Abstract
OBJECTIVE
SGTL2 inhibitors increase urinary glucose excretion and have beneficial effects on cardiovascular and renal outcomes. The underlying mechanism may involve caloric restriction-like metabolic effects due to urinary glucose loss. We investigated the effects of dapagliflozin on 24-h energy metabolism and insulin sensitivity in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
There were 26 patients with type 2 diabetes randomized to a 5-week double-blind, crossover study with a 6- to 8-week washout. Indirect calorimetry was used to measure 24-h energy metabolism and the respiratory exchange ratio (RER), both by whole-room calorimetry and by ventilated hood during a two-step euglycemic-hyperinsulinemic clamp. Results are presented as the differences in least squares mean (95% CI) between treatments.
RESULTS
Evaluable patients (n = 24) had a mean (SD) age of 64.2 (4.6) years, BMI of 28.1 (2.4) kg/m2, and HbA1c of 6.9% (0.7) (51.7 [6.8] mmol/mol). Rate of glucose disappearance was unaffected by dapagliflozin, whereas fasting endogenous glucose production (EGP) increased by dapagliflozin (+2.27 [1.39, 3.14] μmol/kg/min, P < 0.0001). Insulin-induced suppression of EGP (–1.71 [–2.75, –0.63] μmol/kg/min, P = 0.0036) and plasma free fatty acids (–21.93% [–39.31, –4.54], P = 0.016) was greater with dapagliflozin. Twenty-four-hour energy expenditure (–0.11 [–0.24, 0.03] MJ/day) remained unaffected by dapagliflozin, but dapagliflozin reduced the RER during daytime and nighttime, resulting in an increased day-to-nighttime difference in the RER (–0.010 [–0.017, –0.002], P = 0.016). Dapagliflozin treatment resulted in a negative 24-h energy and fat balance (–20.51 [–27.90, –13.12] g/day).
CONCLUSIONS
Dapagliflozin treatment for 5 weeks resulted in major adjustments of metabolism mimicking caloric restriction, increased fat oxidation, improved hepatic and adipose insulin sensitivity, and improved 24-h energy metabolism.
Introduction
Sodium–glucose cotransporter 2 inhibitors (SGLT2is) inhibit glucose and sodium reabsorption in the proximal renal tubules and have been proven a valuable additional treatment for type 2 diabetes to improve glycemic control and also moderately reduce body weight and blood pressure (1). Importantly, SGLT2is have been shown to reduce cardiovascular (CV) risk, including reduced hospitalization for heart failure and reduced risk of kidney disease progression (2,3). How SGLT2is exert their remarkable effects on CV and renal outcomes is not fully understood. For example, only 12% of the relative risk reduction for all-cause death caused by SGLT2is could be explained by the combined changes in conventional CV risk factors (4).
The primary action of SGLT2i on glucose and sodium reabsorption has several metabolic consequences that may help to explain the effects on CV and renal outcome (1). Such metabolic effects may reflect the adaptive response to the loss of ∼50–100 g glucose/day in the urine, resulting in a form of mild caloric restriction and caloric loss overnight. Consistent with this line of thinking, SGLT2i treatment has been reported to result in increased glucagon levels (5) and decreased insulin secretion (6), most likely mediated by decreased plasma glucose levels (7). Other observed metabolic effects include increased endogenous glucose production (EGP) (8), increased fasting fatty acid oxidation (9), and decreased intrahepatic lipid (IHL) content (10). In addition, several (8,9,11) but not all (10) studies have reported improvements in whole-body/peripheral insulin sensitivity. All these separate effects resemble effects that are also observed after caloric restriction, which is also accompanied by enhanced fat oxidation (12), improved metabolic flexibility (13), reductions in plasma glucose, and improvements in insulin sensitivity (13). Despite these separate finding, so far no studies have investigated the hypothesis that SGTL2i treatment of patients with type 2 diabetes can indeed restore the fed-to-fasting cycle in patients with type 2 diabetes and whether the treatment has caloric restriction-like effect on 24-h energy and substrate metabolism, as was hypothesized previously (14).
Therefore, the aim of the current study was to investigate whether SGLT2i treatment for 5 weeks affects 24-h energy balance and substrate metabolism in patients with type 2 diabetes. To this end, whole-body 24-h energy expenditure and substrate oxidation, whole-body and tissue-specific insulin sensitivity, and body composition, including ectopic fat accumulation, were investigated in large detail in patients with type 2 diabetes after 5 weeks of SGLT2i or placebo treatment.
Research Design and Methods
Study Design and Participants
A double-blind, randomized, placebo-controlled, crossover study was conducted at the Metabolic Research Unit Maastricht (MRUM) of Maastricht University between 5 March 2018 and 4 November 2019. Ethics Committee of Maastricht University Medical Centre (Maastricht, the Netherlands) approved the study, which was conducted to conform to the Declaration of Helsinki (15). Upon written informed consent, patients were randomized to double-blind treatment for two treatment periods, each 5 weeks or a maximum duration of 40 days, separated by 6–8 weeks washout (see Supplementary Material for details). End points were assessed at the end of each 5-week period. Male and female patients with type 2 diabetes, diagnosed for at least 6 months, with HbA1c levels between 6 and 9% (42 and 75 mmol/mol), and on a stable dose of metformin for >3 months or drug naïve, were eligible. Detailed eligibility criteria are in Supplementary Table 1.
Procedures
The study comprised seven visits, including screening (visit 1). Visit 2 (or 5), the first visit of the treatment periods, included randomization and measurement of safety markers. A safety visit (visit 3 or 6) was scheduled after 2 weeks, and an end-of-treatment visit (visit 4 or 7) after 5 weeks. All outcome measures were performed at visit 4/7 and were spread over a period of 6–8 days, and visit 4 was followed by a 6–8 weeks wash-out period. Timing of study medication during end of treatment was ∼8:30 a.m., except on the day of the euglycemic-hyperinsulinemic clamp (EHC), when the timing of medication was at 6:30 a.m., before start of tracer infusion.
During the end-of-treatment visit, blood was drawn at 1:30 p.m. for assessment of HbA1c, uric acid, and hs-CRP. Hereafter, participants stayed in a whole-room calorimeter from 6:00 p.m. onward to measure O2 consumption and CO2 production (16). The last 24 h starting at 5:30 a.m. were used for analysis. In the respiration chamber, participants adhered to an activity protocol consisting of multiple times standing upright and including twice a 15-min stepping exercise at 30 steps/min to increase physical activity levels. Physical activity was measured by Actigraphy (ActiGraph, Pensacola, FL). Participants received three standardized meals at 0830, 1300, and at 1800. Twenty-four-hour energy intake was determined from the sleeping metabolic rate (SMR) during the first night multiplied by an activity factor of 1.5, and all meals were completely consumed by the volunteers. Twenty-four hour energy expenditure and RER were calculated using Brouwer et al. (17). Daytime RER was calculated from 0800 until 2200 and nighttime RER from 0000 till 0530. SMR was defined as the two consecutive hours with lowest energy expenditure using the Weir formula (18) and was averaged for two nights. Twenty-four-hour diet-induced thermogenesis (DIT) was determined by plotting energy expenditure against physical activity level, and the intercept of the regression line at the lowest physical activity represents SMR+DIT (19). Glucose and nitrogen were measured in 24-h urine collection (in 6-h aliquots), the latter to calculate daytime and nighttime protein oxidation. Blood samples were drawn at seven time points (see Supplementary Table 2).
After leaving the respiration chamber after an overnight fast, proton MRS was used to quantify IHL content (see Supplementary Materials for details). Subsequently, an EHC with coinfusion of d-glucose [6,6-D2] tracer (0.04 mg/kg ⋅ min) was performed (20). After 3 h, an insulin infusion was started with 10 mU/m2/min for 3 h to assess hepatic insulin sensitivity and subsequently increased to 40 mU/m2/min for 2.5 h to assess whole-body insulin sensitivity. Glucose (20%) was coinfused to keep glucose levels at ∼5 mmol/L. Arterialized blood (hotbox) was drawn every 5–10 min to monitor glucose concentration. During the last 30 min of every steady-state period, blood samples were collected, and indirect calorimetry (Omnical; Maastricht Instruments, Maastricht, the Netherlands) was performed to assess substrate utilization according to Péronnet et al. (21). Nonoxidative glucose disposal (NOGD) was defined as the Rd corrected for urinary glucose loss minus carbohydrate oxidation. Metabolic flexibility was defined as the change in the RER from basal to high-insulin stimulated state. Isotopic enrichment of plasma glucose was determined using electron ionization gas chromatography-mass spectrometry (22). Calculation of glucose Ra and Rd was performed according to Steele’s single pool nonsteady state equations (23). Basal Rd values were corrected for fasting urinary glucose excretion during the night, and Rd values during insulin infusion were corrected for urinary glucose excretion during the clamp. In two participants, glucose excretion during the clamp could not be measured in one of the periods, and fasted urinary glucose excretion was used, multiplied by the percentage average difference on urinary glucose excretion in the fasted (2.209 and 0.008 μmol/kg/min on dapagliflozin and placebo) and insulin-stimulated state (2.656 and 0.006 μmol/kg/min on dapagliflozin and placebo). Exclusion of these two subjects did not alter the EHC results.
On day 6, 7, or 8 during the end-of-treatment visit, a DEXA scan (Hologic, Marlborough, MA) was used to determine body composition.
Changes in vital signs and safety laboratory values were monitored during the study. Adverse events leading to discontinuation from the study as well as serious adverse events and cases of suspected diabetic ketoacidosis were collected.
Biochemical Analysis
Details of the biochemical analysis are outlined in Supplementary Material.
Statistics
The evaluable analysis set, consisting of patients with at least one dose of the investigational product (per protocol) and no important protocol deviations, was used for the statistical analyses, using SAS version 9.04 software and performed by IQVIA Biostatistics, Reading, U.K. The expected difference between treatment groups was estimated using a linear mixed-effects model. This model had treatment group, treatment sequence, and period as fixed effects, as well as a random intercept for each subject. If deviations from normality were detected, a nonparametric test of treatment difference against zero was performed (Wilcoxon signed rank test) using all of the data and ignoring the sequence. The least-squares means (LSM) and the corresponding 95% CIs for treatment effect in the respective treatment periods are presented. The difference in the LSM between the two treatments was computed, with the corresponding 95% CI and P value tabulated. A two-sided 0.05 level was considered statistically significant. We used the Benjamini-Hochberg procedure for multiple testing and found that adjusted P values were in agreement with unadjusted P values. For power calculation, see the Supplementary Material.
The trial is registered with ClinicalTrials.gov, number NCT03338855.
Data and Resource Availability
Data underlying the findings described in this manuscript may be available upon request in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
Results
The study enrolled 38 patients, and 26 patients were randomized (Supplementary Fig. 1). Twelve patients were ineligible for participation. Two patients were excluded from the evaluable data set because of important protocol deviations. One of the excluded participants discontinued the study after completing one period as a result of a change in medical treatment affecting insulin sensitivity. The other patient was excluded because safety and efficacy assessments were not performed as defined in the protocol.
Baseline characteristics of the 24 evaluable patients are reported in Supplementary Table 3. The patients had a mean (SD) age of 64.2 (4.6) years, BMI of 28.1 (2.4) kg/m2 and HbA1c of 6.9% (0.7) (51.7 [6.8] mmol/mol). Patients had a mean estimated glomerular filtration rate of 141 (13.0) mL/min. There were 17 patients on metformin treatment, and 7 patients did not use any antidiabetic drugs. No other antidiabetic drugs were used.
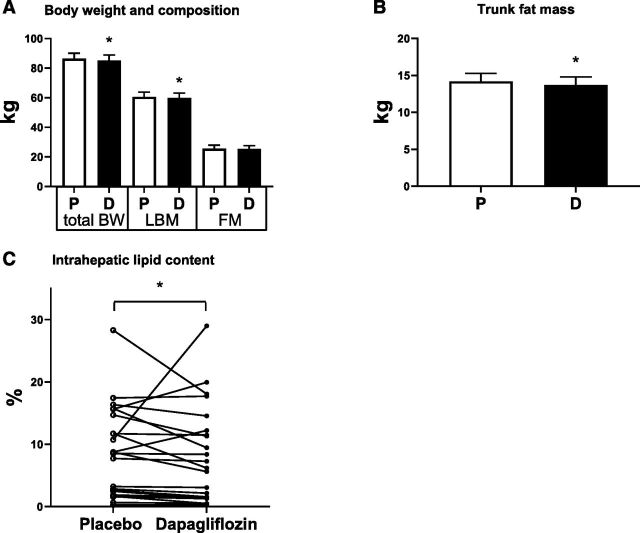
Compliance was >95% during both treatment periods. Body weight was significantly reduced by dapagliflozin treatment compared with placebo (–1.26 (–1.85, –0.66) kg, P = 0.0003) (Fig. 1A). Body weight was measured also at the beginning of each period, and no carryover effects as a result of the treatment sequence were noticed. Assessment of body composition by DEXA showed significantly reduced lean mass by dapagliflozin treatment compared with placebo (–0.67 [–1.29, –0.04] kg, P = 0.038) (Fig. 1A), whereas whole-body fat mass was not significantly affected by dapagliflozin treatment (Fig. 1A). Trunk fat mass (–0.48 [–0.89, –0.07] kg, P = 0.023) (Fig. 1B) as well as IHL content, as measured by 1H-MRS, were lower after dapagliflozin treatment in 18 of 22 patients (P = 0.036) (Fig. 1C).
Figure 1.
Body composition (n = 24) (A), fat mass (n = 24) (B), and IHL (C) content upon placebo (P) and dapagliflozin (D) treatment (n = 22). BW, body weight; FM, fat mass; LBM, lean body mass. Results are in LSM and 95% CI, obtained through a linear mixed model, with the exception of IHL, where a Wilcoxon paired rank sum test was used. *P < 0.05 is considered significantly different.
Systolic blood pressure after 2 weeks of treatment was significantly lower after dapagliflozin treatment (–6.77 [–12.09, –1.45] mmHg, P = 0.015) (Supplementary Table 4), but diastolic blood pressure was not significantly affected by dapagliflozin (–1.85 [–5.68, 1.98] mmHg, P = 0.33) (Supplementary Table 4). Systolic blood pressure (–3.58 [–10.33, 3.16] mmHg, P = 0.28) (Supplementary Table 4) or diastolic blood pressure (–0.65 [–4.61, 3.31] mmHg, P = 0.74) (Supplementary Table 4) after 5 weeks of treatment was not significantly changed after dapagliflozin treatment. Levels of plasma hsCRP (0.22 [–0.45, 0.90] mg/L, P = 0.50) (Supplementary Table 5) and HbA1c (–0.07% [–0.22, 0.08], P = 0.33) (Supplementary Table 5), measured during the end-of-treatment visits, were not significantly altered with dapagliflozin treatment. HbA1c was measured also at the beginning of each period, and no carryover effects as a result of treatment sequence were noticed with respect to HbA1c levels. Urate levels were lower after dapagliflozin treatment (–56.8 [–75.4, –38.1] µmol/L, P < 0.0001) (Supplementary Table 5). Furthermore, levels of hemoglobin were higher with dapagliflozin treatment (0.19 [0.02, 0.35] mmol/L, P = 0.03) (Supplementary Table 5), while there was no significant change in erythrocyte volume fraction (0.004 [–0.006, 0.014] L/L, P = 0.43) (Supplementary Table 5).
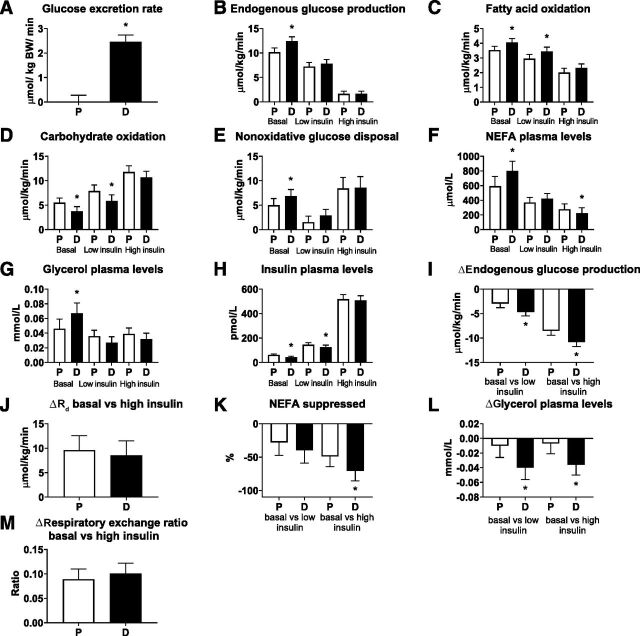
A two-step EHC with indirect calorimetry was performed after an overnight fast when the patients had been in the respiration chamber for 36 h. Urinary glucose excretion during the clamp increased after dapagliflozin treatment (2.46 [2.06, 2.86] µmol/kg/min, P < 0.0001) (Supplementary Table 6 and Fig. 2A). Basal or fasting EGP was higher after dapagliflozin treatment (2.27 [1.39, 3.14] µmol/kg/min, P < 0.0001) (Supplementary Table 6 and Fig. 2B), an increase that was similar in magnitude to the increase in urinary glucose excretion rate. Basal Rd, corrected for urinary glucose loss, was similar between the dapagliflozin and placebo treatment periods (0.11 [–1.12, 1.25] µmol/kg/min, P = 0.85) (Supplementary Table 6). Fasting fat oxidation was higher (0.53 [0.22, 0.85] µmol/kg/min, P = 0.0022) (Supplementary Table 6 and Fig. 2C), while fasting carbohydrate oxidation was lower after dapagliflozin treatment compared with placebo (–1.73 [–2.72, –0.74], µmol/kg/min, P = 0.0016) (Supplementary Table 6 and Fig. 2D). NOGD in the fasted state was higher with dapagliflozin treatment versus placebo (1.85 [0.45, 3.24] µmol/kg/min, P = 0.012) (Supplementary Table 6 and Fig. 2E). Fasting nonesterified fatty acid (NEFA) levels (208.88 [53.88, 363.89] µmol/L, P = 0.011) (Supplementary Table 6, Fig. 2F) and fasting glycerol levels (0.022 [0.005, 0.039] mmol/L, P = 0.013) (Supplementary Table 6 and Fig. 2G) were all significantly higher after dapagliflozin treatment compared with placebo. Fasting insulin levels were lower after dapagliflozin treatment compared with placebo (–18.18 [–23.07, –13.28] pmol/L, P < 0.0001) (Supplementary Table 6 and Fig. 2H).
Figure 2.
Urinary glucose excretion (A), EGP (B), fatty acid (FA) oxidation (C), carbohydrate oxidation (D), NOGD (E), plasma NEFA levels (F), plasma glycerol levels (G), plasma insulin levels (H), ΔEGP (I), ΔRd (J), percentage of NEFA suppression (K), Δsuppression of glycerol plasma levels (L), and ΔRER (M) measured during a two-step EHC upon placebo (P) and dapagliflozin (D) treatment. Results (n = 22) are in LSM and 95% CI, obtained through a linear mixed model. *P < 0.05 is considered significantly different.
Next, a low dose of insulin (10 mU/m2/min) was infused for 3 h to assess hepatic insulin sensitivity. The insulin-induced suppression (ΔEGPlow-basal) was larger with dapagliflozin treatment compared with placebo (–1.71 [–2.78, –0.63] µmol/kg/min, P = 0.0036) (Supplementary Table 6 and Fig. 2I), suggestive of enhanced hepatic insulin sensitivity. Improvements in insulin suppression of EGP were observed despite lower plasma insulin levels after dapagliflozin treatment (–20.73 [–37.62, –3.83] pmol /L, P = 0.019) (Supplementary Table 6 and Fig. 2H). In the low-insulin state, fat oxidation was significantly higher (0.50 [0.11, 0.89] µmol/kg/min, P = 0.015) (Supplementary Table 6 and Fig. 2C), whereas carbohydrate oxidation was significantly lower after dapagliflozin treatment compared with placebo (–2.03 [–3.85, –0.21] µmol/kg/min, P = 0.030) (Supplementary Table 6 and Fig. 2D).
To assess peripheral insulin sensitivity, a high dose (40 mU/m2/min) of insulin was subsequently infused for 2.5 h. Insulin sensitivity expressed as the change in Rd from basal to the high-insulin state and corrected for urinary glucose loss (ΔRdhigh-basal) was not significantly affected by dapagliflozin (–1.07 [–3.18, 1.05] µmol/kg/min, P = 0.33) (Supplementary Table 6). Plasma insulin levels during the high-insulin infusion were not affected by dapagliflozin (–9.73 [–36.54, 17.09] pmol/L, P = 0.46) (Supplementary Table 6 and Fig. 2H), and ΔRdhigh-basal corrected for steady-state insulin concentrations was not different between the treatment arms (–0.0029 [–0.0083, 0.0024], P = 0.27) (Supplementary Table 6 and Fig. 2J). Plasma NEFA was more suppressed upon the high-insulin infusion after dapagliflozin treatment compared with placebo (–21.93% [–39.31, –4.54], P = 0.016) (Supplementary Table 6 and Fig. 2K), resulting in lower NEFA levels during the high-insulin state after dapagliflozin treatment versus placebo (–53.21 [–101.2, –5.23] µmol/L, P = 0.031) (Supplementary Table 6 and Fig. 2F). Similarly, plasma glycerol levels were more suppressed by the high-insulin infusion after dapagliflozin treatment compared with placebo (–0.029 [–0.050, –0.008] mmol/L, P = 0.0085) (Supplementary Table 6 and Fig. 2L). Differences in fatty acid oxidation (0.30 [–0.01, 0.60] µmol/kg/min, P = 0.055) (Supplementary Table 6 and Fig. 2C) or carbohydrate oxidation (–1.11 [–2.33, 0.11] µmol/kg/min, P = 0.071) (Supplementary Table 6 and Fig. 2D) between dapagliflozin and placebo during the high-insulin state did not reach statistical significance. NOGD during the high-insulin infusion rate was not changed by dapagliflozin (0.16 [–1.89, 2.22] µmol/kg/min, P = 0.87) (Supplementary Table 6 and Fig. 2E). The insulin-induced increase in carbohydrate oxidation (ΔCHOox high-basal, 0.77 [–0.37, 1.92] µmol/kg/min, P = 0.17) and reduction in fatty acid oxidation (ΔFAox high-basal, –0.28 [–0.65, 0.09] µmol/kg/min, P = 0.13) were more pronounced after dapagliflozin treatment but did not reach statistical significance (Supplementary Table 6). Changes in RER between basal and high-insulin state (as a measure or metabolic flexibility) were numerically larger after dapagliflozin treatment but did not reach statistical significance (0.01 [–0.01, 0.03], P = 0.18) (Supplementary Table 6 and Fig. 2M).
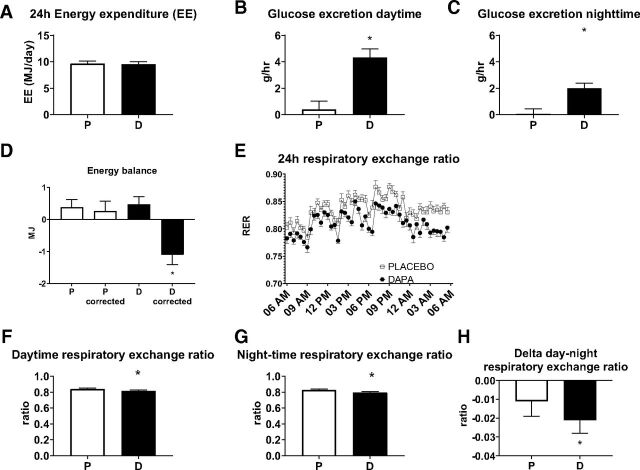
To investigate the effect of dapagliflozin on whole-body substrate metabolism in detail, patients stayed in a respiration chamber for 36 h, and measurements were performed during the last 24 h of the stay. Twenty-four-hour total energy expenditure was not significantly affected by dapagliflozin treatment compared with placebo (–0.11 [–0.25, 0.03] MJ/day, P = 0.11) (Supplementary Table 7 and Fig. 3A). The sleeping metabolic rate was not significantly affected by dapagliflozin treatment (P = 0.36) (Supplementary Table 7). Food intake was similar during both treatment periods, and activity levels did not differ between the two periods (6.33 [–9.65, 22.32] counts/min, P = 0.42). Diet-induced thermogenesis was not significantly affected by dapagliflozin treatment (P = 0.64) (Supplementary Table 7).
Figure 3.
Twenty-four-hour energy expenditure (n = 24) (A), glucose excretion daytime (n = 23) (B), glucose excretion nighttime (n = 23) (C), energy balance (n = 24) (D), 24-h RER plot (n = 24) (E), daytime RER (n = 24) (F), nighttime RER (n = 24) (G), and ΔRER between day and night (n = 24) (H). Results are in LSM and 95% CI, obtained through a linear mixed model. *P < 0.05 is considered significantly different.
Twenty-four-hour urinary glucose loss was significantly higher by dapagliflozin treatment compared with placebo (3.53 [3.04, 4.00] g/h, P < 0.0001) (Supplementary Table 7). Interestingly, urinary glucose loss during dapagliflozin treatment was approximately twofold higher at daytime versus nighttime (4.3 [3.7, 5.0] g/h vs. 2.0 [1.6, 2.4] g/h) (Fig. 3B and C). After correction for energy lost as urinary glucose, the energy balance was negative in the dapagliflozin period (Fig. 3D).
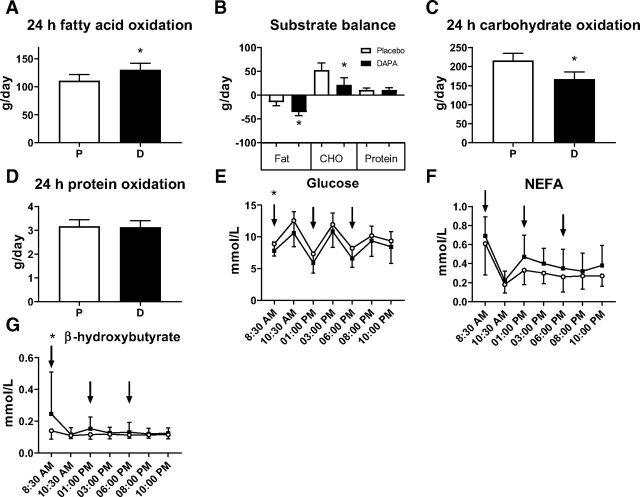
Twenty-four-hour RER was lower after dapagliflozin treatment (–0.02 [–0.03, –0.01], P = 0.0001) (Supplementary Table 7 and Fig. 3E). This effect was observed both during daytime (P = 0.0001) (Supplementary Table 7 and Fig. 3F) and nighttime (P < 0.0001) (Supplementary Table 7 and Fig. 3G). RER was lower at night compared with daytime, and the decrease in RER from day to nighttime, which can be considered as a marker of metabolic flexibility, was larger after dapagliflozin compared with placebo (–0.010 [–0.017, –0.002], P = 0.016) (Supplementary Table 7 and Fig. 3H). Twenty-four-hour fatty acid oxidation was higher after dapagliflozin treatment (19.70 [12.48, 26.92] g/day, P < 0.0001) (Supplementary Table 7 and Fig. 4A), resulting in a negative fat balance after dapagliflozin treatment (−20.51 [−27.90, −13.12] g/day) (Fig. 4B). The higher fat oxidation was observed both during daytime (20.19 [12.57, 27.81] g/day, P < 0.0001) (Supplementary Table 7) and nighttime (22.04 [13.83, 30.26] g/day, P < 0.0001) (Supplementary Table 7). Consequently, 24-h carbohydrate oxidation was lower after dapagliflozin treatment (–49.39 [–69.02, –29.77] g/day, P < 0.0001) (Supplementary Table 7 and Fig. 4C), and carbohydrate balance, corrected for urinary glucose loss, remained positive during both treatment periods (Fig. 4B). A lower carbohydrate oxidation after dapagliflozin treatment was observed both during daytime (–53.35 [–75.68, –31.03] g/day, P < 0.0001) (Supplementary Table 7) and nighttime (–47.69 [–67.64, –27.74] g/day, P < 0.0001) (Supplementary Table 7). Twenty-four-hour protein oxidation was not significantly affected by dapagliflozin treatment versus placebo (–0.91 [–7.30, 5.47] g/day, P = 0.77) (Supplementary Table 7 and Fig. 4D) and did not differ between treatment periods neither during daytime (–1.99 [–8.86, 4.87] g/day, P = 0.55) (Supplementary Table 7) nor during nighttime (–0.192 [–8.40, 8.04] g/day, P = 0.96) (Supplementary Table 7).
Figure 4.
A: Twenty-four-hour fatty acid oxidation measured during the stay in the respiration chamber after placebo (P) or dapagliflozin (D) treatment. Substrate balance (n = 24) (B), 24-h carbohydrate oxidation (n = 24) (C), 24-h protein oxidation (n = 24) (D), plasma glucose (n = 21) (E), NEFA (n = 23) (F), and β-hydroxybutyrate levels (G) as measured in the respiration chamber (n = 23). Blood draws at 8:30 a.m., 1:00 p.m., and 6:00 p.m. were taken before meals. Results are in LSM and 95% CI, obtained through a linear mixed model. *P < 0.05 is considered significantly different.
While in the respiration chamber, blood was sampled seven times during the day. Fasting glucose levels were lower after dapagliflozin (P < 0.0001) (Supplementary Table 5), and the area under the curve (AUC) for the plasma glucose profile during the day was significantly lower with dapagliflozin treatment (P = 0.0006) (Supplementary Table 5 and Fig. 4E). Fasting NEFA levels were unaffected after dapagliflozin treatment (P = 0.22) (Supplementary Table 5); however, the AUC for the plasma NEFA profile during the day was higher after dapagliflozin (P = 0.0026) (Supplementary Table 5 and Fig. 4F). Fasting (P = 0.045) (Supplementary Table 5) and AUC of plasma levels of β-hydroxybutyrate during the day were higher after dapagliflozin treatment (P = 0.047) (Supplementary Table 5 and Fig. 4G). The AUC for plasma FGF21 was not different with dapagliflozin (P = 0.16) (Supplementary Table 5), while the AUC for plasma glucagon was higher with dapagliflozin treatment (P = 0.039) (Supplementary Table 5).
No serious adverse events, adverse events leading to discontinuation, or events of diabetic ketoacidosis were reported. One suspected diabetic ketoacidosis was diagnosed as a gastroenteritis that was not regarded as due to study treatment.
Conclusions
SGLT2is are indicated for glucose control in type 2 diabetes and have recently been shown to have unanticipated favorable effects on CV and renal outcomes (2,3,24–26). The primary pharmacological action of SGLT2is cause glucose and energy loss in urine, which would mimic daily mild caloric restriction but also result in extra nighttime loss of energy. Here, we demonstrate that dapagliflozin has beneficial effects on 24-h energy and substrate metabolism that mimic the effects previously observed with mild caloric restriction or time-restricted feeding (12,27,28).
We confirm in a controlled setting the pharmacological action of SGLT2is, leading to a loss of urinary glucose of ∼90 g/day. Interestingly, the rate of urinary glucose loss appeared to be twice as large during daytime compared with nighttime, which is a novel finding in this study. A plausible explanation for the differences in daytime and nighttime glucose excretion is the diurnal change in dapagliflozin concentration and, therefore, the lower nighttime concentration of dapagliflozin (29). Furthermore, higher (dietary) carbohydrate availability and higher plasma glucose levels or GFR during daytime may contribute to larger urinary glucose loss. We also observed higher glucose excretion rates during the insulin phase compared with the basal phase of the glucose clamp, suggesting that higher daytime insulin levels may also be involved.
A glucose loss of 90 g/day would result in an energy deficit of ∼1,500 kJ/day and an expected decrease in body weight of ∼1.5–2.0 kg during the treatment period, which is close to what we report here. However, there was no significant effect of dapagliflozin treatment on body fat, but a small but significant decrease in lean body mass, which most likely reflects a loss of extracellular water, as recently reported after 14 days of dapagliflozin treatment (30). Interestingly, we found that the SGLT2i did not result in compensatory reductions in 24-h energy expenditure, sleeping metabolic rate, or diet-induced thermogenesis. Because food intake and activity was kept constant while in the respiration chamber, the overall 24-h energy balance was negative after dapagliflozin treatment, inducing a state of caloric restriction. By design, we provided a diet relatively high in carbohydrate, and the overall 24-h carbohydrate balance did not become negative by dapagliflozin treatment. This suggests that the increase in 24-h fat oxidation cannot solely be attributed to a lack of substrate availability for glucose oxidation, because it is well known that the intake of carbohydrates stimulates their own oxidation (31).
However, in the postabsorptive state—specifically during the night when the energy and carbohydrate balance is per definition negative—dapagliflozin may have resulted in a more pronounced negative energy balance. Indeed, a novel finding is that fat oxidation was more strongly increased at nighttime, leading to a distinct fed-to-fasting transition with dapagliflozin treatment. These findings are interesting, because we recently showed that the 24-h variability in substrate oxidation—as reflected by measurement of 24-h RER—is markedly blunted in volunteers with prediabetes compared with lean, healthy volunteers. This suggests that in prediabetes the diurnal oscillations in the fed-fasting cycle are blunted (32), but similar measurements have not been done in patients with type 2 diabetes. However, it has been shown in type 2 diabetes that 24-h fluctuations in glycogen content are blunted (31), and it is tempting to speculate that dapagliflozin helps to restore the 24-h rhythmicity in glycogen metabolism since reduced glycogen levels trigger an increased fat oxidation. Moreover, a typical fed-fasting cycle, with alternating periods of energy excess and restriction, triggers a cascade of molecular pathways that are involved in the maintenance of optimal cellular functioning and thereby affect whole-body metabolic health. Indeed, inducing alternating periods of transient energy deficit also characterize interventions such as exercise training, caloric restriction, or time-restricted feeding, which all have been shown to improve cardiometabolic health (27,28,33). Thus, the beneficial health effects of dapagliflozin in type 2 diabetes may be due to the restoration of a blunted fed-to-fasting transition. Future investigations including molecular analysis of underlying mechanisms are needed to further investigate this finding.
In the current study, we did not find a beneficial effect of SGLT2is on peripheral insulin sensitivity. Lack of improvement in peripheral insulin sensitivity was also observed by Latva-Rasku et al. (10), whereas other studies with SGLT2is reported improvements in peripheral insulin sensitivity (8,9,34). Possible explanations for the discrepant findings include differences in baseline glucose control of the patients and different rates of insulin infusion. In the current study, the patients’ baseline HbA1c level was 6.9% (51.7 mmol/mol), which is lower than reported in studies that did show an improved peripheral insulin sensitivity, in which baseline HbA1c levels averaged 7.5% (58 mmol/mol) (34), 8.4–8.8% (68–73 mmol/mol) (8), and 8.5% (69 mmol/mol) (9). Worse baseline glucose control would result in larger improvement in glucose control by the intervention and therefore reduced glucotoxicity that could help to explain improved insulin sensitivity. Furthermore, the insulin infusion rate during the high-insulin phase of the glucose clamp in our study (40 mU/m2/min) was lower compared with the infusion rate in those studies that did show improved insulin sensitivity: 80 mU/m2/min (8,9) and 120 mU/m2/min (34). Although the insulin infusion in our study tended to suppress fat oxidation more during dapagliflozin treatment compared with placebo, the lower insulin infusion rate of 40 mU/m2/min did not completely suppress fat oxidation, and absolute values for fat oxidation during the clamp remained higher with dapagliflozin treatment compared with placebo; this elevated fat oxidation could impair skeletal muscle glucose uptake via substrate competition. Similar changes have been observed in athletes in the morning after running a marathon when fat oxidation and plasma NEFA levels were also elevated—probably due to the negative energy balance—and insulin sensitivity was paradoxically reduced (35).
The higher fat oxidation and lower carbohydrate oxidation in the fasting state as well as the unchanged fasting Rd corrected for urinary glucose loss also led to the unexpected observation of increased NOGD in the fasted state.
In line with previous studies investigating the effect of SGLT2is on EGP (8,9), an increased EGP in the overnight fasted state of similar magnitude as the urinary glucose excretion rate was observed. The liver is the main organ responsible for EGP and is dependent on the combined effects of glycogenolysis and gluconeogenesis. We did not determine glycogenolysis, but enhanced protein oxidation upon SGTL2 inhibition has been suggested to contribute to the elevated gluconeogenesis and, thereby, elevated EGP (14). However, in the current study, protein oxidation based on urinary nitrogen excretion was not affected by dapagliflozin treatment, and therefore, a major contribution of glycogenolysis to EGP cannot be excluded. Alternatively, gluconeogenesis can be fueled by adipose tissue lipolysis, and glycerol could become a major contributor of carbon for gluconeogenesis (31). Interestingly, dapagliflozin improved adipose tissue insulin sensitivity toward suppression of lipolysis reflected in larger suppression of NEFA and glycerol during insulin stimulation, which was paralleled by enhanced suppression of EGP. These novel findings indicate regulation of gluconeogenesis by dapagliflozin via changed adipose tissue insulin sensitivity and suggest that the enhanced hepatic insulin sensitivity reported here and previously (36) can be explained by improved adipose tissue insulin sensitivity. In addition, improved adipose tissue insulin sensitivity toward suppression of lipolysis, together with enhanced fat oxidation, suggests an increased fatty acid turnover, which may also explain the lower IHL content found in this and previous studies (10,36), despite higher fasting and daytime free fatty acid levels. Because IHL content is known to correlate with hepatic insulin sensitivity (37), enhanced fatty acid turnover may be another factor contributing to improved hepatic insulin sensitivity.
A limitation of the study is the short duration of treatment. Patients receiving SGLT2 inhibition for 6 months have been shown to increase food intake to compensate for the glucose losses (38). It has been suggested that the increased compensatory food intake resulting in stabilization of weight occurs ∼2–3 months after initiation of treatment with SGLT2is (39). Therefore, the metabolic adaptations described in this study may look differently after 3 months of treatment. Furthermore, patients included in this study were well controlled and had relatively low HbA1c, so effects of SGLT2 inhibition on HbA1c and glucose control are limited in this study. In addition, the better glucose control of volunteers in this study may have affected the results, such as effects of treatment on peripheral insulin sensitivity, and limits direct comparison with studies that included less well-controlled patients.
To summarize, dapagliflozin treatment for 5 weeks resulted in caloric restriction-like effects on 24-h substrate flexibility as a result of increased urinary glucose loss. Consistent with a caloric restriction-like effect, dapagliflozin also reduced hepatic lipid content and improved hepatic and adipose tissue insulin sensitivity toward suppression of lipolysis. These results may provide new insights in the mechanisms underlying the beneficial health effects of SGTL2is, although further studies are needed to provide support for this hypothesis.
Article Information
Acknowledgments. The authors would like to thank all study participants and staff in the Maastricht University Medical Centre. The authors also thank staff at AstraZeneca and IQVIA for their valuable support.
Duality of Interest. This clinical trial was funded by AstraZeneca. R.E. and J.O. are employed by AstraZeneca and are AstraZeneca shareholders. No other potential conflicts of interest relevant to this article were reported.
The study funder was involved in the design of the study, the interpretation of data, and writing the report and did not impose any restrictions regarding the publication of the report.
Author Contributions. Y.J.M.O.d.K., M.d.L., B.D., M.K.C.H., J.H., V.B.S.-H., E.P., and P.S. designed and performed the experiments. Y.J.M.O.d.K., M.d.L., B.D., V.B.S.-H., E.P., and P.S. analyzed the data. Y.J.M.O.d.K., J.O., and P.S. drafted the manuscript. R.E., M.K.C.H., J.H., V.B.S.-H., B.H., J.O., E.P., and P.S. designed and conceived the study. All authors reviewed and approved the final version of the manuscript. P.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT03338855, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.14058038.
Y.J.M.O.d.K., M.d.L., and B.D. share equal authorship.
References
- 1. Bolinder J, Ljunggren Ö, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014;16:159–169 [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 4. Coleman RL, Gray AM, Broedl Md UC, et al. Can the cardiovascular risk reductions observed with empagliflozin in the EMPA-REG OUTCOME trial be explained by concomitant changes seen in conventional cardiovascular risk factor levels? Diabetes Obes Metab 2020;22:1151–1156 [DOI] [PubMed] [Google Scholar]
- 5. Alatrach M, Laichuthai N, Martinez R, et al. Evidence against an important role of plasma insulin and glucagon concentrations in the increase in EGP caused by SGLT2 inhibitors. Diabetes 2020;69:681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lundkvist P, Pereira MJ, Kamble PG, et al. Glucagon levels during short-term SGLT2 inhibition are largely regulated by glucose changes in patients with type 2 diabetes. J Clin Endocrinol Metab 2019;104:193–201 [DOI] [PubMed] [Google Scholar]
- 8. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daniele G, Xiong J, Solis-Herrera C, et al. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care 2016;39:2036–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Latva-Rasku A, Honka MJ, Kullberg J, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care 2019;42:931–937 [DOI] [PubMed] [Google Scholar]
- 11. Merovci A, Abdul-Ghani M, Mari A, et al. Effect of dapagliflozin with and without acipimox on insulin sensitivity and insulin secretion in T2DM males. J Clin Endocrinol Metab 2016;101:1249–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Most J, Redman LM. Impact of calorie restriction on energy metabolism in humans. Exp Gerontol 2020;133:110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson ML, Distelmaier K, Lanza IR, et al. Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes 2016;65:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esterline RL, Vaag A, Oscarsson J, Vora J. MECHANISMS IN ENDOCRINOLOGY: SGLT2 inhibitors: clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol 2018;178:R113–R125 [DOI] [PubMed] [Google Scholar]
- 15. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194 [DOI] [PubMed] [Google Scholar]
- 16. Schoffelen PF, Westerterp KR, Saris WH, Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol (1985) 1997;83:2064–2072 [DOI] [PubMed] [Google Scholar]
- 17. Brouwer E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (Oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl 1957;6:795–802 [PubMed] [Google Scholar]
- 18. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westerterp KR, Wilson SA, Rolland V. Diet induced thermogenesis measured over 24h in a respiration chamber: effect of diet composition. Int J Obes Relat Metab Disord 1999;23:287–292 [DOI] [PubMed] [Google Scholar]
- 20. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 21. Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci 1991;16:23–29 [PubMed] [Google Scholar]
- 22. Ackermans MT, Pereira Arias AM, Bisschop PH, Endert E, Sauerwein HP, Romijn JA. The quantification of gluconeogenesis in healthy men by (2)H2O and [2-(13)C]glycerol yields different results: rates of gluconeogenesis in healthy men measured with (2)H2O are higher than those measured with [2-(13)C]glycerol. J Clin Endocrinol Metab 2001;86:2220–2226 [DOI] [PubMed] [Google Scholar]
- 23. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 1959;82:420–430 [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 25. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 26. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 27. Cioffi I, Evangelista A, Ponzo V, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med 2018;16:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr 2017;37:371–393 [DOI] [PubMed] [Google Scholar]
- 29. Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009;85:520–526 [DOI] [PubMed] [Google Scholar]
- 30.Scholtes RA, Muskiet MHA, van Baar MJB, et al. Natriuretic effect of two weeks of dapagliflozin treatment in patients with type 2 diabetes and preserved kidney function during standardized sodium intake: results of the DAPASALT trial. Diabetes Care 2021;44:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen MC, Vatner DF, Shulman GI.. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 2017;13:572–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wefers J, Connell NJ, Fealy CE, et al. Day-night rhythm of skeletal muscle metabolism is disturbed in older, metabolically compromised individuals. Mol Metab 2020;41:101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2015;4:e002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudaliar S, Henry RR, Boden G, et al. Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin. Diabetes Technol Ther 2014;16:137–144 [DOI] [PubMed] [Google Scholar]
- 35.Tuominen JA, Ebeling P, Bourey R, et al. Postmarathon paradox: insulin resistance in the face of glycogen depletion. Am J Physiol 1996;270:E336–E343 [DOI] [PubMed] [Google Scholar]
- 36. Cusi K, Bril F, Barb D, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab 2019;21:812–821 [DOI] [PubMed] [Google Scholar]
- 37. Targher G, Byrne CD. Clinical Review: nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab 2013;98:483–495 [DOI] [PubMed] [Google Scholar]
- 38. Matsuba I, Kanamori A, Takihata M, et al. Canagliflozin increases calorie intake in type 2 diabetes without changing the energy ratio of the three macronutrients: CANA-K study. Diabetes Technol Ther 2020;22:228–234 [DOI] [PubMed] [Google Scholar]
- 39. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]