Abstract
Background
Limited clinical data are available on bone loss in ankylosing spondylitis (AS) patients with hip involvement, especially for bone strength. The purpose of this study was to analyze bone strength and bone turnover markers in AS patients with hip involvement.
Material/Methods
The stiffness index (SI) calculated by quantitative ultrasound (QUS) was used to compare the bone strength between patients with AS with radiographic hip involvement (RHI-AS, BASRI-hip ≥2) and those without radiographic hip involvement (WORHI-AS, BASRI-hip ≤1). The Spearman correlation test was used to evaluate the association between SI and bone turnover markers [TP1NP, OC, β-CTx, 25(OH)VD3, and PTH].
Results
RHI-AS (BASRI-hip ≥2) patients accounted for 52.2% (177/339) of all patients. There was no significant difference in most of the basic clinical features between RHI-AS and WORHI-AS patients, except for age and BMI. After adjusting for confounding factors (age and BMI), the stiffness index (SI) of RHI-AS patients was significantly lower than that of WORHI-AS patients (ORadj=0.982, 95% CIadj=0.968~0.997, Padj=0.017). The Z scores calculated by SI were lower in RHI-AS patients (ORadj=0.802, 95% CIadj=0.679~0.949, Padj=0.01). Among the 5 bone turnover markers in the RHI-AS patients, only 25(OH)VD3 had a correlation with SI (rho=0.279, P=0.001).
Conclusions
AS patients have lower bone strength once the disease progresses to include radiologic hip involvement. Treatment of vitamin D deficiency may be an effective way to improve bone strength in AS patients with hip involvement.
Keywords: 24,25-Dihydroxyvitamin D 3; Bone Diseases, Metabolic; Hip Joint; Osteoporosis; Spondylitis, Ankylosing
Background
Ankylosing spondylitis (AS) is a common immune disease that mainly damage the spine and is characterized by sacroiliac arthritis, peripheral arthritis, enthesis, and iritis in young men, which can cause a heavy symptomatic burden and affect the quality of remaining life [1].
Hip involvement is frequently found in AS patients, with a prevalence rate of approximately one-third, which is the main cause of disability [2]. The main manifestations of joint damage in AS are synovitis and bone erosion in the hip joint, which is different from the new bone formation in the spine [3]. The hip is the largest full-motion joint and the most burdened joint in the human body, and destruction of the hip structure can cause balance disorders and abnormalities in posture and gait [4]. Therefore, it is important to understand the bone strength of AS patients with hip involvement because these patients are more likely to fall during exercise.
Decreased bone mineral density (BMD) has been recognized as a common complication of AS, which can lead to osteoporosis and subsequent vertebral fragility fractures [5]. However, BMD can only reflect bone quantity, which accounts for 70% of bone strength, and cannot reflect bone quality, which accounts for 30% of bone strength [6]. Bone quality related to bone elasticity is also important to bone strength, and its destruction contributes to fragility fractures [7].
Quantitative ultrasound (QUS) is used to study bone strength because of its comprehensive ability to measure both bone quantity and bone quality [7]. However, few studies have explored bone strength in patients with AS, especially those with hip involvement. The purpose of this study was to analyze the bone strength measured by QUS in AS patients with hip involvement and to evaluate the correlation between bone turnover markers and bone strength.
Material and Methods
Patient Inclusion Criteria
We retrospectively analyzed bone strength and bone turnover markers in ankylosing spondylitis patients who visited Beijing Jishuitan Hospital from 2013-1-1 to 2019-12-31. A total of 2752 patient visits over 18 years old who were diagnosed with ankylosing spondylitis were screened by a single-center hospital information system. There were 485 patients left after eliminating duplicate visit records and obtaining initial visit records. Of the 485 patients, 392 were applied to the classification criteria of AS (1984 modified New York). Finally, 339 cases were ultimately included in the present study after the implementation of the exclusion criteria. The exclusion criteria were as follows: 1) incomplete data, totaling 28 cases; 2) bone tumor, metabolic bone diseases, tuberculosis, or hematological cancer, totaling 11 cases; and 3) other rheumatic immune diseases, totaling 14 cases.
The ethics approval number for this retrospective clinical study is 202003-13.
Clinical Information
Basic clinical information was obtained from the electronic medical records, including sex, age, course of disease, smoking, body mass index (BMI, kg/m2), family history of AS, C-reactive protein (CRP), human leukocyte antigen B27 (HLA-B27) status, and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). The mSASSS score was done on medical imaging systems. The mSASSS score ranges from 0 to 72 and was divided into 2 parts. The first part was the lower margin of the second cervical vertebra to the upper margin of the first thoracic vertebra (0–36), and the second part was the lower margin of the 12th thoracic vertebra to the upper margin of the sacrum (0–36). The scoring criteria were as follows: 0 (normal), 1 (squaring), 2 (syndesmophyte), and 3 (ankylosis).
Definition of Radiographic Hip Involvement
The BASRI-hip score based on X-ray was used to evaluate radiographic hip involvement. The BASRI-hip score was divided into 5 grades and corresponding to 0–4 points: 0) normal, there was no radiological change of the hip joint; 1) suspicious, there may be local joint space narrowing; 2) mild, the lesion of hip joint was obvious, but the circumferential joint space was more than 2 mm; 3) moderate, the circumferential joint space was less than 2 mm and the bone-on-bone apposition was less than 2 cm; and 4) severe, hip fusion or bone-on-bone apposition greater than 2 cm, or with indications of total hip replacement. A BASRI-hip greater than or equal to 2 point was defined as ankylosing spondylitis with radiographic hip involvement (RHI-AS), while a BASRI-hip less than or equal to 1 point was defined as ankylosing spondylitis without radiographic hip involvement (WORHI-AS). Two rheumatologists independently read the X-rays on medical imaging systems, and 5 rheumatologists voted for the final decision (3 or more votes) when the 2 disagreed.
Measurement of Bone Strength
Bone strength was evaluated by the stiffness index of the calcaneus using an Achilles QUS system (GE Healthcare, Waukesha, WI, USA). The T score represented the stiffness index, with values higher or lower than the reference average of young adults expressed as standard deviation (SD). The Z score represented the stiffness index, with values higher or lower than the reference average of patients of the same age and race expressed as standard deviation (SD). According to the operating instructions of the Achilles QUS system, the reduction of the T score by 1–2.5 standard deviations (−2.5<T<−1) was equivalent to osteopenia, the reduction of the T score by at least 2.5 standard deviations (T≤−2.5) was equivalent to osteoporosis, the reduction of the Z score by at least 2 standard deviations (Z≤−2) was equivalent to “below the expected range for age”.
Detection of Bone Turnover Markers
Five bone turnover markers were assessed by commercial kits (Cobas e601 automated immunoassay analyzer, Roche, Germany) using the electrochemiluminescence immunoassay method: TP1NP (total procollagen I N-terminal propeptide, 15.3–52.7 ng/ml), β-CTx (beta C-terminal cross-linked telopeptides of type I collagen, <0.584 ng/ml), OC (osteocalcin, 14.0–46.0 ng/ml), 25(OH)VD3 (25-dihydroxyvitamin D3, 20.0–40.0 ng/ml), and PTH (parathyroid hormone, 15.0–65.0 pg/ml).
Statistical Analysis
The Kolmogorov-Smirnov test was used to test the normality of continuous data. Means±SD was used when the continuous data were normally distributed, and medians (Q1, Q3) were used when the data were non-normally distributed. The classification data were expressed as the number of cases and percentage. The t test was used to compare continuous data in accordance with normal distribution, while the Mann-Whitney U test was used to compare continuous data in non-normal distribution. The chi-squared test was used to compare the percentage of classification data. The correction for confounding factors between the 2 groups was by binary logistic regression. Spearman correlation testing was used to evaluate the correlation between different bone turnover markers and bone strength. Locally-weighted polynomial regression (LOESS) was used to fit the curves. P values of less than 0.05 were considered to indicate statistically significant differences.
Results
Demographic Analysis for all AS Patients
In the present study, there were 283 males (83.5%) and 56 females (16.5%). The mean age was 32 (25, 42) years, the course of AS was 9 (5, 15) years, and radiographic hip involvement (BASRI-hip ≥2) was noted in 52.2% (177/339) of all cases. The number of cases with BASRI-hip score from 0 to 4 were 136 (40.1%), 26 (7.7%), 95 (28%), 71 (20.9%), and 11 (3.2%), respectively.
Comparison of Basic Clinical Information
There was no significant difference in most of the basic clinical features between RHI-AS and WORHI-AS, including sex, course of disease, family history, smoking history, HLA-B27 and CRP (Table 1). However, the age of RHI-AS was lower than that of WORHI-AS [27(23,37) vs 37(28, 48) years, P<0.001], and the BMI of RHI-AS was lower than that of WORHI-AS [21.0(18.7, 24.5) vs 23.5(21.2, 26.8) kg/m2, P<0.001] (Table 1).
Table 1.
Information for AS with and without radiographic hip involvement.
| RHI-AS (n=177) | WORHI-AS (n=162) | P | |
|---|---|---|---|
| Male, n (%) | 154 (87.0) | 129 (76.9) | 0.068 |
| Age (years), median (Q1, Q3) | 27 (23, 37) | 37 (28, 48) | <0.001 |
| Course of disease (years), median (Q1, Q3) | 9 (5, 14) | 9 (4, 16) | 0.785 |
| BMI (kg/m2), mean±SD | 21.0 (18.7, 24.5) | 23.5 (21.2, 26.8) | <0.001 |
| Family history, n (%) | 21 (11.9) | 31 (19.1) | 0.063 |
| Smoking history, n (%) | 97 (54.8) | 72 (44.4) | 0.057 |
| HLA-B27 (positive), n (%) | 167 (94.4) | 147 (90.7) | 0.204 |
| CRP (mg/dl), median (Q1, Q3) | 26 (12.1, 54.3) | 20.6 (9.8, 57.8) | 0.284 |
| BASDAI, median (Q1, Q3) | 3.8 (2.4, 5.3) | 3.8 (2.2, 5.2) | 0.893 |
| mSASSS, median (Q1, Q3) | 25 (14.5, 35) | 23 (14.8, 33.5) | 0.821 |
RHI-AS – ankylosing spondylitis with radiographic hip involvement; WORHI-AS – ankylosing spondylitis without radiographic hip involvement.
Comparison of Bone Strength
The stiffness index of RHI-AS was significantly lower than that of WORHI-AS (ORadj=0.982, 95% CIadj=0.968~0.997, Padj=0.017), regardless of adjustment for confounding factors (age and BMI). The difference between patients with RHI-AS and WORHI-AS was more significant in the Z score, which represents bone strength compared with people of the same age and race. The Z scores of patients with RHI-AS were lower than those of patients with WORHI-AS (ORadj=0.802, 95% CIadj=0.679~0.949, Padj=0.01), and the incidence of “below the expected range for age” (Z≤−2) in the RHI-AS group was significantly higher than that in the WORHI-AS group (ORadj=1.891, 95% CIadj=1.079~3.313, Padj=0.026). Similarly, the T scores of patients with RHI-AS were lower than those of patients with WORHI-AS, but there was no significant difference in the incidence of osteoporosis (T≤−2.5) (Table 2).
Table 2.
Bone strength of AS with and without radiographic hip involvement.
| RHI-AS (n=177) | WORHI-AS (n=162) | Unadjusted | Adjusted for age and BMI | |||
|---|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |||
| SI, median (Q1, Q3) | 77.9 (66, 85.7) | 80.3 (71.6, 89.4) | 0.015 | 0.984 (0.971, 0.997) | 0.017 | 0.982 (0.968, 0.997) |
| Z score | ||||||
| Median (Q1, Q3) | −1.5 (−2.5, −0.6) | −0.8 (−1.7,0.3) | <0.001 | 0.692 (0.592, 0.809) | 0.01 | 0.802 (0.679, 0.949) |
| ≤−2, n (%) | 66 (37.3) | 26 (16.0) | <0.001 | 3.110 (1.852, 5.223) | 0.026 | 1.891 (1.079, 3.313) |
| T score | ||||||
| Median (Q1, Q3) | −2.2 (−3.2, −1.5) | −1.8 (−2.6, −1) | 0.002 | 0.780 (0.667, 0.913) | 0.005 | 0.781 (0.658, 0.927) |
| ≤−2.5, n (%) | 76 (42.9) | 56 (34.6) | 0.115 | 1.424 (0.918, 2.211) | 0.115 | 1.478 (0.909, 2.404) |
SI – Stiffness index; RHI-AS – ankylosing spondylitis with radiographic hip involvement; WORHI-AS – ankylosing spondylitis without radiographic hip involvement.
Comparison of Bone Turnover Markers
The TP1NP and OC of patients with RHI-AS seemed to be higher than those of patients with WORHI-AS, but there was no significant difference after adjusting for age and BMI, and the adjusted ORs (95% CIs) were 1.006 (0.997, 1.014) for TP1NP and 1.020 (0.994, 1.047) for OC. Differences between RHI-AS and WORHI-AS were no significant in the other 3 bone turnover markers, including β-CTx, 25(OH)VD3, and PTH (Table 3).
Table 3.
Bone turnover markers of AS with and without radiographic hip involvement.
| RHI-AS(n=177) | WORHI-AS(n=162) | Unadjusted | Adjusted for age and BMI | |||
|---|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |||
| TP1NP (ng/ml), median (Q1, Q3) | 64.41 (48.0, 90.09) | 58.47 (40.2, 69.11) | 0.004 | 1.011 (1.004, 1.019) | 0.185 | 1.006 (0.997, 1.014) |
| β-CTx (ng/ml), median (Q1, Q3) | 0.63 (0.44, 0.83) | 0.64 (0.51, 0.79) | 0.773 | 1.058 (0.723, 1.549) | 0.677 | 1.021 (0.927, 1.124) |
| OC (ng/ml), median (Q1, Q3) | 24.07 (17.13, 31.44) | 20.43 (15.77, 25.5) | 0.001 | 1.041 (1.016, 1.067) | 0.135 | 1.020 (0.994, 1.047) |
| 25(OH)VD3 (ng/ml), median (Q1, Q3) | 15.11 (10.55, 24.16) | 16.08 (11.41, 24.52) | 0.307 | 0.988 (0.965, 1.011) | 0.229 | 0.985 (0.961, 1.010) |
| PTH (pg/ml), median (Q1, Q3) | 33.65 (25.4, 47.23) | 37.0 (25.8, 50.9) | 0.255 | 0.992 (0.979, 1.006) | 0.683 | 1.003 (0.989, 1.018) |
RHI-AS – ankylosing spondylitis with radiographic hip involvement; WORHI-AS – ankylosing spondylitis without radiographic hip involvement; TP1NP – total procollagen I N-terminal propeptide; β-CTx – beta C-terminal cross-linked telopeptides of type I collagen; OC – osteocalcin; 25(OH)VD3 – 25-dihydroxyvitamin D3, PTH – parathyroid hormone.
Correlation Between Bone Strength and Bone Turnover Markers
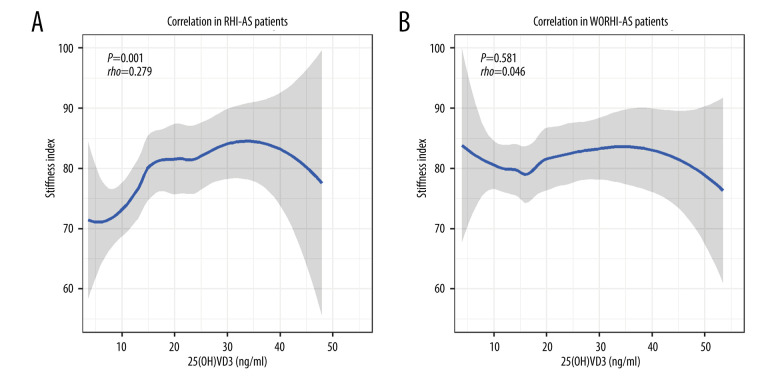
In RHI-AS patients, only 25(OH)VD3 had a correlation with SI (rho=0.279, P=0.001), while the other 4 bone turnover markers showed no correlation with the stiffness index. In WORHI-AS patients, no bone turnover markers were correlated with the stiffness index (Table 4). The correlation curves between 25(OH)VD3 and stiffness index are shown in Figure 1.
Table 4.
Correlation between stiffness index and bone turnover markers.
| RHI-AS (n=177) | WORHI-AS (n=162) | |||
|---|---|---|---|---|
| rho | P | rho | P | |
| TP1NP (ng/ml) | −0.105 | 0.198 | 0.076 | 0.367 |
| β-CTx (ng/ml) | −0.104 | 0.204 | 0.068 | 0.417 |
| OC (ng/ml) | 0.032 | 0.699 | −0.071 | 0.394 |
| 25(OH)VD3 (ng/ml) | 0.279 | 0.001 | 0.046 | 0.581 |
| PTH (pg/ml) | −0.086 | 0.29 | −0.007 | 0.934 |
Rho – Spearman’s rank correlation coefficient; RHI-AS – ankylosing spondylitis with radiographic hip involvement; WORHI-AS – ankylosing spondylitis without radiographic hip involvement; TP1NP – total procollagen I N-terminal propeptide; β-CTx – beta C-terminal cross-linked telopeptides of type I collagen; OC – osteocalcin; 25(OH)VD3 – 25-dihydroxyvitamin D3; PTH – parathyroid hormone.
Figure 1.
(A, B) Correlation curves between 25(OH)VD3 and stiffness index in AS patients. Locally-weighted polynomial regression (LOESS) was used to fit the curves.
Discussion
Hip involvement is the common clinical manifestations of ankylosing spondylitis, which can lead to disability, with an incidence ranging from 19% to 47% as evaluated by X-ray [2,8]. The hip, which is a ball (femoral head) and socket (acetabulum) joint that connects the trunk and lower limbs, plays an important role in maintaining balance and supporting bodyweight. AS patients with hip involvement are thus more likely to fall and have fractures; therefore, knowledge of bone strength in AS patients with hip involvement is highly important and should be emphasized.
Bone quantity and bone quality are the 2 main components of bone strength. Bone mineral density (BMD), the per-volume unit of bone mineral content, is often used to reflect bone quantity, and a few studies have explored the bone mineral density of AS patients with hip involvement. Wang et al found that hip involvement was an important risk factor for decreased femoral BMD independent of age and BMI in 504 AS patients who were followed up for 5 years (OR=1.313, P=0.028) [9]. However, it is not sufficient to reflect bone strength only by BMD; bone quality is related to bone elasticity and influenced by bone microarchitecture and geometry, which is also an important component of bone strength. Quantitative ultrasonography (QUS) is a convenient method to evaluate bone strength that combines bone quantity and bone quality [7,10]. Using the stiffness index measured by QUS to evaluate bone strength, the present study found that the bone strength of AS patients with radiographic hip involvement was significantly lower than that of AS patients without radiographic hip involvement, even after adjusting for age and BMI (ORadj=0.982, 95% CIadj=0.968~0.997, Padj=0.017). These results indicate that patients with hip involvement may have worse bone quantity and bone quality than those without hip involvement.
The reason for lower bone strength in RHI-AS patients remains unclear. We concluded that there may be 2 reasons for this ambiguity: Exercise at a young age can improve bone quality and prevent osteoporosis, and these protective benefits will continue into old age [11]. The destruction of the hip structure decreases the ability of AS patients to exercise, and some RHI-AS patients spend a long time in wheelchairs or are bedridden. Another factor is the pathological condition of inflammation, which can be severe in patients with WORHI-AS. Under chronic inflammatory conditions, osteoclast differentiation factor RANKL, which is produced by immunocytes, can promote the differentiation of macrophages to osteoclasts, resulting in bone loss [12]. RHI-AS patients have more severe symptoms than WORHI-AS patients, which is indicative of a more severe inflammatory state [8,13].
Among bone turnover markers, total collagen I N-terminal peptide (TP1NP) and osteocalcin (OC) seemed to be higher in RHI-AS patients than in WORHI-AS patients, but the statistical difference was not significant after adjusting for age and BMI. There was no significant difference in the level of 25(OH)VD3 between RHI-AS patients and WORHI-AS patients. We found that 25(OH)VD3 was correlated with the stiffness index in RHI-AS patients (rho=0.279, P=0.001), but this correlation was not established in WORHI-AS patients. These seemingly contradictory results are related to the complex relationship between 25(OH)VD3 and SI. In RHI-AS patients, Figure 1A indicates that SI increased with 25(OH)VD3 elevation when 25(OH)VD3 was relatively low, while SI decreased when 25(OH)VD3 was excessive. In WORHI-AS patients, Figure 1B indicates that SI fluctuated slightly as 25(OH)VD3 gradually increased. The relationship between SI and 25 (OH)VD3 was not simply linear, and showed different forms in different types of AS patients. Thus, even though the RHI-AS patients had lower SI, it did not necessarily lead to a significant difference in overall 25 (OH)VD3 levels between the RHI-AS group and WORHI-AS group. These results suggest that 25(OH)VD3 may play a more important role in bone metabolism when the disease progresses to radiologic hip damage in AS patients. These results also suggest that proper supplementation of vitamin D may be beneficial for bone strength, while excessive supplementation may have the opposite effect. When the BMD decreases to the threshold of osteoporosis, 25(OH)VD3 is an independent risk factor for fragile fracture [14], so supplementation with vitamin D may be an important treatment to prevent osteoporotic fracture in patients with RHI-AS.
The limitations of this study may include the following aspects. First, radiologic hip involvement is a relatively late stage of hip damage in AS, and whether the conclusions of this paper are applicable to AS patients with early hip involvement should be further studied. Second, fragile fractures were not analyzed in the present study due to the limitations of data sources and research methods.
Conclusions
AS patients have lower bone strength once the disease progresses to radiologic hip involvement. Treatment of vitamin D deficiency may be an effective way to improve bone strength in RHI-AS patients.
Footnotes
Conflicts of Interest
None.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Source of support: Beijing JST Research Funding (ZR-201915)
References
- 1.Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71:1599–613. doi: 10.1002/art.41042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vander Cruyssen B, Vastesaeger N, Collantes-Estevez E. Hip disease in ankylosing spondylitis. Curr Opin Rheumatol. 2013;25:448–54. doi: 10.1097/BOR.0b013e3283620e04. [DOI] [PubMed] [Google Scholar]
- 3.Jeong H, Eun YH, Kim IY, et al. Characteristics of hip involvement in patients with ankylosing spondylitis in Korea. Korean J Intern Med. 2017;32:158–64. doi: 10.3904/kjim.2015.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, Li J, Xia Z, Xu W. The gait deviations of ankylosing spondylitis with hip involvement. Clin Rheumatol. 2019;38:1163–75. doi: 10.1007/s10067-018-4401-y. [DOI] [PubMed] [Google Scholar]
- 5.Hu LY, Lu T, Chen PM, et al. Should clinicians pay more attention to the potential underdiagnosis of osteoporosis in patients with ankylosing spondylitis? A national population-based study in Taiwan. PLoS One. 2019;14:e0211835. doi: 10.1371/journal.pone.0211835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirzaali MJ, Libonati F, Ferrario D, et al. Determinants of bone damage: An ex-vivo study on porcine vertebrae. PLoS One. 2018;13:e0202210. doi: 10.1371/journal.pone.0202210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raum K, Grimal Q, Varga P, et al. Ultrasound to assess bone quality. Curr Osteoporos Rep. 2014;2:154–62. doi: 10.1007/s11914-014-0205-4. [DOI] [PubMed] [Google Scholar]
- 8.Vander Cruyssen B, Muñoz-Gomariz E, Font P, et al. Hip involvement in ankylosing spondylitis: epidemiology and risk factors associated with hip replacement surgery. Rheumatology (Oxford) 2010;49:73–81. doi: 10.1093/rheumatology/kep174. [DOI] [PubMed] [Google Scholar]
- 9.Wang DM, Zeng QY, Chen SB, et al. Prevalence and risk factors of osteoporosis in patients with ankylosing spondylitis: A 5-year follow-up study of 504 cases. Clin Exp Rheumatol. 2015;33:465–70. [PubMed] [Google Scholar]
- 10.Falardeau T, Belanger P. Ultrasound tomography in bone mimicking phantoms: Simulations and experiments. J Acoust Soc Am. 2018;144:2937–46. doi: 10.1121/1.5079533. [DOI] [PubMed] [Google Scholar]
- 11.Pagnotti GM, Styner M, Uzer G, et al. Combating osteoporosis and obesity with exercise: Leveraging cell mechanosensitivity. Nat Rev Endocrinol. 2019;15:339–55. doi: 10.1038/s41574-019-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover-role of the immune system. Nat Rev Endocrinol. 2016;12:518–32. doi: 10.1038/nrendo.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Zheng W, Zhang C, Li J, et al. Radiographic hip involvement in ankylosing spondylitis: Factors associated with severe hip diseases. J Rheumatol. 2015;42:106–10. doi: 10.3899/jrheum.140428. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-López FR, Chedraui P, Pilz S. Vitamin D supplementation after the menopause. Ther Adv Endocrinol Metab. 2020;11:1–13. doi: 10.1177/2042018820931291. [DOI] [PMC free article] [PubMed] [Google Scholar]