
- 5. Ding M, Fratiglioni L, Johnell K, et al. Atrial fibrillation, antithrombotic treatment, and cognitive aging: a population‐based study. Neurology. 2018;91:e1732‐e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calderon‐Larranaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2017;72:1417‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc. 2015;63:1783‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vassilaki M, Aakre JA, Mielke MM, et al. Multimorbidity and neuroimaging biomarkers among cognitively normal persons. Neurology. 2016;86:2077‐2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calderon‐Larranaga A, Vetrano DL, Ferrucci L, et al. Multimorbidity and functional impairment‐bidirectional interplay, synergistic effects and common pathways. J Intern Med. 2019;285:255‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marengoni A, Roso‐Llorach A, Vetrano DL, et al. Patterns of multimorbidity in a population‐based cohort of older people: sociodemographic, lifestyle, clinical, and functional differences. J Gerontol A Biol Sci Med Sci. 2020;75:798‐805. [DOI] [PubMed] [Google Scholar]
- 11. Prados‐Torres A, Calderon‐Larranaga A, Hancco‐Saavedra J, Poblador‐Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67:254‐266. [DOI] [PubMed] [Google Scholar]
- 12. Sankowski R, Mader S, Valdes‐Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age‐associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4‐9. [DOI] [PubMed] [Google Scholar]
- 14. Lagergren M, Fratiglioni L, Hallberg IR, et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res. 2004;16:158‐168. [DOI] [PubMed] [Google Scholar]
- 15. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition ‐ an ESPEN Consensus Statement. Clin Nutr. 2015;34:335‐340. [DOI] [PubMed] [Google Scholar]
- 16. Kravitz BA CM, Kawas CH. Elevated C‐reactive protein levels are associated with prevalent dementia in the oldest‐old. Alzheimers Dement. 2009;5(4):318‐323. 10.1016/j.jalz.2009.04.1230. PMID: 19560102; PMCID: PMC2740472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guisado‐Clavero M, Roso‐Llorach A, Lopez‐Jimenez T, et al. Multimorbidity patterns in the elderly: a prospective cohort study with cluster analysis. BMC Geriatr. 2018;18:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Violan C, Roso‐Llorach A, Foguet‐Boreu Q, et al. Multimorbidity patterns with K‐means nonhierarchical cluster analysis. BMC Fam Pract. 2018;19:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vetrano DL, Roso‐Llorach A, Fernandez S, et al. Twelve‐year clinical trajectories of multimorbidity in a population of older adults. Nat Commun. 2020;11:3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei MY, Levine DA, Zahodne LB, Kabeto MU, Langa KM. Multimorbidity and Cognitive decline over 14 years in older Americans. J Gerontol A Biol Sci Med Sci. 2020;75:1206‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fabbri E, An Y, Zoli M, et al. Association between accelerated multimorbidity and age‐related cognitive decline in older baltimore longitudinal study of aging participants without dementia. J Am Geriatr Soc. 2016;64:965‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bauer K, Schwarzkopf L, Graessel E, Holle R. A claims data‐based comparison of comorbidity in individuals with and without dementia. BMC Geriatr. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haaksma ML, Rizzuto D, Leoutsakos JS, et al. Predicting cognitive and functional trajectories in people with late‐onset dementia: 2 population‐based studies. J Am Med Dir Assoc. 2019;20:1444‐1450. [DOI] [PubMed] [Google Scholar]
- 24. Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ. 2020;368:l6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aarts S, van den Akker M, Tan FE, Verhey FR, Metsemakers JF, van Boxtel MP. Influence of multimorbidity on cognition in a normal aging population: a 12‐year follow‐up in the Maastricht Aging Study. Int J Geriatr Psychiatry. 2011;26:1046‐1053. [DOI] [PubMed] [Google Scholar]
- 26. Wang JH, Wu YJ, Tee BL, Lo RY. Medical comorbidity in Alzheimer's Disease: a nested case‐control study. J Alzheimers Dis. 2018;63:773‐781. [DOI] [PubMed] [Google Scholar]
- 27. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age‐related cognitive decline. Nat Rev Cardiol. 2015;12:267‐277. [DOI] [PubMed] [Google Scholar]
- 28. Grande G, Rizzuto D, Vetrano DL, et al. Cognitive and physical markers of prodromal dementia: a 12‐year‐long population study. Alzheimers Dement. 2020;16:153‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vicario A, Cerezo GH. At the heart of brain disorders ‐ preventing cognitive decline and dementia. Eur Cardiol. 2015;10:60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh‐Manoux A, Dugravot A, Fournier A, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28‐year follow‐up study. JAMA Psychiatry. 2017;74:712‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 32. Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. BMJ. 2012;344:e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Musicco M, Adorni F, Di Santo S, et al. Inverse occurrence of cancer and Alzheimer disease: a population‐based incidence study. Neurology. 2013;81:322‐328. [DOI] [PubMed] [Google Scholar]
- 34. van der Willik KD, Schagen SB, Ikram MA. Cancer and dementia: two sides of the same coin?. Eur J Clin Invest. 2018;48:e13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68:214‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ganguli M. Cancer and dementia: it's complicated. Alzheimer Dis Assoc Disord. 2015;29:177‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pukkala E, Verkasalo PK, Ojamo M, Rudanko SL. Visual impairment and cancer: a population‐based cohort study in Finland. Cancer Causes Control. 1999;10:13‐20. [DOI] [PubMed] [Google Scholar]
- 38. Mitchell P, Liew G, Gopinath B, Wong TY. Age‐related macular degeneration. Lancet. 2018;392:1147‐1159. [DOI] [PubMed] [Google Scholar]
- 39. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3‐9. [DOI] [PubMed] [Google Scholar]
- 40. Frisina RD, Wheeler HE, Fossa SD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin‐based chemotherapy in survivors of adult‐onset cancer. J Clin Oncol. 2016;34:2712‐2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grande G, Haaksma ML, Rizzuto D, et al. Co‐occurrence of cognitive impairment and physical frailty, and incidence of dementia: systematic review and meta‐analysis. Neurosci Biobehav Rev. 2019;107:96‐103. [DOI] [PubMed] [Google Scholar]
- 42. Sindi S, Kareholt I, Johansson L, et al. Sleep disturbances and dementia risk: a multicenter study. Alzheimers Dement. 2018;14:1235‐1242. [DOI] [PubMed] [Google Scholar]
- 43. Marseglia A, Darin‐Mattsson A, Kalpouzos G, et al. Can active life mitigate the impact of diabetes on dementia and brain aging?. Alzheimers Dement. 2020. [DOI] [PubMed] [Google Scholar]
- 44. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ardura‐Fabregat A, Boddeke E, Boza‐Serrano A, et al. Targeting neuroinflammation to treat Alzheimer's disease. CNS Drugs. 2017;31:1057‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang H, Wu LM, Wu J. Cross‐talk between apolipoprotein E and cytokines. Mediators Inflamm. 2011;2011:949072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease–lessons from pathology. BMC Med. 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vetrano DL, Rizzuto D, Calderon‐Larranaga A, et al. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med. 2018;15:e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiology. 2009;20:863‐871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


