Currently, the gold standard for diagnosing diabetic kidney disease (DKD) is the measurement of the urinary albumin-to-creatinine ratio (UACR), but it has been clarified that there are many cases of decreased renal function and atypical nephropathy without the development of albuminuria (1,2). Given these diversified pathologies of DKD, it is necessary to identify new biomarkers that can predict renal prognosis at an earlier disease stage.
In this retrospective case-control study, we performed posttranslational modification analysis of plasma albumin by electrospray ionization time-of-flight mass spectrometry (3,4) in 257 outpatients with type 2 diabetes at a single center to evaluate association between the posttranslational modifications of albumin and DKD progression. In fact, 257 patients with type 2 diabetes (179 men and 78 women) aged 49–80 years (mean ± SD 67.1 ± 10.7) who visited the Jinnouchi Clinic, Diabetes Care Center, in Kumamoto, Japan, between April 2014 and June 2017 were enrolled, and the follow-up period was 2 years. According to Kidney Disease: Improving Global Outcomes (KDIGO) glomerular filtration rate (GFR) categories in chronic kidney disease, the subjects were classified into five estimated (e)GFR stages (Fig. 1A).
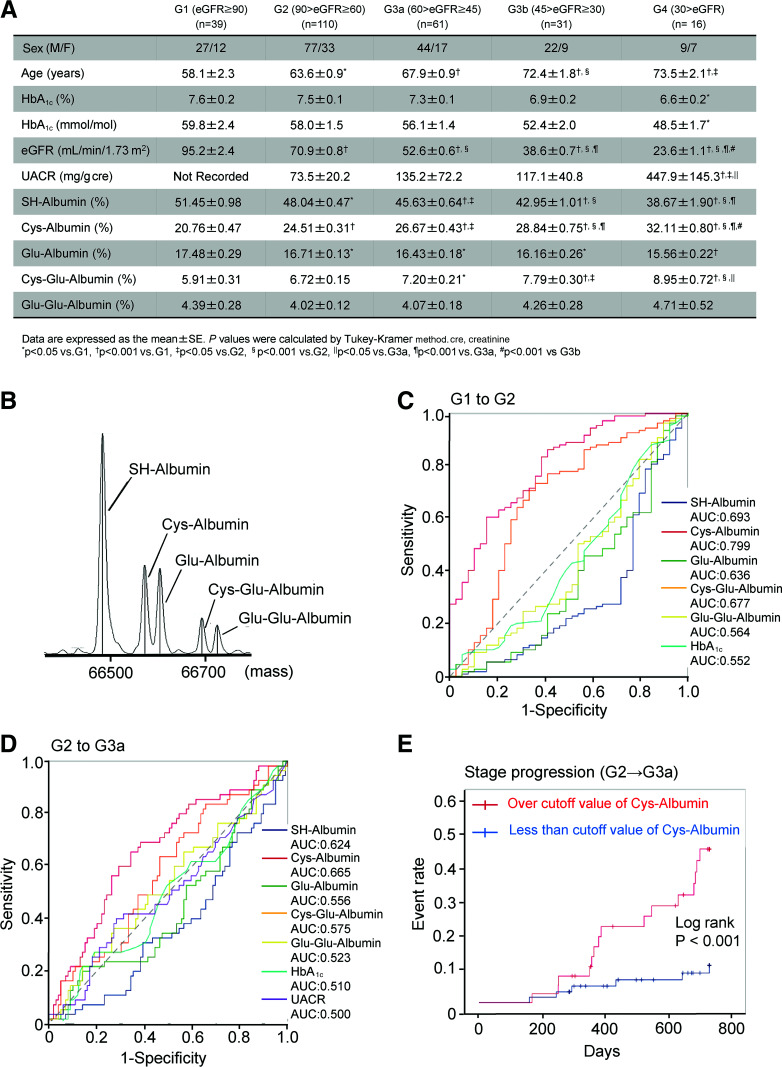
Figure 1.
A: Characteristics of patients enrolled in this study (n = 257; 179 men, 78 women). B: The mass peaks of each posttranslational form of serum albumin in patients with type 2 diabetes. C and D: Receiver operating characteristics curve analysis of each posttranslational modified albumin, HbA1c, and UACR for predicting each nephropathy stage progression in patients with type 2 diabetes with G1 to G2 stage (C) and G2 to G3a stage (D). E: Kaplan-Meier curve analysis for occurrence of nephropathy stage progression (G2 to G3a) in patients with type 2 diabetes with G2 stage of nephropathy who were above or below the cutoff value of Cys-Albumin (log-rank test: P = 0.001).
As a result of posttranslational modification analysis of plasma albumin, five modifications were detected in patients with type 2 diabetes (Fig. 1B): 1) native albumin having free thiol residue at Cys-34 (SH-Albumin); 2) oxidized albumin, which is an oxidative cysteinylation at Cys-34 (Cys-Albumin); 3) glycated albumin in which one molecule of glucose is bound (Glu-Albumin); 4) albumin in which cysteine and glucose each bind one molecule (Cys-Glu-Albumin); and 5) glycated albumin in which two molecules of glucose are bound (Glu-Glu-Albumin). For these albumin peaks, the proportion of each albumin posttranslational modification was calculated using the following formula: each albumin fraction/total albumin fraction (peaks 1–5) × 100.
The percentages of these albumin modifications were compared among all eGFR stages (Fig. 1A). The results showed that SH-Albumin and Glu-Albumin were decreased with the decline of eGFR. On the other hand, Cys-Albumin and Cys-Glu-Albumin were increased. For Glu-Glu-Albumin, no significant differences were observed among the different eGFR stages. In particular, significant changes in Cys-Albumin (P < 0.001) were observed during early-stage progression (G1 to G2).
Receiver operating characteristic analysis showed that, among the five posttranslational modifications of albumin, the diagnostic power of Cys-Albumin was the highest, with an area-under-the-curve (AUC) value of 0.799 for the diagnosis of G2 stage (G1 to G2) by using cutoff value 21.47% (sensitivity = 0.836, specificity = 0.615) (Fig. 1C). Interestingly, for the diagnosis of G3a stage (G2 to G3a), the AUC of Cys-Albumin (AUC = 0.665, cutoff value = 25.69% [sensitivity = 0.655, specificity = 0.657]) was higher than that of UACR (P = 0.012) (Fig. 1D). Among the 22 patients whose stage progressed to G3a after 2 years, 15 patients had higher Cys-Albumin than the cutoff value of 25.69%. Kaplan-Meier analysis showed that the time to stage progression (G2 to G3a) was significantly shorter in this group of patients (Fig. 1E).
Multiple regression analysis demonstrated that Cys-Albumin (partial regression coefficient = –1.687 [SE = 0.279]), standardized partial regression coefficient = –0.414) was most strongly associated with decline in eGFR among Cys-Albumin, age, HbA1c, diabetes duration, systolic blood pressure, BMI, plasma albumin concentration, or UACR as independent variables in patients with type 2 diabetes (R2 = 0.469, P < 0.001).
In this study, Glu-Albumin and Glu-Glu-albumin, which reflect hyperglycemia, were not dominant as diagnostic markers for DKD. These results were presumed to be due to the fact that HbA1c level was well controlled in our study population (Fig. 1A). Previously, we have reported that Cys-Albumin can be used as an oxidative stress marker reflecting the redox state in the body (3,4). The present results of Cys-Albumin reflecting renal function well suggest that oxidative stress is involved in renal dysfunction even in patients with good glycemic control. Therefore, in addition to the conventional management of glycation stress by monitoring HbA1c, the monitoring of oxidative stress such as Cys-Albumin in daily clinical practice may be important for preventing the onset and progression of DKD.
The limitation of this study is that the number of subjects was relatively small. Since this was a retrospective study at a single center, future long-term prospective studies at multiple centers are needed to evaluate the usefulness of Cys-Albumin as an early diagnostic marker for DKD. It would also be necessary to investigate whether Cys-Albumin could serve as an early diagnostic marker for other complications in patients with type 2 diabetes in the future.
In conclusion, our data emphasize that Cys-Albumin has potential to serve as a biomarker for the progression of kidney disease in patients with type 2 diabetes.
Article Information
Acknowledgments. The authors thank the investigators, staff, and trial participants for dedication and commitment to the trial.
Funding. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI grant numbers JP20H03406 and JP19K07166 and JSPS Fellows grant 17J11624.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contribution. T.I. and H.W. wrote the manuscript and researched data. H.K., T.N., K.T., I.F., N.A., Y.S., N.K., and A.M. researched data. H.M., M.T., K.M., T.W., M.F., M.O., M.L.F., H.J., and T.M. contributed to the discussion and reviewed/edited the manuscript. K.O., A.Y., and J.S. researched data, contributed to discussion, and reviewed/edited the manuscript. T.I., H.W., K.O., and T.M. were responsible for the work as a whole, including the study design, access to data, drafting of the article, and critical revision of the manuscript for important intellectual content. All authors approved the version to be published. T.I. and H.W. are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y; Japan Diabetes Clinical Data Management Study Group . Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant 2009;24:1212–1219 [DOI] [PubMed] [Google Scholar]
- 2. Merchant ML, Perkins BA, Boratyn GM, et al. Urinary peptidome may predict renal function decline in type 1 diabetes and microalbuminuria. J Am Soc Nephrol 2009;20:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagumo K, Tanaka M, Chuang VTG, et al. Cys34-cysteinylated human serum albumin is a sensitive plasma marker in oxidative stress-related chronic diseases. PLoS One 2014;9:e85216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watanabe H, Imafuku T, Otagiri M, Maruyama T. Clinical implications associated with the posttranslational modification–induced functional impairment of albumin in oxidative stress–related diseases. J Pharm Sci 2017;106:2195–2203 [DOI] [PubMed] [Google Scholar]