Abstract
OBJECTIVE
Emerging data from animal and human pilot studies suggest potential benefits of glucagon-like peptide 1 receptor agonists (GLP-1RA) on lung function. We aimed to assess the association of GLP-1RA and chronic lower respiratory disease (CLRD) exacerbation in a population with comorbid type 2 diabetes (T2D) and CLRD.
RESEARCH DESIGN AND METHODS
A new-user active-comparator analysis was conducted with use of a national claims database of beneficiaries with employer-sponsored health insurance spanning 2005–2017. We included adults with T2D and CLRD who initiated GLP-1RA or dipeptidyl peptidase 4 inhibitors (DPP-4I) as an add-on therapy to their antidiabetes regimen. The primary outcome was time to first hospital admission for CLRD. The secondary outcome was a count of any CLRD exacerbation associated with an inpatient or outpatient visit. We estimated incidence rates using inverse probability of treatment weighting for each study group and compared via risk ratios.
RESULTS
The study sample consisted of 4,150 GLP-1RA and 12,540 DPP-4I new users with comorbid T2D and CLRD. The adjusted incidence rate of first CLRD admission during follow-up was 10.7 and 20.3 per 1,000 person-years for GLP-1RA and DPP-4I users, respectively, resulting in an adjusted hazard ratio of 0.52 (95% CI 0.32–0.85). For the secondary outcome, the adjusted incidence rate ratio was 0.70 (95% CI 0.57–0.87).
CONCLUSIONS
GLP-1RA users had fewer CLRD exacerbations in comparison with DPP-4I users. Considering both plausible mechanistic pathways and this real-world evidence, potential beneficial effects of GLP-1RA may be considered in selection of an antidiabetes treatment regimen. Randomized clinical trials are warranted to confirm our findings.
Introduction
Glucagon-like peptide 1 receptor agonists (GLP-1RA) define a class of glucose-lowering agents indicated to treat patients with type 2 diabetes (T2D). The mechanism of action of GLP-1RA involves stimulation of insulin secretion by activation of the pancreatic glucagon-like peptide 1 receptors in the pancreas (1,2). These receptors are abundantly expressed in multiple organs including stomach (3), heart (4), lung (5), and kidney (6), providing pathways for potential effects of GLP1-RA beyond glucose control. Indeed, based on recent clinical trials evidence, several GLP-1RA (liraglutide, semaglutide, and dulaglutide) have been granted a U.S. Food and Drug Administration indication for major cardiovascular risk prevention in patients with T2D and cardiovascular disease (7).
GLP-1RA may also offer a potential benefit for patients with T2D and comorbid chronic lower respiratory disease (CLRD). In an ex vivo study, GLP-1RA showed a protective effect in attenuating human bronchial hyperresponsiveness (5), a known hallmark of CLRD. Similar potential protective effects were reported in several animal studies (8,9). Moreover, results from a small noncontrolled study have shown a decline in CLRD exacerbations in asthma patients (10). While these results suggest a potential role of GLP-1RA for patients with T2D and CLRD, conclusive population-based evidence is not available. Therefore, the aim of the current study was to assess the association between GLP-1RA use and CLRD exacerbations in a population of patients with T2D and CLRD.
Research Design and Methods
Study Design and Data Source
A new-user active-comparator analysis within a retrospective cohort design was carried out with use of IBM MarketScan Commercial Claims Database data from 2005 to 2017. The data include longitudinal information on health care use of privately insured employees, retirees, and their dependents in the U.S. Data of reimbursed medical services, including diagnoses and procedures associated with in- and outpatient encounters as well as outpatient pharmacy medication-dispensing claims, are available. Each dispensed medication is accompanied by information about the dispensed days of supply, strength, quantity, and route of administration.
Study Sample
The study sample comprised patients aged >17 years who have had at least one inpatient or two outpatient encounters with T2D and CLRD, defined based on the presence of diagnoses or medication dispensing (Supplementary Tables 1 and 2) during the year before index date. Exposure assignment and start of follow-up (index date) were defined based on the first observed prescription for GLP-1RA and dipeptidyl peptidase 4 inhibitors (DPP-4I) (whichever came first) in the database. Patients whose first prescription fill did not follow at least 365 days’ continuous health plan enrollment were excluded to achieve a new-user design. We included patients who used either study drug class as an add-on to other antidiabetes agents, e.g., metformin. Patients using insulin prior to the index date or with a diagnosis of type 1 diabetes were excluded. Patients with a history of cystic fibrosis, lung cancer, pulmonary embolism, and pulmonary hypertension, which may independently worsen lung function, were excluded, as were women with pregnancy at index date. Patients with thyroid carcinoma who would likely be channeled to DPP-4I agents because of the related GLP-1RA contraindication were also excluded. Finally, patients with clinical conditions (except chronic obstructive pulmonary disease [COPD] and asthma) that frequently require chronic systemic corticosteroid treatment, which is protective against lung exacerbations, were excluded.
Exposure Measurement
Patients were included in the GLP-1RA group if they initiated one of the following approved drugs: exenatide, liraglutide, dulaglutide, or albiglutide. If the patients initiated saxagliptin, sitagliptin, linagliptin, or alogliptin then they were assigned to the DPP-4I group. Drug exposure was assumed from the day of a prescription fill until 30 days after exhaustion of the dispensed days’ supply; thus; exposure gaps <30 days were permitted. Switching between drugs in the same exposure group was allowed.
Outcome Measurement
First CLRD hospitalization, the primary study end point, was defined as hospital admission with a principal diagnosis of CLRD or a combination of a principal diagnosis of lower–respiratory tract infection, respiratory distress syndrome, or respiratory failure and a secondary diagnosis of CLRD.
The secondary outcome was the number of CLRD exacerbations requiring hospitalization or an emergency room visit with a principal diagnosis of CLRD or a new prescription fill of systemic corticosteroids (dexamethasone, hydrocortisone, methylprednisolone, prednisolone, or prednisone). Because pulmonary exacerbations among CLRD patients require ∼1 week post–treatment initiation for recovery (11), and clinical guidelines (12) recommend a 10- to 14-day course of oral corticosteroid treatment, we established a minimum 2-week treatment gap requirement to distinguish between episodes. For instance, if a patient received an oral corticosteroid right after hospitalization for CLRD or visited an emergency room a week after discharge, the patients were assumed to have had only one episode.
Study Follow-up
For the primary analysis, patients were followed from the index date until the primary end point, health plan disenrollment, discontinuation of study drug, adding or switching to a medication of the comparator group, hospitalization for reasons other than CLRD exacerbations, or reaching 1 year of follow-up (study end). The patients were censored at hospital admission because claims data lack information on drug use during admission and thus drug exposure assignments would have been unreliable. The study was ended at 1 year of follow-up because we were particularly interested in evaluating outcomes outside of the known impact of GLP-1RA on weight loss, which would be expected to materialize during longer treatment duration. In the analysis of the secondary outcome, the patients were not censored at the first event to allow for counting of all exacerbations during follow-up (Supplementary Fig. 1).
Measurement of Confounders
Based on a literature search and clinical expertise, we identified 45 potential confounders including demographics, clinical conditions, clinical procedures, health services use, and certain prescription medication use. All measured confounders were ascertained during the 1-year baseline period (definitions and lists for the potential confounders and corresponding measurements can be found in Supplementary Appendix 1).
Statistical Analysis
Inverse probability of treatment weights was used to adjust for confounders. The weights were calculated from exposure propensity scores obtained after fitting of a logistic regression model to predict GLP-1RA versus DPP-4I initiation. The weights were stabilized with use of the marginal mean of the propensity scores (stabilized inverse probability treatment weighting [SIPTW]). We further trimmed the top and bottom 1% of the SIPTW. We fitted a Cox proportional hazards model to estimate the adjusted hazard ratio (HR) of first CLRD hospital admission, between GLP-1RA and DPP-4I users. We further stratified the analysis by CLRD type. For our secondary outcome, we used a Poisson regression model to compare CLRD exacerbation rates between the two study groups. Again, SIPTW was used to account for potential confounders. The 95% CIs were estimated with use of the robust variance estimator (13).
Sensitivity Analyses
To evaluate the robustness of our findings, we estimated the propensity score using a machine learning technique, namely, Bayesian additive regression trees (BART), instead of logistic regression. Second, instead of weighting the study sample, we matched GLP-1RA users to DPP-4I users (1:2 match) based on the propensity score. Third, because of the very low sensitivity of obesity and smoking measures in claims data, we used multiple imputation techniques to obtain information on obesity and tobacco use that was available from linked survey data for a subsample of ∼5% of our study population. Fourth, we augmented our prespecified variables that were included in the propensity score with ∼100 additional potential confounders, identified via high-dimensional propensity score technique (14). Fifth, although this has been refuted, DPP-4I have been linked to lower–respiratory tract infections (15–19), and to address potential channeling, we replicated our analysis using sulfonylureas instead of DPP-4I as the comparator group. Sixth, to increase the specificity of the primary outcome, we required CLRD as the principal diagnosis. Seventh, to further balance for potential differences in diabetes duration and severity across comparison groups, we provided results restricted to patients who initiated the study drugs as an addition to only metformin. Eighth, drug discontinuation or switching may be differential between the two groups, and therefore we adjusted for censoring by estimating the inverse probability of censoring weight, which was added as second weight to balance comparison groups. Finally, we used skin infection in a negative outcome analysis to test residual confounding and, particularly, presence of a healthy user effect. Additional information on sensitivity analyses is presented in Supplementary Appendix 2.
All data management was carried out with SAS, version 9.4 (SAS Institute, Cary, NC). Descriptive analyses and inferential models were performed with R software, version 3.4.2 (R Foundation for Statistical Computing) and the following R packages: ipw (20), survey (21), ggplot2 (22), mice (23), and tableone (24).
Results
Descriptive
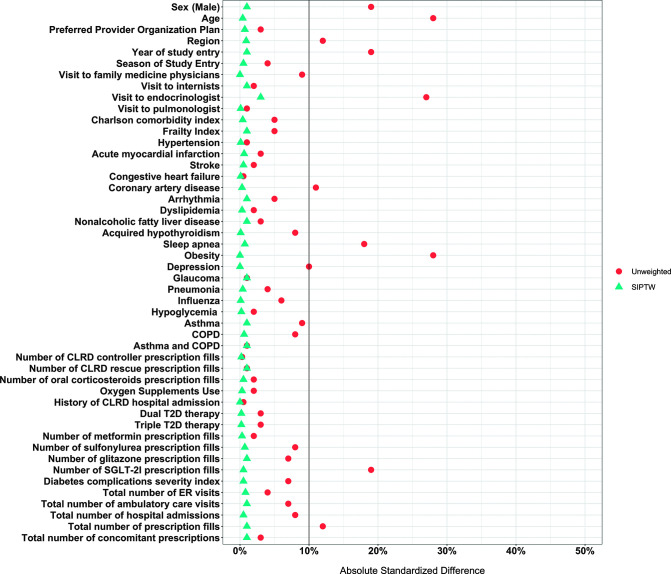
We identified ∼700,000 new users of either GLP-1RA or DPP-4I who had a T2D diagnosis during the 1-year baseline period. After application of our inclusion and exclusion criteria, the study sample consisted of 4,150 GLP-1RA and 12,540 DPP-4I users whom we identified as patients with T2D and CLRD (Supplementary Fig. 2). The majority of the study sample was female: 65% of GLP-1RA and 55% of DPP-4I groups. Obesity was significantly higher among the GLP-1RA (34%) users in comparison with DPP-4I (21%) users. Most of the comorbidities were distributed similarly in the study groups, with similar Charlson comorbidity indices. The use of CLRD medications was also similar between the study groups including use of CLRD controllers, e.g., inhaled corticosteroids, or CLRD rescue medications such as short-acting β-agonists. Finally, study groups showed similar health care services use (Table 1). The distribution of propensity scores, i.e., the probability of receiving GLP-1RA conditional on measured confounders, showed almost complete overlap between the two drug groups (Supplementary Fig. 3). Figure 1 shows the absolute standardized differences of covariates after application of SIPTW: all close to 0. During follow-up, the number of the prescriptions fills was relatively similar across comparison groups (median 7 and 8 for GLP-1RA and DPP-4I users, respectively). The average days’ supply dispensed with each fill was similar as well (median 30 days).
Table 1.
Characteristics of GLP-1RA and DPP-4I users with T2D and comorbid CLRD
| Characteristic | Unweighted sample | SIPTW weighted sample | ||||
|---|---|---|---|---|---|---|
| DPP-4I, N = 12,540 | GLP-1RA, N = 4,150 | ASD | DPP-4I, N = 12,445 | GLP-1RA, N = 4,091 | ASD | |
| Demographics | ||||||
| Male sex, n (%) | 5,628 (44.9) | 1,471 (35.4) | 0.19 | 5,293 (42.5) | 1,679 (41.1) | 0.01 |
| Age, years | 54.33 (7.8) | 52.02 (8.8) | 0.28 | 53 (8) | 53.6 (7.8) | 0.00 |
| Preferred Provider Organization plan, n (%) | 7,657 (61.1) | 2,587 (62.3) | 0.03 | 7,657 (61.5) | 2,508 (61.4) | 0.01 |
| Region, n (%) | ||||||
| Northeast | 2,354 (18.8) | 589 (14.2) | 0.12 | 2,208 (17.7) | 708 (17.3) | 0.01 |
| North central | 3,032 (24.2) | 1,008 (24.3) | 0.00 | 3,009 (24.2) | 989 (24.1) | 0.00 |
| South | 5,366 (42.8) | 1,969 (47.4) | 0.09 | 5,460 (43.9) | 1,814 (44.4) | 0.01 |
| West | 1,636 (13) | 530 (12.8) | 0.01 | 1,620 (13) | 524 (12.8) | 0.01 |
| Unknown | 152 (1.2) | 54 (1.3) | 0.01 | 152 (1.2) | 49 (1.2) | 0.01 |
| Year of study entry, n (%) | ||||||
| 2007 | 1,077 (8.6) | 387 (9.3) | 0.02 | 1,028 (8.2) | 361 (8.8) | 0.01 |
| 2008 | 835 (6.7) | 157 (3.8) | 0.13 | 747 (6) | 250 (6.1) | 0.01 |
| 2009 | 1,010 (8.1) | 154 (3.7) | 0.18 | 876 (7) | 278 (6.8) | 0.01 |
| 2010 | 1,282 (10.2) | 357 (8.6) | 0.05 | 1,231 (9.9) | 396 (9.7) | 0.00 |
| 2011 | 1,584 (12.6) | 359 (8.7) | 0.13 | 1,461 (11.7) | 478 (11.7) | 0.00 |
| 2012 | 1,756 (14.0) | 516 (12.4) | 0.05 | 1,706 (13.7) | 546 (13.4) | 0.01 |
| 2013 | 1,144 (9.1) | 341 (8.2) | 0.03 | 1,111 (8.9) | 358 (8.8) | 0.01 |
| 2014 | 1,206 (9.6) | 413 (10) | 0.01 | 1,217 (9.8) | 398 (9.7) | 0.00 |
| 2015 | 1,126 (9) | 532 (12.8) | 0.12 | 1,238 (9.9) | 402 (9.8) | 0.01 |
| 2016 | 829 (6.6) | 481 (11.6) | 0.17 | 983 (7.9) | 328 (8) | 0.00 |
| 2017 | 691 (5.5) | 453 (10.9) | 0.19 | 850 (6.8) | 288 (7) | 0.00 |
| Season of study entry, n (%) | ||||||
| Winter (December–February) | 3,031 (24.2) | 924 (22.3) | 0.04 | 2,968 (23.8) | 979 (23.9) | 0.00 |
| Spring (March–May) | 3,189 (25.4) | 1,110 (26.7) | 0.03 | 3,193 (25.6) | 1,050 (25.7) | 0.01 |
| Summer (June–August) | 3,216 (25.6) | 1,095 (26.4) | 0.02 | 3,201 (25.7) | 1,042 (25.5) | 0.00 |
| Fall (September–November) | 3,104 (24.7) | 1,021 (24.6) | 0.00 | 3,088 (24.8) | 1,012 (24.8) | 0.01 |
| Visit to family medicine physicians, n (%) | 7,604 (60.6) | 2,694 (64.9) | 0.09 | 7,679 (61.7) | 2,530 (61.9) | 0.00 |
| Visit to internists, n (%) | 6,016 (48) | 2,039 (49.1) | 0.02 | 6,012 (48.3) | 1,990 (48.7) | 0.01 |
| Visit to endocrinologist, n (%) | 1,150 (9.2) | 766 (18.5) | 0.27 | 1,421 (11.4) | 489 (11.9) | 0.03 |
| Visit to pulmonologist, n (%) | 2,182 (17.4) | 747 (18) | 0.01 | 2,177 (17.5) | 713 (17.5) | 0.00 |
| Comorbidities | ||||||
| Charlson comorbidity index | 2.69 (1.2) | 2.63 (1.1) | 0.05 | 2.67 (1.1) | 2.66 (1.1) | 0.00 |
| Frailty index | 0.16 (0.4) | 0.15 (0.4) | 0.05 | 0.16 (0.4) | 0.16 (0.4) | 0.01 |
| Hypertension, n (%) | 8,853 (70.6) | 2,907 (70) | 0.01 | 8,784 (70.5) | 2,859 (70) | 0.00 |
| Acute myocardial infarction, n (%) | 137 (1.1) | 34 (0.8) | 0.03 | 129 (1) | 43 (1) | 0.01 |
| Stroke, n (%) | 304 (2.4) | 87 (2.1) | 0.02 | 293 (2.4) | 94 (2.3) | 0.01 |
| Congestive heart failure, n (%) | 681 (5.4) | 230 (5.5) | 0.00 | 674 (5.4) | 218 (5.3) | 0.00 |
| Coronary artery disease, n (%) | 2,066 (16.5) | 526 (12.7) | 0.11 | 1,929 (15.5) | 621 (15.2) | 0.00 |
| Arrhythmia, n (%) | 1,004 (8) | 282 (6.8) | 0.05 | 957 (7.7) | 307 (7.5) | 0.01 |
| Dyslipidemia, n (%) | 8,525 (68) | 2,771 (66.8) | 0.02 | 8,440 (67.8) | 2,757 (67.5) | 0.00 |
| Nonalcoholic fatty liver disease, n (%) | 612 (4.9) | 229 (5.5) | 0.03 | 631 (5.1) | 217 (5.3) | 0.01 |
| Acquired hypothyroidism, n (%) | 1,782 (14.2) | 709 (17.1) | 0.08 | 1,858 (14.9) | 615 (15) | 0.00 |
| Sleep apnea, n (%) | 2,695 (21.5) | 1,225 (29.5) | 0.18 | 2,923 (23.5) | 987 (24.16) | 0.01 |
| Obesity, n (%) | 2,636 (21) | 1,395 (33.6) | 0.28 | 3,006 (24.1) | 1,008 (24.6) | 0.00 |
| Depression, n (%) | 1,560 (12.4) | 674 (16.2) | 0.10 | 1,672 (13.4) | 560 (13.7) | 0.00 |
| Glaucoma, n (%) | 888 (7.1) | 279 (6.7) | 0.01 | 865 (6.9) | 276 (6.7) | 0.01 |
| Pneumonia, n (%) | 1,050 (8.4) | 307 (7.4) | 0.04 | 1,009 (8.1) | 321 (7.9) | 0.00 |
| Influenza, n (%) | 240 (1.9) | 115 (2.8) | 0.06 | 264 (2.1) | 87 (2.1) | 0.00 |
| Hypoglycemia, n (%) | 83 (0.7) | 35 (0.8) | 0.02 | 88 (0.7) | 30 (0.7) | 0.00 |
| CLRD | ||||||
| CLRD type, n (%) | ||||||
| Asthma | 5,966 (47.6) | 2,153 (51.9) | 0.09 | 6,066 (48.7) | 2,036 (49.8) | 0.01 |
| COPD | 4,906 (39.1) | 1,461 (35.2) | 0.08 | 4,748 (38.1) | 1,529 (37.4) | 0.01 |
| Asthma and COPD | 1,668 (13.3) | 536 (12.9) | 0.01 | 1,635 (13.1) | 518 (12.7) | 0.01 |
| Number of CLRD controller prescription fills* | 2.5 (4.2) | 2.5 (4) | 0.00 | 2.5 (4.1) | 2.5 (4.1) | 0.00 |
| Number of CLRD rescue prescription fills† | 1.7 (2.7) | 1.7 (2.6) | 0.01 | 1,7 (2.6) | 1.7 (2.5) | 0.01 |
| Number of oral corticosteroid prescription fills | 1.3 (2) | 1.3 (1.8) | 0.02 | 1.3 (2) | 1.3 (1.9) | 0.01 |
| Oxygen supplements use, n (%) | 781 (6.2) | 242 (5.8) | 0.02 | 758 (6.1) | 248 (6.1) | 0.00 |
| History of CLRD hospital admission, n (%) | 290 (2.3) | 99 (2.4) | 0.01 | 288 (2.3) | 96 (2.3) | 0.00 |
| T2D | ||||||
| Dual T2D therapy, n (%) | 8,625 (68.8) | 2,802 (67.5) | 0.03 | 8,538 (68.6) | 2,805 (68.7) | 0.00 |
| Triple T2D therapy, n (%) | 3,915 (31.2) | 1,348 (32.5) | 0.03 | 3,912 (31.4) | 1,278 (31.3) | 0.00 |
| Number of metformin prescription fills | 5.5 (4.3) | 5.4 (4.2) | 0.02 | 5.5 (4.2) | 5.5 (4.3) | 0.00 |
| Number of sulfonylurea prescription fills | 3.1 (4.2) | 2.8 (4.1) | 0.08 | 3 (4.2) | 3 (4.3) | 0.01 |
| Number of glitazone prescription fills | 1.3 (3) | 1.1 (2.8) | 0.07 | 1.2 (2.9) | 1.2 (2.9) | 0.01 |
| Number of SGLT-2I prescription fills | 0.2 (1.2) | 0.5 (1.9) | 0.19 | 0.3 (1.5) | 0.3 (1.4) | 0.00 |
| Diabetes Complications Severity Index | 0.6 (0.7) | 0.5 (0.7) | 0.07 | 0.5 (0.7) | 0.5 (0.7) | 0.00 |
| Health care services use in previous year | ||||||
| Total number of ER visits | 0.1 (0.3) | 0.1 (0.3) | 0.04 | 0.1 (0.3) | 0.1 (0.3) | 0.01 |
| Total number of ambulatory care visits | 21.5 (16.7) | 22.7 (17.2) | 0.07 | 21.8 (16.8) | 22.1 (16.7) | 0.01 |
| Total number of hospital admissions | 0.2 (0.6) | 0.2 (0.5) | 0.08 | 0.2 (0.5) | 0.2 (0.5) | 0.00 |
| Total number of prescription fills | 16.2 (6.5) | 17 (6.5) | 0.12 | 16.3 (6.5) | 16.5 (6.2) | 0.01 |
| Total number of concomitant prescriptions | 7.7 (3.3) | 7.8 (3.3) | 0.03 | 7.7 (3.3) | 7.7 (3.2) | 0.01 |
| Eye exam, n (%) | 1,753 (14) | 612 (14.7) | 0.02 | NA | NA | NA |
| Blood glucose test, n (%) | 11,633 (92.8) | 3,895 (93.9) | 0.04 | NA | NA | NA |
| Urinalysis, n (%) | 6,323 (50.4) | 2,085 (50.2) | 0.00 | NA | NA | NA |
Data are mean (SD) unless otherwise indicated. ASD, absolute standardized differences; ER, emergency room; NA, not available (was not included in the SIPTW analysis); SGLT-2I, sodium–glucose cotransporter 2 inhibitors.
Controller medications include inhaled corticosteroids, leukotriene antagonist, long-acting β-agonists, and long-acting muscarinic antagonists.
Rescue medications include inhaled short-acting β-agonists and short-acting muscarinic medications.
Figure 1.
Balance of sample characteristics before and after application of SIPTW. ER, emergency room; SGLT-2I, sodium–glucose cotransporter 2 inhibitors.
Primary Outcome
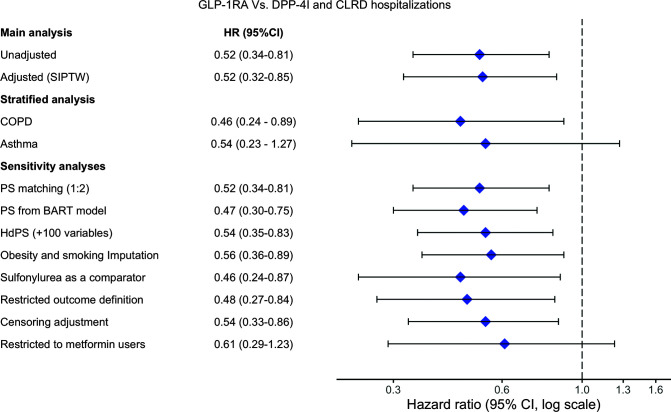
We identified a total of 174 new CLRD hospitalizations in the study sample (Table 2, Supplementary Table 3, and Supplementary Fig. 4) with corresponding incidence rates of 11 per 1,000 person-years (95% CI 7.3–16.6) and 20.6 per 1,000 person-years (95% CI 17.6–24.2) for GLP-1RA and DPP-4I users, respectively. The adjusted SIPTW incidence rates were 10.7 per 1,000 person-years (95% CI 7.1–16.2) and 20.3 per 1,000 person-years (95% CI 17.2–23.8) for GLP-1RA and DPP-4I users, respectively. The adjusted HR showed GLP-1RA use associated with a lower risk for CLRD hospitalizations (HR 0.52, 95% CI 0.32–0.85). When stratified by CLRD type, COPD and asthma patients were consistent with the main analysis but CIs became wider, especially for the asthma group due a small number of events (HR 0.54, 95% CI 0.23–1.27) (Fig. 2).
Table 2.
Risk for CLRD hospitalizations and CLRD exacerbations among GLP-1RA and DPP-4I users
| GLP-1RA | DPP-4I | |
|---|---|---|
| Sample, n | 4,150 | 12,540 |
| Primary outcome: first CLRD hospitalization | ||
| CLRD hospitalization, n | 23 | 151 |
| Days of follow-up, mean | 175 | 203 |
| Person-years, n | 2,089 | 7,328.1 |
| Incidence rate per 1,000 person-years (95% CI) | 11 (7.3–16.6) | 20.6 (17.6–24.2) |
| Unadjusted HR (95% CI) | 0.52 (0.34–0.81) | Reference |
| SIPTW-adjusted HR (95% CI) | 0.52 (0.32–0.85) | Reference |
| Secondary outcome: number of pulmonary exacerbations | ||
| CLRD exacerbations, n | 122 | 528 |
| Days of follow-up, mean | 184 | 215 |
| Person-years, n | 2,096.7 | 7,387.6 |
| Incidence rate per 1,000 person-years (95% CI) | 58.2 (48.7–69.5) | 71.5 (65.6–77.8) |
| Unadjusted RR (95% CI) | 0.81 (0.66–0.99) | Reference |
| SIPTW-adjusted RR (95% CI) | 0.70 (0.57–0.87) | Reference |
RR, rate ratio.
Figure 2.
CLRD hospitalizations (primary outcome) among GLP-1RA vs. DPP-4I users: main and sensitivity analyses. hdPS, high-dimensional propensity score; PS, propensity score.
Secondary Outcome
A total of 650 exacerbations requiring emergency or inpatient care or oral steroid initiation were identified during follow-up (Table 2). GLP-1RA users had ∼13 fewer exacerbations per 1,000 person-years compared with DPP-4I users. The incidence rate ratio of experiencing CLRD exacerbations estimated using SIPTW was 0.70 (95% CI 0.56–0.86).
Sensitivity Analyses
Using BART to estimate propensity scores resulted in an HR of 0.47 (95% CI 0.30–0.75). The estimated HRs from propensity score matching, adjustment with a high-dimensional propensity score, and multiple imputation analyses to incorporate unmeasured information on obesity and smoking were 0.52 (0.34–0.81), 0.54 (0.35–0.83), and 0.56 (0.36–0.89), respectively. Sulfonylurea users showed a slightly higher incidence rate of CLRD hospitalizations in comparison with DPP-4I (26.2 per 1,000 person-years). The estimated HR of CLRD hospitalizations associated with GLP-1RA versus sulfonylurea use was 0.46 (95% CI 0.24–0.87). After restriction of the outcome definition to CLRD diagnoses designated as principal cause for admissions, the HR was 0.48 (95% CI 0.27–0.84). When the analysis was restricted to patients who initiated the study drugs as an add-on to metformin only, the point estimate was consistent with that of the main analysis (HR 0.61, 95% CI 0.29–1.23). Adjusting for potential imbalances in patient characteristics created through informative censoring produced findings similar to those of the main analysis (HR 0.54, 95% CI 0.33–0.86). Finally, evaluation of skin infection, which should not be different between the groups if patients’ health statuses were similar, showed no significant associations. HR of skin infection was 1.15 (95% CI 0.55–2.41) in comparison of GLP-1RA with DPP-4I (Fig. 2).
Conclusions
Based on our analysis of real-world data spanning from 2005 through 2017, we observed that patients with T2D and CLRD who initiated GLP-1RA had a lower incidence rate both of CLRD hospitalizations and of a more broadly defined range of CLRD exacerbations. Various sensitivity analyses demonstrated the robustness of these results. These results, if confirmed in randomized clinical trials, hold the potential to significantly improve respiratory outcomes in patients with T2D and CLRD with GLP-1RA.
Studies pertaining to the effect of GLP-1RA use in CLRD patients are scarce. Existing evidence originates mainly from animal studies (5,9,25). We are aware of only one clinical study investigating the effect of GLP-1RA use in patients with T2D and comorbid asthma, which showed promising findings. This study included only nine asthma patients with obesity, who received GLP-1RA and were followed for 52 weeks without a comparator group, allowing no formal inferences (10). Another prospective study of 30 patients with T2D found improvement in patients’ lung function, yet patients with respiratory diseases were excluded (26). Overall, to date, human studies have not been sufficient to provide clinical utility to prescribers in the management of patients with T2D and comorbid CLRD.
In our study we addressed these shortcomings by investigating a large national real-world T2D population of privately insured patients, establishing sufficient statistical power for studying the risk for CLRD hospitalization. We adopted an active-comparator design, selecting DPP-4I users as the comparator for several clinical and methodological reasons. First, DPP-4I, similar to GLP-1RA, are recommended as a second-line therapeutic option and were available shortly after GLP-RA entered the market, thus placing them at a similar level of adoption and clinical experience in selection of diabetes treatment regimens. Second, it has been shown that DPP-4I use is not associated with lung function change (27); hence, unlike insulin and other comparators that could worsen lung function, DPP-4I serve as an appropriate reference for evaluation of GLP-1RA in real-world settings. Of note, changing our comparator drug to sulfonylureas yielded similar results.
Biological plausibility is pivotal to infer a causal association from clinical observational studies. Glucagon-like peptide 1 (GLP-1) receptors are abundantly expressed in human lungs (28), and when they are activated, bronchodilation and prevention of bronchial hyperresponsiveness occur (29). Moreover, administration of GLP-1RA in patients with T2D has been shown to improve the forced expiratory volume in 1 s and the forced vital capacity (26). Both measures are typically higher in CLRD patients with fewer exacerbations (30). Although DPP-4I inhibit the enzyme that inactivates endogenous GLP-1, which may produce an effect similar to that of GLP-1RA by increasing endogenous GLP-1 hormone levels, endogenous GLP-1 levels are extremely low in comparison with exogenous GLP-1. GLP-1RA have been shown to increase GLP-1 hormone levels by 10-fold, which may result in increased potency in comparison with DPP-4I (31). More recently, the coronavirus disease 2019 pandemic has brought new attention to the role of T2D and antidiabetes drugs in regulating inflammation (32). Our study, in conjunction with these mechanistic pathways and evidence from animal studies, calls for a reappraisal of the GLP-1RA role in CLRD.
One key concern in observational studies is the nonrandom nature of exposure assignment, leading to potential confounding. Comparison group characteristics, displayed in Table 1, fail to provide an explanation for the observed association and suggest limited channeling. We aimed to address unmeasured confounders using several strategies. First, to capture a potential healthy user effect (i.e., GLP-1RA users have a healthier lifestyle) we examined the proportion of patients who had eye exams performed as an indication of better adherence to monitoring recommendations. The proportion was similar between the groups. Second, to align patients with respect to T2D severity and control, we used an active-comparator new-user design. Because uncontrolled glucose levels, which are not captured by claims data, are associated with worsening lung function (33), we ensured that comparison groups had similar diabetes severity indices and a similar history of glucose-lowering agent use. Nevertheless, future studies that capture HbA1c levels over time and integrate this information to further balance comparison groups are warranted. For further addressing the impact of misclassified obesity status, we used internal validation data to improve the measurement. Results showed a slight change in the point estimate, yet conclusions remained the same. We limited time-varying effects such as changes in BMI by restricting study follow-up to 1 year. Finally, we tested whether use of high-dimensional propensity scores, which are used to screen encounter records for any potential predictor of exposure, might capture additional measured confounders or proxies of unmeasured confounders. The adjusted estimate was consistent with the primary analysis. We also evaluated effects on negative outcomes that would be affected by unmeasured risk factors such as healthy lifestyle, cardiovascular risk factors, and diabetes severity and found no associations. Using new-user and active-comparator study designs further mitigated the risk of unmeasured confounders (34).
To address potential outcomes misclassification, we conducted a sensitivity analysis where we required CLRD as principal diagnosis for admission. Requiring CLRD as principal diagnosis for admission has shown high positive predictive value (>90%) (35). Likewise, using COPD codes in the primary position of inpatient encounters has shown high specificity in validation studies (>99%) focused on identifying COPD admission (36). Our study conclusions remained unchanged. Moreover, grouping asthma and COPD in one composite outcome may underestimate or overestimate the GLP-1RA association with each individual condition. We, therefore, stratified the analysis by CLRD condition and found similar results, though nonsignificant among asthma patients due to a small number of hospitalizations in this group. Moreover, we censored at hospitalization to curb the impact of exposure misclassification, which may have resulted in a loss of follow-up and consequently a loss of study events. However, only 0.2% of the study sample was censored due to hospitalization, and this was nondifferential between the study groups. Therefore, censoring patients due to hospitalization likely would not have distorted the observed effect. Finally, we should note that while broadly applicable to privately insured patients in the U.S., our exclusion criteria do not allow inferences for patients with insulin use and thus are not generalizable to patients with advanced stages of T2D.
It might be surprising that, given the observed magnitude of a beneficial effect, limited reports of clinical experience have offered related hypotheses. However, pulmonologists involved in treatment of CLRD patients during admission are likely not the prescribers of GLP-1RA or DPP-4I, and thus the benefit of GLP-1RA may not have been detected in clinical practice. We should furthermore consider the wide CIs of our effect estimates, owing to the small number of available events, which confirm statistical power sufficient for conclusions of beneficial effects but also illustrate the uncertainty around the exact size of such effect.
Considering the presence of comorbidities among patients with T2D, recent clinical guidelines have added directions regarding advantages and disadvantages for each second-line glucose-lowering therapeutic class in terms of effects on comorbidities. For instance, patients with T2D and morbid obesity may initiate GLP-1RA or SGLT-2I to promote weight loss (37). In absence of no prior finding of beneficial effects offered by other drug classes (26,38,39), future clinical guidelines should consider available evidence to provide direction for clinicians who manage patients with T2D and comorbid CLRD.
In summary, in this first comparative effectiveness study, patients with T2D and comorbid CLRD who initiated GLP-1RA had significantly fewer CLRD hospitalizations in comparison with initiators of DPP-4I. This association was reproducible for milder forms of CLRD exacerbation. The results remained unchanged in multiple sensitivity analyses aimed at examining the effect of residual confounding and measurement biases. Considering both plausible mechanistic pathways and this real-world evidence, potential beneficial effects of GLP-1RA should be considered in selection of an antidiabetes treatment regimen. Randomized clinical trials are warranted to confirm our findings.
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.A. conceptualized the study and was supported by K.C. and A.G.W. All authors contributed to the study design and data interpretation. Y.A. performed the statistical analyses and was supported by M.J.D. Y.A. drafted the first version of manuscript. All authors made critical revisions to the manuscript. Y.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14138138.
References
- 1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 2. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 2007;113:546–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broide E, Bloch O, Ben-Yehudah G, Cantrell D, Shirin H, Rapoport MJ. GLP-1 receptor is expressed in human stomach mucosa: analysis of its cellular association and distribution within gastric glands. J Histochem Cytochem 2013;61:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okerson T, Chilton RJ. The cardiovascular effects of GLP-1 receptor agonists. Cardiovasc Ther 2012;30:e146–e155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogliani P, Calzetta L, Capuani B, et al. Glucagon-like peptide 1 receptor: a novel pharmacological target for treating human bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 2016;55:804–814 [DOI] [PubMed] [Google Scholar]
- 6. Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept 2007;141:120–128 [DOI] [PubMed] [Google Scholar]
- 7. Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: An updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab 2019;21:2576–2580 [DOI] [PubMed] [Google Scholar]
- 8. Nguyen D-V, Linderholm A, Haczku A, Kenyon N. Obesity-related, metabolic asthma: a new role for glucagon-like peptide 1 agonists. Lancet Respir Med 2017;5:162–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu T, Wu XL, Zhang W, Xiao M. Glucagon like peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a protein kinase A (PKA)-dependent nuclear factor-κB (NF-κB) signaling pathway in mice. Int J Mol Sci 2015;16:20195–20211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan F, Mat A, Hogan AE, et al. Preliminary asthma-related outcomes following glucagon-like peptide 1 agonist therapy. QJM 2017;110:853–854 [DOI] [PubMed] [Google Scholar]
- 11. Pauwels RA. Similarities and differences in asthma and chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc 2004;1:73–76 [DOI] [PubMed] [Google Scholar]
- 12. Rabe KF, Hurd S, Anzueto A, et al.; Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 [DOI] [PubMed] [Google Scholar]
- 13. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570 [DOI] [PubMed] [Google Scholar]
- 14. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willemen MJ, Mantel-Teeuwisse AK, Straus SM, Meyboom RH, Egberts TC, Leufkens HG. Use of dipeptidyl peptidase-4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care 2011;34:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gamble J-M, Donnan JR, Chibrikov E, Twells LK, Midodzi WK, Majumdar SR. Comparative safety of dipeptidyl peptidase-4 inhibitors versus sulfonylureas and other glucose-lowering therapies for three acute outcomes. Sci Rep 2018;8:15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012;344:e1369. [DOI] [PubMed] [Google Scholar]
- 18. Schernthaner G, Barnett AH, Emser A, et al. Safety and tolerability of linagliptin: a pooled analysis of data from randomized controlled trials in 3572 patients with type 2 diabetes mellitus. Diabetes Obes Metab 2012;14:470–478 [DOI] [PubMed] [Google Scholar]
- 19. Wvan der Zanden R, de Vries F, Lalmohamed A, et al. Use of dipeptidyl-peptidase-4 inhibitors and the risk of pneumonia: a population-based cohort study. PLoS One 2015;10:e0139367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Wal WM, Geskus RB. ipw: An R package for inverse probability weighting. J Stat Softw 2011;43:1–23 [Google Scholar]
- 21. Lumley T. Analysis of complex survey samples. J Stat Softw 2004;9:1–19 [Google Scholar]
- 22. Wickham H, Sievert C. ggplot2: Elegant Graphics for Data Analysis. Cham, Switzerland, Springer, 2016 [Google Scholar]
- 23. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67 [Google Scholar]
- 24. Yoshida K. Package ‘tableone,’ 2020. Accessed 17 March 2020. Available from https://cloud.r-project.org/web/packages/tableone/tableone.pdf
- 25. Viby N-E, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology 2013;154:4503–4511 [DOI] [PubMed] [Google Scholar]
- 26. Rogliani P, Matera MG, Calzetta L, et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir Med 2019;154:86–92 [DOI] [PubMed] [Google Scholar]
- 27. Colice G, Price D, Gerhardsson de Verdier M, et al. The effect of DPP-4 inhibitors on asthma control: an administrative database study to evaluate a potential pathophysiological relationship. Pragmat Obs Res 2017;8:231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Leary NA, Wright MW, Brister JR, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 2016;44:D733–D745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cazzola M, Rogliani P, Calzetta L, Lauro D, Page C, Matera MG. Targeting mechanisms linking COPD to type 2 diabetes mellitus. Trends Pharmacol Sci 2017;38:940–951 [DOI] [PubMed] [Google Scholar]
- 30. Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:245–251 [DOI] [PubMed] [Google Scholar]
- 31. Briere DA, Bueno AB, Gunn EJ, Michael MD, Sloop KW. Mechanisms to elevate endogenous GLP-1 beyond injectable GLP-1 analogs and metabolic surgery. Diabetes 2018;67:309–320 [DOI] [PubMed] [Google Scholar]
- 32. Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev 2020;41:bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2005;161:546–556 [DOI] [PubMed] [Google Scholar]
- 34. Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol 2015;11:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oelsner EC, Loehr LR, Henderson AG, et al. Classifying chronic lower respiratory disease events in epidemiologic cohort studies. Ann Am Thorac Soc 2016;13:1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest 2012;141:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S90–S102 [DOI] [PubMed] [Google Scholar]
- 38. Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res 2015;16:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hitchings AW, Lai D, Jones PW; Metformin in COPD Trial Team . Metformin in severe exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2016;71:587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]