Abstract
OBJECTIVE
To characterize national trends and characteristics of adults with diabetes receiving American Diabetes Association (ADA) guideline-recommended care.
RESEARCH DESIGN AND METHODS
We performed serial cross-sectional analyses of 4,069 adults aged ≥20 years with diabetes who participated in the 2005–2018 National Health and Nutrition Examination Survey (NHANES).
RESULTS
Overall, the proportion of U.S. adults with diabetes receiving ADA guideline-recommended care meeting all five criteria by self-report in the past year (having a primary doctor for diabetes and one or more visits for this doctor, HbA1c testing, an eye examination, a foot examination, and cholesterol testing) increased from 25.0% in 2005–2006 to 34.1% in 2017–2018 (P-trend = 0.004). For participants with age ≥65 years, it increased from 29.3% in 2005–2006 to 44.2% in 2017–2018 (P-trend = 0.001), whereas for participants with age 40–64 and 20–39 years, it did not change significantly during the same time period: 25.2% to 25.8% (P-trend = 0.457) and 9.9% to 26.0% (P-trend = 0.401), respectively. Those who were not receiving ADA guideline-recommended care were more likely to be younger, of lower socioeconomic status, uninsured, newly diagnosed with diabetes, not on diabetes medication, and free of hypercholesterolemia.
CONCLUSIONS
Receipt of ADA guideline-recommended care increased only among adults with diabetes aged ≥65 years in the past decade. In 2017–2018, only one of three U.S. adults with diabetes reported receiving ADA guideline-recommended care, with even a lower receipt of care among those <65 years of age. Efforts are needed to improve health care delivery and equity in diabetes care. Insurance status is an important modifiable determinant of receiving ADA guideline-recommended care.
Introduction
Diabetes is a major public health problem, affecting 13.0% of all adults (34.1 million) in the U.S. (1). It is the leading cause of blindness, end-stage kidney disease, nontraumatic limb amputation, and cardiovascular morbidity and mortality (2,3). The estimated economic cost of diabetes was $327 billion in 2017 (4). Costs for prescription medications, other than glucose-lowering medications, and hospital inpatient care accounted for 60% of the total costs of diabetes (4), highlighting the public health and economic importance of preventing diabetes-related complications.
In the last decade, there have been major advances in diabetes management, including the introduction of new classes of glucose-lowering medications that have been shown to improve cardiovascular and kidney outcomes (5). However, without delivery of quality diabetes care, these advances may not improve health outcomes for all patients. Delivery of comprehensive diabetes care is key to prevent complications and reduce societal costs of diabetes.
The American Diabetes Association (ADA) puts forth Standards of Medical Care for patients with diabetes that provide guidance on comprehensive medical evaluation and assessment of comorbidities (6). Previous studies have focused on individual components of diabetes care to assess quality of care (7–12) or broadly defined linkage to diabetes care as seeing health care professionals for diabetes or receiving pharmacologic treatment for diabetes without considering quality of care (13). However, the extent to which U.S. adults with diabetes receive ADA guideline-recommended health care services in a comprehensive way is not known. A better understanding of the gaps in comprehensive diabetes care may help identify the opportunities to improve clinical outcomes for patients.
The objective of our study was to estimate the prevalence of receipt of guideline-recommended diabetes care, defined based on the ADA Standards of Care (6), among U.S. adults with a diagnosis of diabetes. We conducted serial cross-sectional analyses of data from the 2005–2018 National Health and Nutrition Examination Survey (NHANES) to characterize national trends in the receipt of diabetes care. We also examined predictors for receipt of ADA guideline-recommended care and its association with achievement of treatment goals.
Research Design and Methods
Data Source and Study Population
The NHANES is a cross-sectional, complex survey with interviews, physical examinations, and laboratory tests administered by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (14). The NHANES sampling design consists of four stages to select participants representative of the civilian, noninstitutionalized U.S. population (15).
There were 4,950 NHANES participants with self-reported diabetes and ≥20 years of age between 2005 and 2018. Of these, 715 participants from the 2009–2010 survey cycle were excluded because information used to define the study outcome (i.e., hemoglobin A1c [HbA1c] testing in the past year) was not collected in the 2009–2010 survey. We further excluded 166 participants with missing information on explanatory variables if missing was <2% (Supplementary Table 1). The remaining 4,069 participants were included for analysis.
Outcome: Receipt of ADA Guideline-Recommended Diabetes Care
Based on the ADA Standards of Care and NHANES data availability, we defined receipt of ADA guideline-recommended diabetes care as meeting all five criteria in the past 12 months: 1) having a primary doctor for diabetes care and one or more visits for this doctor; 2) receipt of HbA1c testing; 3) receipt of a foot examination; 4) receipt of an eye examination; and 5) receipt of cholesterol testing. This information was ascertained from the following questions administered to all NHANES participants with a self-reported history of diabetes:
“Is there one doctor or other health professional for your diabetes?” “How many times have you seen this doctor or other health professional in the past 12 months?”;
“During the past 12 months, has a doctor or other health professional checked your glycosylated hemoglobin or “A-one-C”?;
“When was the last time you had an eye exam in which the pupils were dilated?”;
“During the past 12 months, about how many times has a doctor or other health professional checked your feet for any sores or irritations?”; and
“About how long has it been since you last had your blood cholesterol checked?”
Explanatory Variables
Based on the previous literature, we selected potential variables that might influence receipt of care (13,16–18). Age, sex, race/ethnicity, family income, education, and health insurance were self-reported during the questionnaire portion of the survey. BMI was calculated from height and weight. Current smoking was defined by self-report or serum cotinine >10 ng/mL. HbA1c was measured in whole blood and calibrated to account for changes in laboratory methods over time (19). Time since diabetes diagnosis was calculated from self-reported age at diabetes diagnosis. Participants provided medication containers to trained interviewers who entered the names into Lexicon Plus, a database of prescription and nonprescription products available in the U.S. market used by NCHS (20). Antidiabetic medication information was extracted from prescription medication data according to the three-level nested category system of Multum Lexicon (20).
Mean blood pressure values were calculated among participants with at least two systolic and at least two diastolic blood pressure measurements. Hypertension was defined as mean systolic blood pressure ≥130 mmHg, mean diastolic blood pressure ≥80 mmHg, or self-reported blood pressure-lowering medication use (21). Total cholesterol measurements were obtained using standardized procedures, and hypercholesterolemia was defined as total cholesterol ≥240 mg/dL or self-reported cholesterol-lowering medication use. Cardiovascular disease was defined as self-reported coronary heart disease, heart failure, or stroke. The glomerular filtration rate was estimated from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration equation (22). Chronic kidney disease was defined as estimated glomerular rate of <60 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio (ACR) ≥30 mg/g according to the Kidney Disease Improving Global Outcomes guidelines (23). The presence of depressive symptoms were defined as having five or more symptoms from a nine-item instrument to screen depression, the Patient Health Questionnaire (24,25).
Achievement of Treatment Goals
Glycemic goals were defined as HbA1c <7% (<53 mmol/mol) (26). We also examined a less stringent HbA1c goal of <7.5% (58 mmol/mol) as well as poor glycemic control (HbA1c >9% [>75 mmol/mol]), one of the quality measures for adults with diabetes developed by the National Committee for Quality Assurance (27). Blood pressure goals were defined as <140/90 mmHg (28). Cholesterol goals were defined as HDL-cholesterol ≥40 mg/dL (1.0 mmol/L) in men and ≥50 mg/dL (1.3 mmol/L) in women and fasting LDL-cholesterol <100 mg/dL (2.6 mmol/L) (28). In the subset of participants with prevalent cardiovascular disease, we examined fasting LDL-cholesterol <70 mg/dL (1.8 mmol/L) (28). We also examined receipt of cholesterol-lowering medication.
Statistical Analyses
We calculated prevalence estimates and SEs for receipt of diabetes care among participants with self-reported diabetes for each 2-year survey cycle, overall and stratified by age (≥65 years, 40–64 years, or 20–39 years). P values for linear trends were calculated with logistic regression models with the 2-year survey cycle as the explanatory variable (modeled as an ordinal variable). We conducted analyses for receipt of all components of care and for each component individually.
We used the t test for continuous variables and the χ2 test for categorical variables to compare sociodemographic and clinical characteristics of the participants who did versus those who did not report receiving ADA guideline-recommended care. We used logistic regression models with adjustment for age, sex, race/ethnicity, education, and income to identify independent predictors of receipt of all components of care as well as the individual components. In sensitivity analyses, we ran the models with each of education and income separately.
To assess the association between receipt of ADA guideline-recommended care and achievement of treatment goals, we used logistic regression models with adjustment for age, sex, race/ethnicity, education, income, insurance, BMI, smoking, HbA1c (except for glycemic goals), diabetes duration, diabetes medication, hypertension (except for blood pressure goals), hypercholesterolemia (except for cholesterol goals), cardiovascular disease, chronic kidney disease, and depressive symptom. All analyses accounted for the complex survey design of NHANES, and we applied survey weights so that all results are representative of the civilian, noninstitutionalized U.S. population with diabetes (29). We used the Taylor series (linearization) method to obtain SE estimates, in accordance with NCHS recommendations (30). All statistical analyses were conducted using Stata 14 software (StataCorp, College Station, TX).
Results
Among U.S. adults aged ≥20 with a history of diabetes in 2005–2018 (mean age, 59.9 years; women, 49.2%), 29.2% (95% CI 27.0–31.6) reported having received all components comprising our definition of guideline-recommended diabetes care (having seen a doctor for diabetes in the past year, having HbA1c and cholesterol checked, and having foot and eye examinations in the past year).
Trends in Receipt of ADA Guideline-Recommended Diabetes Care
Overall, the proportion of U.S. adults with diabetes who reported receipt of ADA guideline-recommended care (all components) increased significantly over time, from 25.0% (95% CI 18.6–32.9) in 2005–2006 to 34.1% (95% CI 30.6–37.7) in 2017–2018 (P-trend = 0.004) (Fig. 1A). When stratified by age, receipt of recommended care increased only among participants with age ≥65 years, from 29.3% (95% CI 19.6–41.4) in 2005–2006 to 44.2% (95% CI 38.6–50.0) in 2017–2018 (P-trend = 0.001) (Fig. 1B). It remained unchanged among participants with age 40–64 years (25.2% [95% CI 17.5–34.9] in 2005–2006 vs. 25.8% [95% CI 18.0–35.5] in 2017–2018; P-trend = 0.457). Among participants with age 20–39 years, it increased without statistical significance from 9.9% (95% CI 3.1–27.6) in 2005–2006 to 26.0% (95% CI 7.6–60.1; P-trend = 0.401). There was no significant difference in receipt of ADA guideline-recommended care by sex (Supplementary Fig. 1)
Figure 1.
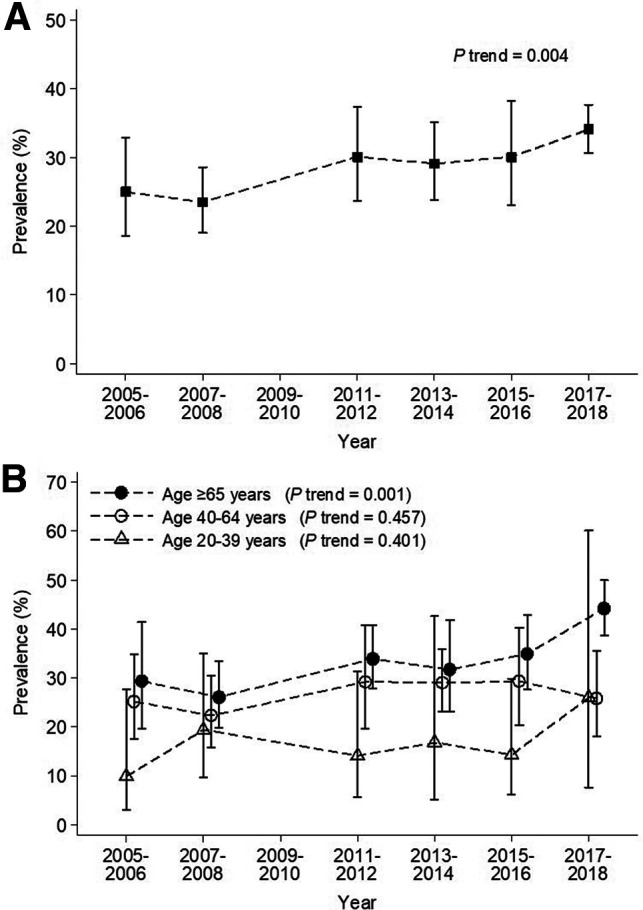
Trend in receipt of ADA guideline-recommended care (all components) among U.S. adults with self-reported diabetes (NHANES 2005–2018). A: Overall participants. B: Participants stratified by age.
Trends in Receipt of Individual Components of ADA Guideline-Recommended Diabetes Care
Among the five components, only receipt of HbA1c testing increased significantly over time, from 64.4% (95% CI 56.4–71.6) in 2005–2006 to 85.3% (95% CI 80.1–89.3) in 2017–2018 (Supplementary Fig. 2A). The increase in receipt of HbA1c testing was observed in both groups of age ≥65 years (64.7% [95% CI 51.5–76.0] to 91.7% [95% CI 86.4–95.0], P-trend <0.001) (Supplementary Fig. 2B) and age 40–64 years (64.6% [95% CI 55.9–72.4] to 81.4% [95% CI 72.7–87.8], P-trend <0.001) (Supplementary Fig. 2C). Among participants with age 20–39 years, it increased without statistical significance from 62.3% (95% CI 35.8–83.0) in 2005–2006 to 70.0% (95% CI 43.5–87.6; P-trend = 0.380) (Supplementary Fig. 2D).
Characteristics of Study Population by Receipt of ADA Guideline-Recommended Diabetes Care
Compared with the participants not receiving recommended diabetes care, those receiving it were significantly older, more often non-Hispanic White, had higher incomes, and more often had a higher education level and health insurance (Table 1). They were less often current smokers or those with poor glycemic control (HbA1c >9%). They were more likely to have a longer duration of diabetes, taken diabetes medications, or to have comorbidities such as hypertension, hypercholesterolemia, or cardiovascular disease.
Table 1.
Characteristics of U.S. adults with diabetes, overall and by receipt of ADA guideline-recommended diabetes care (NHANES 2005–2018*) (N = 4,069)
| Overall Mean or % (SE) | ADA guideline-recommended care | P value | ||
|---|---|---|---|---|
| Mean or % (SE) | ||||
| No | Yes | |||
| Unweighted n | 4,069 | 3,015 | 1,054 | |
| Age (years), mean | 59.9 (0.3) | 59.0 (0.3) | 62.2 (0.5) | <0.001 |
| Age group, % | <0.001 | |||
| 20–39 years | 8.1 (0.5) | 9.5 (0.6) | 4.6 (1.0) | |
| 40–64 years | 52.1 (1.0) | 53.7 (1.2) | 48.2 (2.3) | |
| ≥65 years | 39.8 (1.1) | 36.7 (1.2) | 47.1 (2.1) | |
| Female, % | 49.2 (1.2) | 49.8 (1.4) | 47.7 (1.9) | 0.360 |
| Race/ethnicity, % | <0.001 | |||
| Non-Hispanic White | 61.0 (1.8) | 57.9 (2.0) | 68.7 (2.3) | |
| Non-Hispanic Black | 15.2 (1.2) | 16.0 (1.3) | 13.5 (1.3) | |
| Mexican American | 14.9 (1.2) | 16.9 (1.5) | 9.9 (1.1) | |
| Other | 8.9 (0.7) | 9.3 (0.8) | 7.9 (1.1) | |
| Family income, % | <0.001 | |||
| Below poverty threshold | 15.5 (0.9) | 18.1 (1.1) | 9.2 (1.1) | |
| Above or at poverty threshold | 76.0 (1.2) | 72.9 (1.4) | 83.5 (1.9) | |
| Missing | 8.4 (0.6) | 8.9 (0.8) | 7.3 (1.3) | |
| Education, % | <0.001 | |||
| <High school | 10.1 (0.7) | 12.0 (0.9) | 5.5 (0.8) | |
| High school degree | 38.5 (1.2) | 40.1 (1.4) | 34.7 (2.2) | |
| >High school degree | 51.4 (1.3) | 48.0 (1.6) | 59.8 (2.3) | |
| Health insurance, % | <0.001 | |||
| No insurance | 9.9 (0.6) | 12.6 (0.8) | 3.1 (0.6) | |
| Public insurance | 55.6 (1.1) | 53.4 (1.1) | 61.1 (2.6) | |
| Private insurance | 34.5 (1.1) | 34.0 (1.2) | 35.8 (2.6) | |
| BMI (kg/m2), mean | 32.9 (0.2) | 32.7 (0.2) | 33.4 (0.4) | 0.055 |
| BMI categories, % | 0.161 | |||
| <25 kg/m2 | 11.7 (0.7) | 12.5 (0.8) | 9.9 (1.2) | |
| 25 to <30 kg/m2 | 26.3 (0.9) | 26.9 (1.0) | 25.0 (2.0) | |
| ≥30 kg/m2 | 59.7 (1.2) | 58.3 (1.3) | 63.2 (1.9) | |
| Missing | 2.2 (0.3) | 2.4 (0.3) | 1.9 (0.5) | |
| Current smoker, self-reported or cotinine >10 ng/mL, % | 20.4 (0.9) | 22.6 (1.1) | 15.1 (1.6) | 0.001 |
| HbA1c (%), mean | 7.1 (0.03) | 7.1 (0.05) | 7.1 (0.05) | 0.246 |
| HbA1c categories, % | 0.006 | |||
| <7% (<53 mmol/mol) | 56.9 (1.1) | 56.8 (1.4) | 57.2 (2.3) | |
| 7–8% (53–64 mmol/mol) | 18.5 (1.0) | 17.0 (1.0) | 22.3 (1.8) | |
| 8–9% (64–75 mmol/mol) | 8.8 (0.7) | 9.2 (0.8) | 7.7 (1.0) | |
| >9% (>75 mmol/mol) | 13.1 (0.7) | 14.5 (0.9) | 9.9 (1.1) | |
| Missing | 2.7 (0.3) | 2.6 (0.3) | 3.0 (0.8) | |
| Time since diabetes diagnosis, % | <0.001 | |||
| 0 to <5 years | 29.5 (0.8) | 32.4 (1.1) | 23.6 (1.9) | |
| 5 to <15 years | 40.5 (0.9) | 40.6 (1.1) | 39.6 (2.3) | |
| ≥15 years | 30.1 (0.9) | 26.9 (1.1) | 36.7 (2.0) | |
| Diabetes medication, % | <0.001 | |||
| None | 17.4 (0.8) | 20.8 (1.0) | 9.3 (1.3) | |
| Noninsulin agents only | 54.0 (1.2) | 54.1 (1.) | 53.9 (2.4) | |
| Insulin | 28.5 (0.9) | 25.1 (1.1) | 36.8 (2.3) | |
| Comorbidities, % | ||||
| Hypertension | 79.5 (0.9) | 78.1 (1.1) | 82.9 (1.4) | 0.009 |
| Hypercholesterolemia | 64.8 (1.1) | 60.1 (1.3) | 76.7 (1.8) | <0.001 |
| Cardiovascular disease | 24.0 (0.9) | 22.8 (1.1) | 27.1 (1.9) | 0.042 |
| Chronic kidney disease | 38.9 (1.0) | 39.2 (1.2) | 38.1 (2.1) | 0.907 |
| Missing | 4.6 (0.4) | 4.6 (0.5) | ||
| Having depressive symptoms, % | 16.6 (1.0) | 18.8 (1.3) | 15.4 (1.7) | 0.232 |
| Missing | 6.9 (0.5) | 7.5 (0.6) | 5.7 (1.0) | |
The 2009–2010 survey cycle was not included.
Predictors of Receipt of ADA Guideline-Recommended Diabetes Care
In adjusted analyses, older age, higher income and education, health insurance, longer duration of diabetes, use of diabetes medications, and hypercholesterolemia were significantly associated with receipt of ADA guideline-recommended diabetes care (Table 2). Sex, race/ethnicity, BMI, smoking, HbA1c, hypertension, cardiovascular disease, chronic kidney disease, and depressive symptoms were not associated with receipt of diabetes care. The results were materially unchanged when education and income were separately modeled (Supplementary Table 2).
Table 2.
Prevalence and ORs of receipt of ADA guideline-recommended care among U.S. adults with self-reported diabetes (NHANES 2005–2018*) (N = 4,069)
| Prevalence % (SE) | Unadjusted OR (95% CI) | Adjusted† OR (95% CI) | |
|---|---|---|---|
| Age, years | |||
| 20–39 | 16.7 (3.3) | 1 [Reference] | 1 [Reference] |
| 40–64 | 27.1 (1.7) | 1.84 (1.11–3.06) | 1.72 (1.04–2.84) |
| ≥65 | 34.6 (1.5) | 2.64 (1.66–4.18) | 2.19 (1.31–3.66) |
| Sex | |||
| Male | 30.1 (1.6) | 1 [Reference] | 1 [Reference] |
| Female | 28.4 (1.4) | 0.92 (0.77–1.10) | 1.00 (0.82–1.20) |
| Race/ethnicity | |||
| Non-Hispanic White | 32.9 (1.8) | 1 [Reference] | 1 [Reference] |
| Non-Hispanic Black | 25.9 (1.4) | 0.71 (0.57–0.89) | 0.87 (0.69–1.10) |
| Mexican American | 19.4 (1.6) | 0.49 (0.38–0.64) | 0.81 (0.61–1.08) |
| Other | 26.1 (2.7) | 0.72 (0.51–1.01) | 0.82 (0.58–1.16) |
| Family income | |||
| Below poverty threshold | 17.4 (1.9) | 1 [Reference] | 1 [Reference] |
| ≥1 times poverty threshold | 32.1 (1.4) | 2.25 (1.69–2.99) | 1.67 (1.23–2.27) |
| Missing | 25.2 (4.0) | 1.60 (1.01–2.51) | 1.22 (0.75–2.00) |
| Education | |||
| <High school | 16.0 (1.9) | 1 [Reference] | 1 [Reference] |
| High school degree | 26.3 (1.8) | 1.88 (1.38–2.55) | 1.61 (1.16–2.24) |
| >High school degree | 34.0 (1.8) | 2.71 (1.92–3.83) | 2.17 (1.52–3.11) |
| Health insurance | |||
| No insurance | 9.3 (1.7) | 1 [Reference] | 1 [Reference] |
| Public insurance | 32.1 (1.4) | 4.64 (3.10–6.92) | 3.23 (2.02–5.14) |
| Private insurance | 30.3 (2.4) | 4.27 (2.76–6.59) | 3.08 (1.96–4.85) |
| BMI categories, kg/m2 | |||
| <25 | 24.7 (2.8) | 1 [Reference] | 1 [Reference] |
| 25 to <30 | 27.8 (2.1) | 1.17 (0.82–1.67) | 1.06 (0.74–1.52) |
| ≥30 | 30.9 (1.4) | 1.36 (1.00–1.86) | 1.25 (0.91–1.73) |
| Missing | 25.1 (6.0) | 1.02 (0.51–2.03) | 0.93 (0.43–2.00) |
| Current smoker | |||
| Yes | 21.6 (2.4) | 1 [Reference] | 1 [Reference] |
| No | 31.2 (1.2) | 1.65 (1.23–2.21) | 1.35 (0.99–1.84) |
| HbA1c categories | |||
| <7% (<53 mmol/mol) | 29.4 (1.5) | 1 [Reference] | 1 [Reference] |
| 7–8% (53–64 mmol/mol) | 35.2 (2.3) | 1.30 (1.01–1.68) | 1.36 (1.06–1.75) |
| 8–9% (64–75 mmol/mol) | 25.7 (3.4) | 0.83 (0.58–1.19) | 0.96 (0.66–1.37) |
| >9% (>75 mmol/mol) | 22.0 (2.4) | 0.68 (0.49–0.93) | 0.87 (0.62–1.24) |
| Missing | 32.4 (6.8) | 1.15 (0.63–2.13) | 1.27 (0.67–2.43) |
| Time since diabetes diagnosis | |||
| 0 to <5 years | 22.1 (2.1) | 1 [Reference] | 1 [Reference] |
| 5 to <15 years | 29.0 (1.7) | 1.44 (1.11–1.87) | 1.39 (1.06–1.81) |
| ≥15 years | 36.6 (2.0) | 2.04 (1.55–2.68) | 1.92 (1.44–2.58) |
| Diabetes medication | |||
| None | 15.6 (2.0) | 1 [Reference] | 1 [Reference] |
| Noninsulin agents only | 29.2 (1.7) | 2.24 (1.59–3.14) | 1.98 (1.40–2.80) |
| Insulin | 37.7 (2.3) | 3.28 (2.31–4.66) | 3.19 (2.22–4.57) |
| Hypertension | |||
| No | 24.4 (2.1) | 1 [Reference] | 1 [Reference] |
| Yes | 30.5 (1.2) | 1.35 (1.08–1.70) | 1.18 (0.93–1.49) |
| Hypercholesterolemia | |||
| No | 19.7 (1.5) | 1 [Reference] | 1 [Reference] |
| Yes | 34.4 (1.4) | 2.13 (1.73–2.63) | 1.95 (1.57–2.41) |
| Cardiovascular disease | |||
| No | 28.1 (1.3) | 1 [Reference] | 1 [Reference] |
| Yes | 32.9 (2.1) | 1.26 (1.01–1.57) | 1.12 (0.86–1.45) |
| Chronic kidney disease | |||
| No | 29.6 (1.5) | 1 [Reference] | 1 [Reference] |
| Yes | 28.7 (1.6) | 0.96 (0.78–1.17) | 0.88 (0.72–1.08) |
| Missing | 29.2 (5.0) | 0.98 (0.61–1.59) | 0.92 (0.55–1.56) |
| Depressive symptoms | |||
| No | 30.2 (1.4) | 1 [Reference] | 1 [Reference] |
| Yes | 27.0 (2.5) | 0.86 (0.64–1.15) | 1.04 (0.77–1.41) |
| Missing | 24.0 (3.7) | 0.73 (0.47–1.12) | 0.94 (0.62–1.43) |
The 2009–2010 survey cycle was not included.
Adjusted for age, sex, race/ethnicity, family income, education, and health insurance.
Predictors of Receipt of Individual Components of ADA Guideline-Recommended Care
Of the five individual components of care, cholesterol testing (78.5%) and an eye examination (62.6%) in the past year had the highest and lowest prevalence, respectively (Table 3). Older age was a significant predictor of all the components of care, except HbA1c testing. Non-Hispanic Black and Mexican American adults with diabetes were significantly less likely to report HbA1c and cholesterol testing than non-Hispanic White adults with diabetes. Mexican Americans were significantly less likely to have a primary doctor for diabetes (with one or more visits) and a foot examined compared with non-Hispanic Whites. Health insurance was strongly associated with all of the components of care. The results were materially unchanged when education and income were separately modeled (Supplementary Tables 3 and 4).
Table 3.
Overall prevalence and adjusted ORs of receipt of each component of ADA guideline-recommended care among U.S. adults with self-reported diabetes (NHANES 2005–2018*) (N = 4,069)
| Diabetes doctor | HbA1c testing | Eye examination | Foot examination | Cholesterol testing | |
|---|---|---|---|---|---|
| Overall prevalence, % (SE) | 69.1 (1.0) | 75.0 (0.9) | 62.6 (1.2) | 70.3 (1.2) | 78.5 (1.0) |
| Adjusted OR (95% CI)† | |||||
| Age, years | |||||
| 20–39 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 40–64 | 1.88 (1.33–2.65) | 1.09 (0.74–1.61) | 1.44 (1.02–2.03) | 1.22 (0.87–1.72) | 2.43 (1.56–3.79) |
| ≥65 | 1.53 (1.06–2.22) | 1.12 (0.70–1.80) | 2.36 (1.62–3.44) | 1.66 (1.17–2.34) | 3.43 (2.17–5.41) |
| Sex | |||||
| Male | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Female | 1.11 (0.92–1.35) | 1.06 (0.86–1.30) | 1.04 (0.87–1.24) | 0.95 (0.77–1.16) | 1.10 (0.87–1.38) |
| Race/ethnicity | |||||
| Non-Hispanic White | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Non-Hispanic Black | 1.11 (0.92–1.34) | 0.54 (0.46–0.73) | 1.12 (0.89–1.41) | 1.02 (0.79–1.32) | 0.73 (0.56–0.95) |
| Mexican American | 0.73 (0.59–0.92) | 0.67 (0.54–0.84) | 0.87 (0.68–1.11) | 0.69 (0.55–0.86) | 0.63 (0.48–0.83) |
| Other | 1.00 (0.72–1.38) | 1.12 (0.77–1.63) | 0.98 (0.71–1.35) | 0.85 (0.63–1.15) | 0.75 (0.53–1.06) |
| Family income | |||||
| Below poverty threshold | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥1 times poverty threshold | 1.39 (1.08–1.78) | 1.57 (1.27–1.95) | 1.57 (1.25–1.96) | 1.28 (0.98–1.69) | 1.25 (0.97–1.60) |
| Missing | 1.52 (1.07–2.18) | 0.97 (0.64–1.48) | 1.08 (0.78–1.49) | 0.93 (0.65–1.32) | 1.59 (1.10–2.32) |
| Education | |||||
| <High school | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| High school degree | 1.01 (0.77–1.33) | 1.65 (1.25–2.16) | 1.09 (0.85–1.40) | 1.15 (0.88–1.51) | 0.94 (0.69–1.30) |
| >High school degree | 1.23 (0.90–1.68) | 3.31 (2.32–4.71) | 1.31 (1.01–1.68) | 1.29 (0.97–1.73) | 1.40 (1.06–1.84) |
| Health insurance | |||||
| No insurance | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Public insurance | 2.79 (2.07–3.75) | 2.64 (1.91–3.63) | 2.76 (2.09–3.65) | 2.64 (2.03–3.43) | 2.98 (2.09–4.25) |
| Private insurance | 2.34 (1.77–3.10) | 2.97 (2.04–4.31) | 2.04 (1.51–2.76) | 2.39 (1.75–3.25) | 3.76 (2.63–5.38) |
| BMI categories, kg/m2 | |||||
| <25 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 25 to <30 | 1.12 (0.82–1.53) | 1.38 (1.01–1.88) | 0.90 (0.66–1.23) | 0.84 (0.60–1.18) | 1.04 (0.76–1.42) |
| ≥30 | 1.55 (1.17–2.05) | 1.43 (1.12–1.91) | 1.10 (0.81–1.48) | 0.94 (0.67–1.32) | 1.18 (0.91–1.53) |
| Missing | 1.35 (0.73–2.49) | 0.92 (0.54–1.56) | 0.98 (0.53–1.81) | 0.74 (0.36–1.53) | 0.73 (0.37–1.43) |
| Current smoker | |||||
| Yes | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| No | 1.16 (0.94–1.43) | 1.17 (0.94–1.46) | 1.52 (1.24–1.86) | 1.15 (0.88–1.49) | 1.27 (0.96–1.67) |
| HbA1c categories | |||||
| <7% (<53 mmol/mol) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 7–8% (53–64 mmol/mol) | 2.14 (1.72–2.66) | 1.68 (1.30–2.16) | 1.22 (0.91–1.62) | 1.60 (1.14–2.25) | 1.05 (0.79–1.38) |
| 8–9% (64–75 mmol/mol) | 1.81 (1.34–2.42) | 1.57 (1.08–2.28) | 1.02 (0.73–1.42) | 1.52 (1.03–2.24) | 0.90 (0.60–1.33) |
| >9% (>75 mmol/mol) | 1.22 (0.90–1.64) | 1.24 (0.96–1.60) | 0.94 (0.69–1.27) | 1.17 (0.88–1.55) | 0.72 (0.53–0.99) |
| Missing | 1.33 (0.76–2.32) | 0.83 (0.45–1.52) | 1.04 (0.63–1.70) | 1.07 (0.60–1.92) | 1.43 (0.75–2.73) |
| Time since diabetes diagnosis | |||||
| 0 to <5 years | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 5 to <15 years | 1.43 (1.12–1.82) | 1.43 (1.13–1.80) | 1.23 (0.92–1.65) | 1.36 (1.09–1.71) | 0.82 (0.61–1.09) |
| ≥15 years | 1.20 (0.95–1.52) | 1.91 (1.46–2.51) | 1.75 (1.27–2.42) | 1.90 (1.46–2.48) | 0.94 (0.69–1.29) |
| Diabetes medication | |||||
| None | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Noninsulin agents only | 3.35 (2.54–4.42) | 2.04 (1.62–2.58) | 1.47 (1.11–1.94) | 1.99 (1.51–2.62) | 2.23 (1.60–3.13) |
| Insulin | 5.25 (3.94–6.99) | 4.29 (3.21–5.72) | 2.25 (1.61–3.14) | 3.64 (2.56–5.16) | 1.93 (1.34–2.78) |
| Hypertension | |||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.24 (1.03–1.51) | 1.33 (1.06–1.66) | 1.03 (0.84–1.25) | 1.23 (0.99–1.53) | 1.38 (1.09–1.74) |
| Hypercholesterolemia | |||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.44 (1.14–1.81) | 1.55 (1.28–1.88) | 1.24 (0.98–1.56) | 1.40 (1.15–1.70) | 2.82 (2.30–3.46) |
| Cardiovascular disease | |||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.08 (0.82–1.42) | 0.80 (0.63–1.00) | 1.24 (0.98–1.56) | 1.21 (0.96–1.53) | 1.42 (1.12–1.79) |
| Chronic kidney disease | |||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 0.93 (0.75–1.15) | 0.94 (0.77–1.14) | 1.02 (0.82–1.27) | 1.12 (0.90–1.40) | 0.74 (0.58–0.94) |
| Missing | 0.92 (0.60–1.43) | 0.97 (0.60–1.57) | 1.06 (0.67–1.68) | 0.86 (0.55–1.36) | 0.68 (0.41–1.12) |
| Depressive symptoms | |||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 0.80 (0.61–1.05) | 0.88 (0.71–1.11) | 0.99 (0.79–1.25) | 0.96 (0.73–1.25) | 0.92 (0.70–1.20) |
| Missing | 1.09 (0.77–1.54) | 0.68 (0.51–0.92) | 0.89 (0.63–1.26) | 1.13 (0.81–1.60) | 1.17 (0.81–1.68) |
The 2009–2010 survey cycle was not included.
Adjusted for age, sex, race/ethnicity, family income, education, and health insurance. Bold values indicate statistical significance.
Receipt of ADA Guideline-Recommended Care and Achievement of Treatment Goals
Participants who received ADA guideline-recommended care were more likely to achieve HbA1c <7.5% (<58 mmol/mol) (adjusted odds ratio [OR] 1.52; 95% CI 1.17–1.98), blood pressure <140/90 mmHg (adjusted OR, 1.47; 95% CI 1.11–1.95), and LDL-cholesterol <100 mg/dL (<2.6 mmol/L) (adjusted OR 1.47; 95% CI 1.03–2.10) and receive cholesterol-lowering medication (adjusted OR 1.79; 95% CI 1.29–2.48) (Supplementary Table 5). Receipt of ADA guideline-recommended care was inversely associated with HbA1c >9% (75 mmol/mol) (adjusted OR 0.59; 95% CI 0.42–0.84).
Conclusions
The prevalence of receipt of ADA guideline-recommended diabetes care in U.S. adults with self-reported diabetes increased during the period of 2005–2018, driven by an increase in receipt of care among older adults. Despite this increase, in 2017–2018, only 34.1% of adults with diabetes reported having received all of the five components of ADA guideline-recommended care. Among the five components, only HbA1c testing increased significantly during the study period. Identified predictors of receipt of ADA guideline-recommended care in our model suggest that adults with diabetes who are socioeconomically disadvantaged and do not have health insurance are less likely to receive recommended diabetes care. Health insurance is an important modifiable predictor of receipt of ADA guideline-recommended care. Taken together, our results show substantial gaps in care. Efforts focused on improving diabetes preventive care services for adults with lower socioeconomic status or lacking health insurance are needed.
Our study adds to a previous population-based study from Torino, Italy, reporting 35.8% of patients with diabetes underwent a comprehensive assessment, defined as receipt of HbA1c testing, an eye examination, cholesterol testing, and albuminuria testing during a 1-year period (31). Despite some differences in the components of care, the proportion of patients with diabetes who received comprehensive diabetes care is similar to our study. The Torino study found that older patients were less likely to receive comprehensive care and that socioeconomic status was not associated with receipt of care. In contrast, our study showed that younger patients were less likely to receive quality care and that lower socioeconomic status was significantly associated with a lack of receiving care. These differences may be partly attributable to the differences in health care systems between the two countries: a private, partial coverage system in the U.S. versus a national health system in Italy. Indeed, health insurance status was strongly associated with receipt of ADA guideline-recommended diabetes care as well as each of its five components in our U.S.-based study. The recent NHANES 2005–2016 analysis by Kazemian et al. (13) showed a strong association of insurance status with any interaction with the health care system for diabetes. Our study adds to this study by showing that insurance status is an important modifiable determinant to improve access to quality diabetes care.
We did not find racial/ethnic differences in receipt of ADA guideline-recommended diabetes care (all components) in our study. However, there were significant racial/ethnic differences in some specific components of care. Mexican Americans had lower access to a diabetes doctor than non-Hispanic Whites, and non-Hispanic Black and Mexican American adults were both less likely to receive HbA1c and cholesterol testing than non-Hispanic Whites. Our findings are consistent with previous reports of poorer glycemic and lipid control among Black and Mexican American adults with diabetes compared with their White counterparts (32–35). Previous studies also showed that Mexican American and Black adults with diabetes underwent major amputation more frequently compared with their White counterparts (36,37). In our study, we observed that Mexican Americans with diabetes were less likely to report a recent foot examination compared with non-Hispanic White counterparts, but there was no Black-White difference in foot examination.
This analysis has several limitations. First, although annual albuminuria testing is recommended by the ADA guidelines, we could not examine receipt of albuminuria testing because this information was not collected in NHANES. Second, information was not available on health care provider type or geographic area. Third, the cross-sectional study design does not allow for us establish the temporality of associations when identifying factors associated with receipt of care. Lastly, we assessed process measures of diabetes care, but improvements in such processes do not necessarily translate into better outcomes (7,38–40). However, our study showed that the receipt of ADA guideline-recommended care was significantly associated with better glycemic, blood pressure, and cholesterol control although causal relationship may not be established due to the nature of cross sectional study design.
Despite its limitations, our study draws upon a number of strengths. The NHANES is a nationally representative survey and provides unbiased, nationally representative estimates. We evaluated characteristics of diabetes in detail, including HbA1c, duration of diabetes, diabetes medication use, and the presence of comorbidities. To our knowledge, our study is the first to evaluate national trends of receipt of diabetes care in both a comprehensive way as well as its individual components.
In conclusion, receipt of ADA guideline-recommended diabetes care has increased in the last decade. However, two of three U.S. adults with diabetes still do not receive guideline-recommended care. Adults <65 years of age, those with less severe diabetes, and those who are socioeconomically disadvantaged or uninsured were less likely to report having received care. Health insurance is an important modifiable determinant of receipt of care. Improving access to health insurance may improve uptake of preventive care practices in adults with diabetes.
Article Information
Funding. J.-I.S. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK121825.
Duality of Interest. The project described was supported by a research grant from Merck to Johns Hopkins Bloomberg School of Public Health (principal investigator: J.-I.S.). J.-I.S., S.H.G., and E.S. received research funding from Merck for the submitted work. G.F. and S.R. are employees of Merck & Co., Inc., Kenilworth, NJ. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.-I.S. designed the study, supervised the analyses, and drafted and revised the manuscript. D.W. and N.D. analyzed data, interpreted data, and provided critical comments on the manuscript. G.F., M.E.G., S.H.G., and S.R. interpreted data and provided critical comments on the manuscript. E.S. designed the study, supervised the analysis, and revised the manuscript. J.-I.S. and E.S. and are the guarantors of this work, and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14120087.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020: Estimates of Diabetes and its Burden in the United States. Accessed 9 September 2020. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 2. Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med 1993;328:1676–1685 [DOI] [PubMed] [Google Scholar]
- 3. Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009;119:1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S111–S124 [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association . 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S40–S52 [DOI] [PubMed] [Google Scholar]
- 7. Grant RW, Buse JB; University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team . Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care 2005;28:337–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagpal J, Bhartia A. Quality of diabetes care in the middle- and high-income group populace: the Delhi Diabetes Community (DEDICOM) survey. Diabetes Care 2006;29:2341–2348 [DOI] [PubMed] [Google Scholar]
- 9. TRIAD Study Group . Health systems, patients factors, and quality of care for diabetes: a synthesis of findings from the TRIAD study. Diabetes Care 2010;33:940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 11. Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014;161:681–689 [DOI] [PubMed] [Google Scholar]
- 12. Fang M. Trends in diabetes management among US adults: 1999-2016. J Gen Intern Med 2020;35:1427–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005-2016. JAMA Intern Med 2019;179:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . About the National Health and Nutrition Examination Survey. Accessed 14 August 2020. Available from https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 15. Centers for Disease Control and Prevention . NHANES Tutorials - Module 2: Sample Design. Accessed 14 August 2020. Available from https://wwwn.cdc.gov/nchs/nhanes/tutorials/Module2.aspx
- 16. Fenton JJ, Von Korff M, Lin EH, Ciechanowski P, Young BA. Quality of preventive care for diabetes: effects of visit frequency and competing demands. Ann Fam Med 2006;4:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernandez-Boussard T, Ahmed SM, Morton JM. Obesity disparities in preventive care: findings from the National Ambulatory Medical Care Survey, 2005-2007. Obesity (Silver Spring) 2012;20:1639–1644 [DOI] [PubMed] [Google Scholar]
- 18. McMorrow S, Kenney GM, Goin D. Determinants of receipt of recommended preventive services: implications for the Affordable Care Act. Am J Public Health 2014;104:2392–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Selvin E, Wang D, Lee AK, Bergenstal RM, Coresh J. Identifying trends in undiagnosed diabetes in U.S. adults by using a confirmatory definition: a cross-sectional study. Ann Intern Med 2017;167:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . NHANES 2005-2006: Prescription Medications Data Documentation, Codebook, and Frequencies. Accessed 4 September 2020. Available from https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/RXQ_RX_D.htm
- 21. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150 [Google Scholar]
- 24. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroenke K, Spitzer RL. The PHQ-9: a new depression and diagnostic severity measure. Psychiar Ann 2002;32:509–521 [Google Scholar]
- 26. American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S73–S84 [DOI] [PubMed] [Google Scholar]
- 27. National Committee for Quality Assurance . Comprehensive Diabetes Care (CDC). Accessed 14 January 2021. Available from https://www.ncqa.org/hedis/measures/comprehensive-diabetes-care/
- 28. American Diabetes Association . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S125–S150 [DOI] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention . NHANES Tutorial: Software Tips. Accessed 4 September 2020. Available from https://wwwn.cdc.gov/nchs/nhanes/tutorials/softwaretips.aspx
- 30. Centers for Disease Control and Prevention . NHANES Tutorial: Module 4: Variance Estimation. Accessed 4 September 2020. Available from https://wwwn.cdc.gov/nchs/nhanes/tutorials/module4.aspx
- 31. Gnavi R, Picariello R, la Karaghiosoff L, Costa G, Giorda C. Determinants of quality in diabetes care process: the population-based Torino Study. Diabetes Care 2009;32:1986–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med 2006;166:675–681 [DOI] [PubMed] [Google Scholar]
- 33. Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care 2006;29:531–537 [DOI] [PubMed] [Google Scholar]
- 34. Jackson GL, Edelman D, Weinberger M. Simultaneous control of intermediate diabetes outcomes among Veterans Affairs primary care patients. J Gen Intern Med 2006;21:1050–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saydah S, Cowie C, Eberhardt MS, De Rekeneire N, Narayan KM. Race and ethnic differences in glycemic control among adults with diagnosed diabetes in the United States. Ethn Dis 2007;17:529–535 [PubMed] [Google Scholar]
- 36. Tan TW, Shih CD, Concha-Moore KC, et al. Disparities in outcomes of patients admitted with diabetic foot infections. PLoS One 2019;14:e0211481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodney PP, Dzebisashvili N, Goodman DC, Bronner KK. Variation in the Care of Surgical Conditions: Diabetes and Peripheral Arterial Disease: A Dartmouth Atlas of Health Care Series. Accessed 14 September 2020. Available from https://www.dartmouthatlas.org/downloads/reports/Diabetes_report_10_14_14.pdf [PubMed]
- 38. Mangione CM, Gerzoff RB, Williamson DF, et al.; TRIAD Study Group . The association between quality of care and the intensity of diabetes disease management programs. Ann Intern Med 2006;145:107–116 [DOI] [PubMed] [Google Scholar]
- 39. Ackermann RT, Thompson TJ, Selby JV, et al.; Translating Research into Action for Diabetes (TRIAD) Study Group . Is the number of documented diabetes process-of-care indicators associated with cardiometabolic risk factor levels, patient satisfaction, or self-rated quality of diabetes care? The Translating Research into Action for Diabetes (TRIAD) study. Diabetes Care 2006;29:2108–2113 [DOI] [PubMed] [Google Scholar]
- 40. Landon BE, Hicks LS, O’Malley AJ, et al. Improving the management of chronic disease at community health centers. N Engl J Med 2007;356:921–934 [DOI] [PubMed] [Google Scholar]


