Abstract
Background:
Our study aim was to evaluate neuromuscular ultrasound (NMUS) for the assessment of taxane chemotherapy-induced peripheral neuropathy (CIPN), the dose-limiting toxicity of this agent.
Methods:
This cross-sectional study of breast cancer patients with taxane CIPN measured nerve cross-sectional area (CSA) by NMUS and compared with healthy historical controls. Correlations were determined between CSA and symptom scale, nerve conduction studies, and intraepidermal nerve fiber density (IENFD).
Results:
A total of 20 participants reported moderate CIPN symptoms at a median of 3.8 months following the last taxane dose. Sural nerve CSA was 1.2 mm2 smaller than healthy controls (P ≤ .01). Older age and time since taxane were associated with smaller sural nerve CSA. For each 1 mm2 decrease in sural nerve CSA, distal IENFD decreased by 2.1 nerve/mm (R2 0.30; P = .04).
Conclusions:
These data support a sensory predominant taxane neuropathy or neuronopathy and warrant future research on longitudinal NMUS assessment of CIPN.
Keywords: breast cancer, chemotherapy, neuropathy, taxane, ultrasound
1 |. INTRODUCTION
Chemotherapy-induced peripheral neuropathy (CIPN) is a common toxicity of many classes of chemotherapy, including taxanes, platinums, vinca alkaloids, and others. Taxanes, such as paclitaxel and docetaxel, have had an increasing role in the treatment of breast cancer and are now used in the majority of patients receiving perioperative chemotherapy.1 When chemotherapy cannot be given as intended due to dose reductions, it is associated with worse progression-free and overall survival.2 Although spontaneous recovery is possible, in roughly half of cases functional disability and risk of falls from CIPN persists for years.3,4 Currently, there is no predictive method to identify patients who are at high risk for developing permanent, debilitating CIPN.
There are few clinically available point-of-care tests to objectively predict, diagnose, or monitor CIPN. Self-reported symptom scales such as the European Organization for Research and Treatment of Cancer CIPN 20 Quality of Life Questionnaire (QLQ)5 are potentially predictive of CIPN but have inter-scale and inter-observer variability.6,7 Quantitative sensory testing fails to predict the severity of CIPN.8,9 Nerve conduction studies (NCSs) are insensitive to small fiber changes; typical findings include mild conduction velocity slowing at sites of entrapment or reduction of sural sensory responses.10–12 Skin biopsy for measuring intraepidermal nerve fiber density (IENFD) is the gold standard for the diagnosis of sensory-predominant small fiber neuropathy but is not an ideal test due to its invasive nature.3,6
Studies have found that neuromuscular ultrasound (NMUS) can detect peripheral nerve lesions generally with enlargement of CSA in patients with demyelinating hereditary,13,14 inflammatory,15,16 entrapment,17 and diabetic neuropathies18 though reduction of CSA has also been reported (eg, amyotrophic lateral sclerosis [ALS],19 spinocerebellar ataxia syndrome20–23). CSA is generally larger in demyelinating than axonal polyneuropathies24 and more commonly reported in large fiber neuropathies, though one study of patients with small fiber neuropathies demonstrated enlargement of CSA compared with matched-healthy controls.25
Less is known about the usefulness of NMUS for assessing the effect of chemotherapy upon peripheral nerves. Four studies have used NMUS for the detection of CIPN among gastrointestinal cancer patients who received oxaliplatin. Compared with historical reference values, three studies found no consistent change in CSA,24,26 although one did note an increase in peripheral nerve CSA at compression sites (median nerve at wrist, ulnar nerve at elbow).27 The fourth study compared neurological measures before and after chemotherapy and found increased CSA of the tibial nerve and at one compression site of the fibular nerve (fibular head), but did not find changes in CSA in other nerves (sural, median, ulnar, radial).28 Importantly, the mechanism and symptoms of neurotoxicity are markedly different between taxanes (eg, microtubular stabilization, hypoesthesia) and oxaliplatin (eg, inhibition of sodium channels; cold-induced paresthesias). Therefore, while prior studies of oxaliplatin CIPN are certainly informative, taxane-specific studies are needed to further characterize this syndrome.
In this study, our aim was to test NMUS as a noninvasive, point-of-care test to measure nerve CSA in patients with active taxane CIPN symptoms with the hypothesis that nerve CSA would be altered.
2 |. METHODS
2.1 |. Eligibility characteristics
Our single-arm, prospective pilot study enrolled breast cancer patients with self-reported symptomatic CIPN for comprehensive neurological assessment, including NMUS. The trial was conducted in accordance with the International Conference on Good Clinical Practice Standards and the Declaration of Helsinki. The protocol was approved by institutional review board and ethics committees of our institution and the trial was registered on ClinicalTrials.gov (NCT03139435). All patients provided informed consent. Eligible patients were ≥18-year-old women with breast cancer (any stage) and clinical symptoms of peripheral neuropathy due to either previous or current taxane exposure, as clinically attributed by the patient and their medical oncologist. We noted the presence of comorbidities (such as diabetes), which can predispose toward peripheral neuropathy with nerve enlargement.18 We excluded patients with a self-reported or documented history of pre-existing peripheral neuropathy before initiation of the taxane. Patients were followed, either by telephone or in person, for 30 days to ensure the biopsy site healed.
2.2 |. Assessments
Participants underwent a cross-sectional assessment that included focused history, neurological exam, and diagnostic testing by a neurologist at a single visit. NMUS with a GE S8 system and a routine 15-MHz linear transducer (GE Healthcare, Chicago, IL) was used to measure CSA at nerve sites as per previously described methodology and institutional practice.29 These sites were at the posterior distal leg (sural nerve), the ankle (tibial nerve), and the forearm and distal wrist crease (DWC; median nerve). The median nerve was assessed at two places to control for enlargement that can occur just proximal to entrapment at the carpal tunnel (at the DWC) due to subclinical carpal tunnel syndrome. Reference values for NMUS were from a published historical dataset.29 The 20-item self-reported neuropathy scale (QLQ-CIPN20) was administered as previously described with the exception of the hearing loss item; this item was excluded from data collection because taxanes are not associated with ototoxicity.30
NCS assessed the tibial motor nerve, median motor nerve, and the sural sensory nerve (antidromic, taken at two sites on the ankle) as per AANEM standards31 and compared with reference values from healthy adults.32 IENFD was measured with two skin biopsies (4–5 mm), one at the upper thigh and the second on the ipsilateral distal leg in sural nerve territory (10 cm above lateral malleolus). As previously described, biopsied tissues were cut into 50-μM sections for H&E staining and immunostaining with an antibody to protein gene product (PGP) 9.5, a neuron- and neuroendocrine cell-specific ubiquitin carboxy-terminal hydrolase expressed throughout the peripheral nervous system.33
Slides were processed and read by two collaborators in dermatopathology with experience in reading skin biopsy samples for IENFD. The same two dermatopathologists reviewed all cases. IENFD was measured as the linear density (per millimeter) of PGP 9.5-positive fibers that crossed the dermal–epidermal junction. Serum and additional unstained biopsy slides were stored and transferred for exploratory analyses. Although not blinded to the diagnosis of CIPN, the examiner performing NMUS did not review the patient’s symptoms before performing the study and was not aware of the cumulative dose, timing since taxane therapy, severity of clinical symptoms, or IENFD results at the time of testing. Dermatopathologists were not blinded to the patient but did not have access and were blinded to the NCS and NMUS data at the time of their analysis.
2.3 |. Study endpoints
The primary outcome was tibial nerve CSA by NMUS compared with historical controls. Secondary outcomes included sural nerve CSA by NMUS and determination of whether tibial or sural nerve CSA correlated with QLQ-CIPN20, NCS, or IENFD. Exploratory outcomes included measures of median nerve CSA.
2.4 |. Statistical analysis
The tibial nerve CSA, sural nerve CSA, and sural nerve amplitude were compared with historical data29,34 from healthy patients using two-sample t-test; primary historical data were used for the analysis of CSA measures. Phase of treatment was defined as active (≤30 days since last taxane administration), recent (>30 and ≤180 days), and remote (>180 days). Comparison of CSA, QLQ scales, and IENFD measures between different phases of taxane exposure were performed using the Kruskal-Wallis tests. Spearman’s rank correlation coefficients were used to study the associations between two measures (eg, distal and proximal IENFD). Linear regression models were used to examine the associations between the dependent variable (nerve CSA) and covariates of interest (NCS measures, self-reported neuropathy scale, and IENFD). Clinical factors (eg, age) that were thought to be potentially associated with nerve CSA were fitted in the model one at a time.
3 |. RESULTS
Between May 4, 2017, and November 13, 2018, 25 patients were screened for eligibility; 3 did not meet inclusion criteria, and 2 declined to participate as summarized in CONSORT flow diagram (Figure 1). Recruitment was by provider referral and by paper fliers posted in both patient and provider areas of the oncology clinic. All patients completed their planned evaluation and follow-up. One patient was excluded from median nerve analysis due to symptoms and NCS findings that were diagnostic of carpal tunnel syndrome.
FIGURE 1.
CONSORT flow diagram
A total of 20 patients underwent evaluation with their baseline clinical characteristics summarized in Table 1. Average cumulative taxane dose was 817 mg/m2; for reference, the typical dosing regimen for adjuvant paclitaxel in breast cancer is 80 mg/m2/week for 12 weeks (total cumulative dose of 960 mg/m2). CIPN patients reported moderate-to-severe CIPN symptoms by QLQ-CIPN20, which were predominantly sensory (19.1 ± 4.9; max 32) as opposed to motor (15.6 ± 5.8; max 32) or autonomic (3.3 ± 1.6; max 8). All patients had normal strength (5/5) in foot dorsiflexion; deep tendon reflexes at the ankles were absent in 13/20 (65%), reduced in 4/20 (20%).
TABLE 1.
Demographic and disease characteristics of the participants
| Parameter | Mean (SD) or n (%) | |
|---|---|---|
| Age, mean (SD) | 55.4 (10.5) | |
| BMI, mean (SD) | 29.8 (6.9) | |
| Stage of breast cancer, n (%) | Stage I | 8 (40%) |
| Stage II | 5 (25%) | |
| Stage III | 2 (10%) | |
| Stage IV | 5 (25%) | |
| Prior radiation therapy, n (%) | 10 (50%) | |
| Prior surgery for breast cancer, n (%) | 19 (95%) | |
| Prior hormone therapy for breast cancer, n (%) | 8 (40%) | |
| Prior lines of systemic cancer therapy, median (IQR) | 1(1–3) | |
| Most recent taxane, n (%) | Paclitaxel | 10 (50%) |
| Docetaxel | 8 (40%) | |
| Nab-paclitaxel | 2 (10%) | |
| Cumulative taxane dose, mg/m2, mean (SD) | 817.3 (414.9) | |
| Months since last taxane dose, median (IQR) | 3.8 (1.0–6.1) | |
| Relevant comorbidities, n (%) | Diabetes, no end-organ damage | 4 (20%) |
| Hypothyroidism | 3 (15%) | |
| Depression | 3 (15%) | |
| Alcoholism | 1(5%) | |
| Cerebovascular disease | 1 (5%) | |
| Vitamin B12 deficiency | 1 (5%) | |
IQR, interquartile range; Nab, nanoparticle albumin–bound.
Sural nerve amplitude was significantly lower (mean 10.9 ± 5.8 μV) than healthy historical controls (mean 17.2 ± 10.1 μV; P < .01) with 8 patients below the normal range (<7.1 μV).34 There were no associations between tibial nerve CSA and tibial compound muscle action potential amplitude (Spearman’s r = 0.12), distal latency (−0.24), or conduction velocity (0.21). There were no associations between sural nerve CSA and sural sensory nerve action potential (SNAP) amplitude (Spearman’s r = 0.20), latency (−0.26), or conduction velocity (0.27). Sural SNAP amplitude was not associated with symptom severity by QLQ-CIPN20 (Spearman rs [18] = −0.23).
Distal IENFD had mean 6.6 ± 3.8 nerves/mm (range, 2.4–15 nerves/mm) with 30% of participants below the normal range, defined as the 5th percentile among historical healthy controls (3.8 nerves/mm). Proximal IENFD had mean 11.0 ± 6.0 nerves/mm (range, 9–24 nerves/mm) with 15% of participants below the normal range, defined as the 5th percentile among historical healthy controls (5.2 nerves/mm).35 Distal and proximal IENFD were associated with each other (Spearman rs [19] P = .03). Neither distal nor proximal IENFD was associated with symptom severity by QLQ CIPN20 (Spearman rs [19] = −0.16; 0.12, respectively) or with cumulative dose (Spearman rs [20] = 0.42; 0.19, respectively). Proximal IENFD was negatively associated with tibial amplitude (Spearman r = −0.46; P = .04); otherwise, there was no association between IENFD (either proximal or distal) and any other NCS measures.
CSA was evaluated by NMUS at 74 nerve sites among the 20 patients with no repeated measurements. The average sural nerve CSA was significantly smaller than healthy historical controls (Table 2). Older age was associated with significantly smaller sural nerve CSA (Spearman rs [20] = −0.65, P ≤ .01). More days since taxane chemotherapy was also associated with smaller sural nerve CSA (rs [20] = −0.46; P = .04). Sural nerve CSA was not significantly associated with either body mass index (BMI) (Spearman rs [20] = 0.25) or cumulative taxane dose (Spearman rs [20] = 0.40); neither were the CSA of other nerves. Unadjusted sural nerve CSA did not have an association with distal IENFD, but when controlled for age and days from last taxane administration, distal IENFD reduced by 2.1 nerve/mm for each 1 mm2 decrease in sural nerve CSA (Table 3).
TABLE 2.
Comparison of nerve CSA by NMUS between CIPN patients and healthy historical controls
| CIPN patients, mm2, mean (SD) | Healthy controls, mm2, mean (SD) | t value | P value | |
|---|---|---|---|---|
| Tibial nerve CSA | 13.0 (3.6) | 14.0 (4.3) | 0.94 | .35 |
| Sural nerve CSA | 4.1 (1.3) | 5.3 (1.8) | 2.85 | <.01* |
| Median nerve CSA (forearm) | 7.3 (1.4) | 7.7 (1.7) | 0.89 | .37 |
| Median nerve CSA (DWC) | 12.5 (3.9) | 10.1 (2.7) | −2.39 | .03** |
Statistically significant at α ≤ .05. Df = 138.
Statistically significant at α ≤ .05. Satterthwaite unequal variances; Df = 16.
TABLE 3.
Multiple regression analysis of factors to predict distal IENFD
| Univariate |
Multivariate model 1* |
Multivariate model 2† |
||||
|---|---|---|---|---|---|---|
| Factors | Beta | P value | Beta | P value | Beta | P value |
| Sural CSA | 0.35 | .13 | 0.99 | .15 | 2.10 | .04‡ |
| Days since last taxane | −0.29 | .21 | −0.01 | .25 | 0.19 | .13 |
| Age | −0.12 | .62 | −0.01 | .09 | ||
Model 1: R2 = 0.19; F(2,19) = 2.03; P = .16.
Model 2: R2 = 0.30; F(3,19) = 2.32; P = .11.
Statistically significant at α ≤ .05.
Descriptive data of nerve findings by phase of treatment (active, recent, remote) are summarized in Table 4. When compared by phase of treatment and independently from each other, tibial nerve CSA (P = .44), sural nerve CSA (P = .17), proximal IENFD (P = 0.43), distal IENFD (P = .32), QLQ sensory scale (P = .46), and sural amplitude (P = .45) did not meet statistical significance by Kruskal-Wallis one-way analysis of variance.
TABLE 4.
Comparison of findings between different phases of taxane exposure
| Active taxane, mean (SD) | Recent taxane, mean (SD) | Remote taxane, mean (SD) | |
|---|---|---|---|
| No. of participants | 6 | 9 | 5 |
| Cumulative dose (taxane mg/m2) | 995.8 (95.8) | 713.9 (440.7) | 789.0 (587.1) |
| Tibial nerve CSA (mm2) | 14.2 (4.8) | 13.2 (3.2) | 11.2 (2.3) |
| Sural nerve CSA (mm2) | 4.8 (1.6) | 3.9 (0.6) | 3.6 (1.5) |
| Forearm median nerve CSA (mm2) | 7.3 (1.9) | 8 (1.0) | 6.2 (0.4) |
| DWC median nerve CSA* (mm2) | 13.8 (6.5) | 13.6 (3.0) | 11.0 (2.4) |
| QLQ sensory scale | 17.2 (4.3) | 20.4 (5.2) | 19.2 (5.4) |
| QLQ motor scale | 12.3 (3.4) | 18.6 (6.6) | 14.2 (4.4) |
| QLQ autonomic scale | 2.5 (0.5) | 3.9 (1.8) | 3.2 (1.6) |
| Proximal IENFD (nerves/mm) | 13.1 (6.7) | 8.9 (3.6) | 12.2 (8.5) |
| Distal IENFD (nerves/mm) | 8.9 (4.9) | 5.5 (2.9) | 5.9 (3.0) |
P value significant to <.05 (.0219) by Kruskal–Wallis one-way analysis of variance.
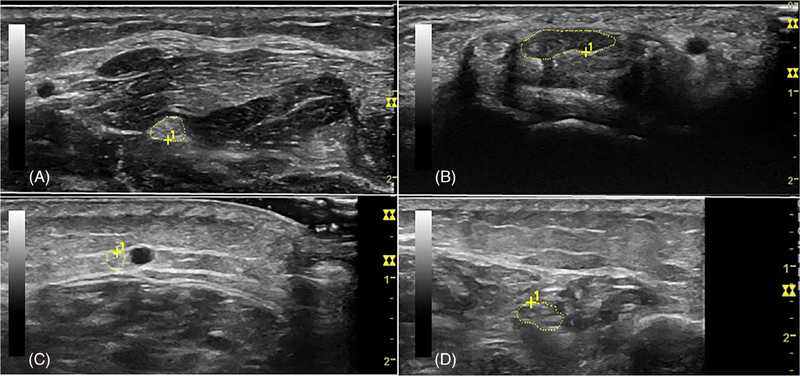
Median nerve CSA at the compressive site (DWC) was larger than among healthy historical controls, whereas at the noncompressive site (forearm), there was no significant difference. These results were the same even when including the participant with carpal tunnel syndrome (n = 20), with median nerve CSA at the DWC larger than healthy historical controls (P = .02) and no significant difference at the forearm (P = .41). Representative NMUS imaging is shown in Figure 2. Descriptive data from all participants is shown in Supporting Information Table S1, which is available online.
FIGURE 2.
Representative imaging by NMUS. A, Median nerve at forearm in participant 3 (CSA, 9 mm2). B, Median nerve at wrist in participant 3 (CSA, 25 mm2). C, Sural nerve in participant 6 (CSA, 4 mm2). D, tibial nerve in participant 6 (CSA, 19 mm2)
The study was well-tolerated without any adverse effects and with a high uptake among participants with just 2 of 25 participants declining to complete the study protocol after registration. Skin biopsy led to one adverse event in a patient who had redness and irritation attributed to an inflammatory local reaction and not an infection.
4 |. DISCUSSION
The key findings of this study are that, compared with healthy historical controls, patients with a small fiber neuropathy caused by taxane exposure had (1) decreased sural nerve size, (2) increased median nerve size but only at a compression site, and (3) NMUS findings that correlated well with IENFD loss on skin biopsies. Reduced CSA was specific to the sural sensory nerve and was not seen in the tibial nerve. This is likely reflective of the predominantly sensory manifestation of taxane CIPN, which was confirmed in our sample using a validated symptom scale.
NMUS assessment of the median nerve at its compression site (DWC) found that CIPN patients had increased CSA compared with healthy controls. When moved proximally to assess the median nerve at a noncompressive site (forearm), there was no difference in CSA between CIPN patients and healthy controls. This discrepancy is often seen with NMUS evaluation of symptomatic carpal tunnel syndrome and other polyneuropathies.36 These findings suggest that, at compression sites, CIPN may aggravate nerve entrapment and cause an increase in CSA of peripheral nerves. This discrepancy in the effects of CIPN between compression and noncompression anatomic sites has been described in NCS11 and was suggested in a prior study of oxaliplatin CIPN.27
The finding of decreased peripheral nerve CSA is uncommon and has been reported in ALS19 and spinocerebellar ataxia syndrome.20–23 In vitro37 and animal studies38 have shown that the neurotoxicity of microtubule-targeting agents (eg, taxanes) is predominantly due damage to the dorsal root ganglion (DRG).39,40 This leads to axonal loss in the longest sensory nerves and contributes to the clinical symptoms consistent with a ganglionopathy. In ALS, the reduction in nerve CSA is thought to reflect nerve atrophy from lower motor neuron (LMN) dropout41 and may have utility as a marker of LMN involvement.42 We hypothesize that the decreased CSA observed in our study may reflect the sensory ganglionopathy of taxane CIPN with sensory nerve atrophy. Because this study was limited to taxane CIPN, these results are not broadly applicable to other classes of chemotherapies whose neurotoxic mechanisms vary.
Multivariable analysis was notable for two confounding factors between CIPN and CSA, that is, age and time since taxane exposure. Age has previously been reported as having a very small negative correlation with peripheral nerve CSA.29 The association with time is a novel finding; we hypothesize that this may be due to the gradual atrophy of damaged peripheral nerves following the initial insult. This is supported by the finding of decreased distal IENFD among patients with more time since taxane. Future studies will need to further account for these confounding factors.
There were several limitations of this pilot study, most of which were inherent to its design and small sample size. This study was designed to provide a broader evaluation of the pathophysiology of CIPN by including participants at all timepoints during and after taxane (active, recent, remote). However, this heterogeneity decreased the statistical power to detect changes in CSA, which may only occur in a time-specific manner. Because there is no intervention available for CIPN once the patient has completed taxane chemotherapy, there is little clinical utility for improved evaluation of chronic CIPN. Therefore, future studies of the clinical use of NMUS, including one that is currently enrolling patients,43 will focus on patients with CIPN who are being considered for additional taxane chemotherapy so that dosing may be adjusted accordingly. This study was limited to female participants; reassuringly, in the prior study of healthy controls, there was no significant difference in nerve CSA by sex.29 Prior studies44 have reported variability among healthy controls with sural nerve CSA approximately 2–6 mm2, our healthy control data had a mean of around 5 mm2. Our study was not funded to support collection of another set of matched control values, which is why we used our previously published set.29 A newer high-resolution probe could also be considered for future studies, as ultrahigh-frequency NMUS has been found to be useful for assessing changes at the fascicular level.45
CIPN is a common complication of breast cancer treatment with a critical need for a better diagnostic imaging modality. NMUS was found to be a rapid, noninvasive assessment of CIPN, which was well-tolerated and available at the point-of-care using a routine 15-Hz linear probe. This study’s findings warrant future research on the longitudinal assessment of sural nerve CSA in CIPN.
Supplementary Material
Acknowledgments
Funding information
Department of Defense, Grant/Award Numbers: W81XWH-19-1-0045, W81XWH-17-1-0541, W81XWH-14-1-0403
CONFLICT OF INTEREST
Michael Cartwright has intellectual property with Elsevier for a textbook on Neuromuscular Ultrasound. Francis Walker has received honoraria and support for expenses from Grifols and AskBio; research funding from Vaccinex, TEVA Pharmaceuticals, and Pfizer; intellectual property with Elsevier and UpToDate; and support with interest-free equipment loans from Natus, Monarch Medical, and Terason. Yusuke Shiozawa has received research funding from TEVA Pharmaceuticals. The remaining authors have no conflicts of interest.
Abbreviations:
- AANEM
American Association of Neuromuscular & Electrdiagnostic Medicine
- ALS
amyotrophic lateral sclerosis
- BMI
body mass index
- CIPN
chemotherapy-induced peripheral neuropathy
- CSA
cross-sectional area
- DRG
dorsal root ganglion
- DWC
distal wrist crease
- H&E
hematoxylin and eosin
- IENFD
intraepidermal nerve fiber density
- LMN
lower motor neuron
- NCS
nerve conduction study
- NMUS
neuromuscular ultrasound
- PGP
protein gene product
- QLQ
Quality of Life Questionnaire
- SNAP
sural sensory nerve action potential
Footnotes
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18): 2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90 (16):1205–1211. [DOI] [PubMed] [Google Scholar]
- 3.Osmani K, Vignes S, Aissi M, et al. Taxane-induced peripheral neuropathy has good long-term prognosis: a 1- to 13-year evaluation. J Neurol. 2012;259(9):1936–1943. [DOI] [PubMed] [Google Scholar]
- 4.Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, Functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35(23):2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res. 2013;22(10):2787–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63 (6):419–437. [DOI] [PubMed] [Google Scholar]
- 7.Reeves BN, Dakhil SR, Sloan JA, et al. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer. 2012;118(20):5171–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsyth PA, Balmaceda C, Peterson K, Seidman AD, Brasher P, DeAngelis LM. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neurooncol. 1997;35 (1):47–53. [DOI] [PubMed] [Google Scholar]
- 9.Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125 (3):767–774. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol. 1994;35(3):304–311. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Stubblefield MD, Custodio CM, Hudis CA, Seidman AD, DeAngelis LM. Electrophysiological features of taxane-induced polyneuropathy in patients with breast cancer. J Clin Neurophysiol. 2013; 30(2):199–203. [DOI] [PubMed] [Google Scholar]
- 12.Kroigard T, Schroder HD, Qvortrup C, et al. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. Eur J Neurol. 2014;21(4): 623–629. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber S, Oldag A, Kornblum C, et al. Sonography of the median nerve in CMT1A, CMT2A, CMTX, and HNPP. Muscle Nerve. 2013;47 (3):385–395. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright MS, Brown ME, Eulitt P, Walker FO, Lawson VH, Caress JB. Diagnostic nerve ultrasound in Charcot-Marie-Tooth disease type 1B. Muscle Nerve. 2009;40(1):98–102. [DOI] [PubMed] [Google Scholar]
- 15.Beekman R, van den Berg LH, Franssen H, Visser LH, van Asseldonk JT, Wokke JH. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology. 2005;65(2): 305–307. [DOI] [PubMed] [Google Scholar]
- 16.Zaidman CM, Pestronk A. Nerve size in chronic inflammatory demyelinating neuropathy varies with disease activity and therapy response over time: a retrospective ultrasound study. Muscle Nerve. 2014;50 (5):733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cartwright MS, Walker FO. Neuromuscular ultrasound in common entrapment neuropathies. Muscle Nerve. 2013;48(5):696–704. [DOI] [PubMed] [Google Scholar]
- 18.Breiner A, Qrimli M, Ebadi H, et al. Peripheral nerve high-resolution ultrasound in diabetes. Muscle Nerve. 2017;55(2):171–178. [DOI] [PubMed] [Google Scholar]
- 19.Cartwright MS, Walker FO, Griffin LP, Caress JB. Peripheral nerve and muscle ultrasound in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44(3):346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leadbetter R, Weatherall M, Pelosi L. Nerve ultrasound as a diagnostic tool for sensory neuronopathy in spinocerebellar ataxia syndrome. Clin Neurophysiol. 2019;130(4):568–572. [DOI] [PubMed] [Google Scholar]
- 21.Pelosi L, Leadbetter R, Mulroy E, Chancellor AM, Mossman S, Roxburgh R. Peripheral nerve ultrasound in cerebellar ataxia neuropathy vestibular areflexia syndrome (CANVAS). Muscle Nerve. 2017;56 (1):160–162. [DOI] [PubMed] [Google Scholar]
- 22.Pelosi L, Mulroy E, Rodrigues MJ, Roxburgh RH. Neuronopathy and neuropathy in autosomal dominant spino-cerebellar ataxia (SCA): a preliminary peripheral nerve ultrasound study. Clin Neurophysiol. 2017;128(12):2436–2437. [DOI] [PubMed] [Google Scholar]
- 23.Pelosi L, Mulroy E, Leadbetter R, et al. Peripheral nerves are pathologically small in cerebellar ataxia neuropathy vestibular areflexia syndrome: a controlled ultrasound study. Eur J Neurol. 2018;25(4): 659–665. [DOI] [PubMed] [Google Scholar]
- 24.Zaidman CM, Al-Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve. 2009;40(6):960–966. [DOI] [PubMed] [Google Scholar]
- 25.Ebadi H, Siddiqui H, Ebadi S, Ngo M, Breiner A, Bril V. Peripheral nerve ultrasound in small fiber polyneuropathy. Ultrasound Med Biol. 2015;41(11):2820–2826. [DOI] [PubMed] [Google Scholar]
- 26.Grimm A, Decard BF, Axer H, Fuhr P. The Ultrasound pattern sum score - UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves. Clin Neurophysiol. 2015;126(11):2216–2225. [DOI] [PubMed] [Google Scholar]
- 27.Briani C, Campagnolo M, Lucchetta M, et al. Ultrasound assessment of oxaliplatin-induced neuropathy and correlations with neurophysiologic findings. Eur J Neurol. 2013;20(1):188–192. [DOI] [PubMed] [Google Scholar]
- 28.Pitarokoili K, Hoffken N, Lonneker N, et al. Prospective study of the clinical, electrophysiologic, and sonographic characteristics of oxaliplatin-induced neuropathy. J Neuroimaging. 2019;29(1):133–139. [DOI] [PubMed] [Google Scholar]
- 29.Cartwright MS, Passmore LV, Yoon JS, Brown ME, Caress JB, Walker FO. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve. 2008;37(5):566–571. [DOI] [PubMed] [Google Scholar]
- 30.Eckhoff L, Knoop A, Jensen MB, Ewertz M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer. 2015;51(3):292–300. [DOI] [PubMed] [Google Scholar]
- 31.American Association of Neuromuscular & Electrdiagnostic Medicine (AANEM). Proper performance and interpretation of electrodiagnostic studies. [Corrected]. Muscle Nerve. 2015;51(3):468–471. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Andary M, Buschbacher R, et al. Electrodiagnostic reference values for upper and lower limb nerve conduction studies in adult populations. Muscle Nerve. 2016;54(3):371–377. [DOI] [PubMed] [Google Scholar]
- 33.Lauria G, Devigili G. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat Clin Pract Neurol. 2007;3(10):546–557. [DOI] [PubMed] [Google Scholar]
- 34.Benatar M, Wuu J, Peng L. Reference data for commonly used sensory and motor nerve conduction studies. Muscle Nerve. 2009;40(5): 772–794. [DOI] [PubMed] [Google Scholar]
- 35.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55(12):1513–1520. [DOI] [PubMed] [Google Scholar]
- 36.Shen J, Cartwright MS. Neuromuscular ultrasound in the assessment of polyneuropathies and motor neuron disease. J Clin Neurophysiol. 2016;33(2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scuteri A, Nicolini G, Miloso M, et al. Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Res. 2006;26(2A):1065–1070. [PubMed] [Google Scholar]
- 38.Zhang H, Li Y, de Carvalho-Barbosa M, et al. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain. 2016;17(7):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolini G, Monfrini M, Scuteri A. Axonal transport impairment in chemotherapy-induced peripheral neuropathy. Toxics. 2015;3(3): 322–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malacrida A, Meregalli C, Rodriguez-Menendez V, Nicolini G. Chemotherapy-induced peripheral neuropathy and changes in cytoskeleton. Int J Mol Sci. 2019;20(9):pii E2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes SL, Simon NG. Clinical and research applications of neuromuscular ultrasound in amyotrophic lateral sclerosis. Degener Neurol Neuromuscul Dis. 2019;9:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schreiber S, Abdulla S, Debska-Vielhaber G, et al. Peripheral nerve ultrasound in amyotrophic lateral sclerosis phenotypes. Muscle Nerve. 2015;51(5):669–675. [DOI] [PubMed] [Google Scholar]
- 43.U.S.National Library of Medicine. Acupuncture in reducing chemotherapy-induced peripheral neuropathy in participants with stage I-III breast cancer. https://ClinicalTrials.gov/show/NCT03505671. Accessed February 17, 2020. [Google Scholar]
- 44.Rbia N, Nijhuis THJ, Roukema GR, Selles RW, van der Vlies CH, Hovius SER. Ultrasound assessment of the sural nerve in patients with neuropathic pain after ankle surgery. Muscle Nerve. 2018;57(3): 407–413. [DOI] [PubMed] [Google Scholar]
- 45.Cartwright MS, Baute V, Caress JB, Walker FO. Ultrahigh-frequency ultrasound of fascicles in the median nerve at the wrist. Muscle Nerve. 2017;56(4):819–822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.