Abstract
OBJECTIVE
To determine whether interrupting sitting with brief bouts of simple resistance activities (SRAs) at different frequencies improves postprandial glucose, insulin, and triglycerides in adults with medication-controlled type 2 diabetes (T2D).
RESEARCH DESIGN AND METHODS
Participants (n = 23, 10 of whom were female, with mean ± SD age 62 ± 8 years and BMI 32.7 ± 3.5 kg · m−2) completed a three-armed randomized crossover trial (6- to 14-day washout): sitting uninterrupted for 7 h (SIT), sitting with 3-min SRAs (half squats, calf raises, gluteal contractions, and knee raises) every 30 min (SRA3), and sitting with 6-min SRAs every 60 min (SRA6). Net incremental areas under the curve (iAUCnet) for glucose, insulin, and triglycerides were compared between conditions.
RESULTS
Glucose and insulin 7-h iAUCnet were attenuated significantly during SRA6 (glucose 17.0 mmol · h · L−1, 95% CI 12.5, 21.4; insulin 1,229 pmol · h · L−1, 95% CI 982, 1,538) in comparison with SIT (glucose 21.4 mmol · h · L−1, 95% CI 16.9, 25.8; insulin 1,411 pmol · h · L−1, 95% CI 1,128, 1,767; P < 0.05) and in comparison with SRA3 (for glucose only) (22.1 mmol · h · L−1, 95% CI 17.7, 26.6; P = 0.01) No significant differences in glucose or insulin iAUCnet were observed in comparison of SRA3 and SIT. There was no statistically significant effect of condition on triglyceride iAUCnet.
CONCLUSIONS
In adults with medication-controlled T2D, interrupting prolonged sitting with 6-min SRAs every 60 min reduced postprandial glucose and insulin responses. Other frequencies of interruptions and potential longer-term benefits require examination to clarify clinical relevance.
Introduction
High volumes of time spent in sedentary behaviors (sitting) are associated with poorer cardiometabolic risk profiles and a higher type 2 diabetes (T2D) incidence (1–4). Additionally, sedentary behavior is highly prevalent in high-risk groups such as those with abnormal glucose metabolism and T2D (5–8). Limiting sedentary time has been identified as an important lifestyle component in the prevention of cardiovascular disease and T2D (9).
In addition to addressing the reduction of overall sitting time, addressing the pattern in which it is accumulated has also been identified as important for diabetes management (10). Observational studies have reported that in the general population, those who regularly interrupt their sitting time have more favorable cardiometabolic risk profiles (11–14). In healthy adults and those at high risk of developing T2D, experimental evidence has shown beneficial impacts on postprandial cardiometabolic risk markers when prolonged sitting is interrupted with short, frequent activity bouts (15–19). These studies have used varying durations, frequencies, and modalities of interruptions, with differing findings. Most have used active interruptions involving walking, but recent studies have examined body weight resistance–type exercises, which can markedly increase muscle activity and have the practical advantage of being in a fixed position with minimal disruption to work tasks or leisure pursuits. In studies of those with T2D, interruptions of 3 min every 30 min with either light walking or simple resistance activities (SRAs) significantly attenuated postprandial glucose and insulin responses in comparison with prolonged sitting (20). This duration and frequency have been recommended for the prevention and management of T2D (21). However, it is unclear whether a specific prescriptive recommendation such as this will be applicable across a wide range of populations (16,20,22). In T2D specifically, the optimal frequency of interruptions to prolonged sitting remains uncertain, as few experimental studies have examined frequency-specific effects when total interruption time and modality were standardized. This is an important T2D management consideration, since in free-living circumstances it is well established that bouts of prolonged sitting can vary on a given day and across different behavioral contexts. Incorporation of less frequent interruptions into daily life may be perceived as more achievable for those with T2D. Thus, a better understanding of the impact of more or less frequent active interruptions to sitting time will be informative for T2D management.
We examined whether, in comparison with uninterrupted sitting, the frequency (every 30 min or every 60 min) of interruptions to sitting time involving SRAs differentially affected whole-trial and meal-specific postprandial metabolic responses in overweight or obese adults with medication-controlled T2D. We hypothesized that postprandial glucose, insulin, and triglyceride responses would be attenuated with active interruptions to prolonged sitting, irrespective of the frequency at which they occur.
Research Design and Methods
Study Design
This randomized crossover trial took place at the Baker Heart and Diabetes Institute (Baker Institute) and was approved by the Alfred Ethics Committee (HREC 50/17, March 2017). Written informed consent was obtained from all participants during the initial screening visit. This study was prospectively registered with the Australian New Zealand Clinical Trials Registry (www.isrctn.org, clinical trial no. ACTRN12617000392369). The outcomes reported here are prespecified secondary outcomes. In addition to these, the primary outcome of femoral artery flow-mediated dilation was measured with an ultrasound probe at five time points, the results of which have previously been published (23).
Participants
Twenty-four overweight or obese adults with T2D were recruited through various methods at the Baker Institute. An initial telephone interview determined eligibility to attend a medical screening and familiarization visit at the Baker Institute clinics. Eligibility to attend the screening visit was based on being age 35–70 years; having a BMI 25–40 kg · m−2; having been diagnosed with T2D for at least 3 months; taking at least one, but no more than three, antihyperglycemic agents (not insulin); self-reporting spending at least 5 h per day sitting in total, being insufficiently physically active (performing <150 min of moderate-vigorous physical activity [MVPA] per week), and not being employed in a physically active occupation; speaking English; not smoking; not being pregnant or planning pregnancy in the next 3 months; being free from any self-reported contraindications to physical activity (e.g., severe arthritis, muscle/joint/tendon injuries), kidney, or liver diseases; not having had cancer within the last 5 years; not excessively consuming alcohol or using recreational drugs; and living within a 40-km radius of the Baker Institute.
Screening and Eligibility Criteria
Final eligibility was determined based on a finger-prick HbA1c result between 6.5% and 10% (Afinion AS100; Abbott Diagnostics) at Baker Pathology. Demographic information was collected, and measurements taken included height; weight; waist, hip, and neck circumference; blood pressure; resting electrocardiogram; and a physician-conducted clinical examination. Participants were excluded on the basis of an abnormal electrocardiogram or blood pressure >160/100 mmHg. Participants were then familiarized with testing day procedures; this included guided practice of the SRAs for active interruptions trial conditions. A participant flow diagram is presented in Fig. 1. Due to slow initial recruitment rates, the eligibility criteria of the original study design were expanded to include a wider T2D population. Additional information regarding these changes is provided in the Supplementary Material.
Figure 1.
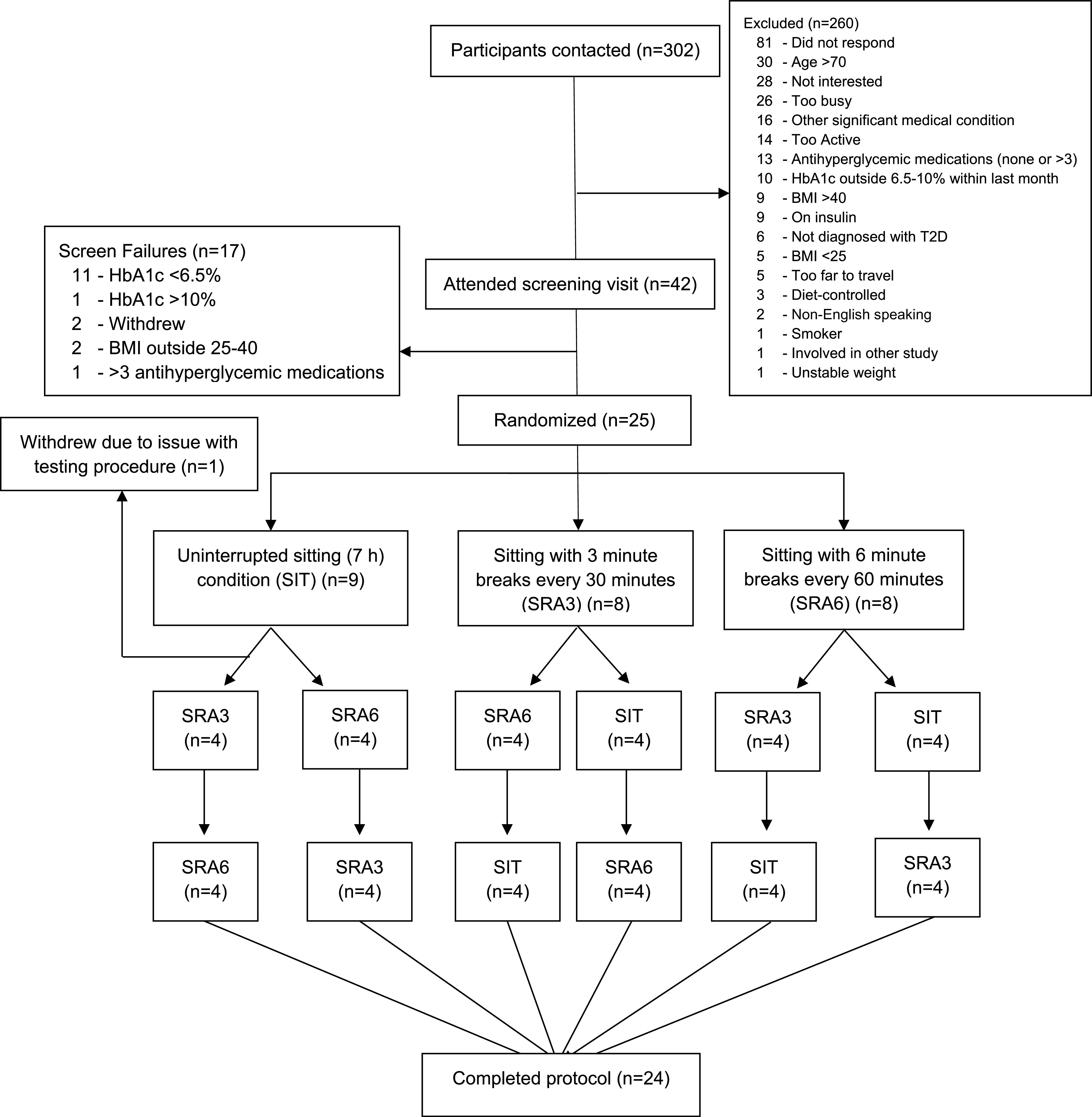
—Participant flow diagram. BMI values are weight in kilograms divided by the square of height in meters.
Randomization Protocol
Figure 1 illustrates that participants were randomly assigned to complete the three trial conditions in one of six possible orders, with use of a balanced block randomization. The randomization sequence was generated with an online statistical package (www.randomization.com) and was prepared by an independent third party. Prior to the participant’s first experimental condition, the trial coordinator and the third party would reveal the randomization sequence. This method of randomization blinds participants to the full order of conditions until the morning of the second experimental visit.
Pre–Experimental Condition Standardization Protocols
Prior to each experimental condition, participants were asked to complete a 3-day food diary, avoid MVPA for 48 h preceding each of the conditions, and abstain from alcohol and caffeine for the 24 h prior. They wore an ActiGraph (GTX3+; ActiGraph, Pensacola, FL) on the wrist for monitoring of sleep (24) in the free-living setting (data not shown). An activPAL4 (PAL technologies, Glasgow, U.K.) monitor was worn for measurement of sitting, standing, stepping, and physical activity data during the habitual period, the 48-h restricted period preceding the testing visits, and for validation of the postural changes (interruptions) involved in the activity protocol during the experimental visit.
Experimental Protocol
Each participant completed three 8-h experimental conditions (including a 1-h initial steady-state period), separated by a 6- to 14-day washout period. The three conditions were as follows:
SIT: on arrival at the clinic, participants confirmed adherence to lead-in protocols. After a steady-state period of 1 h, they remained seated for the duration of the visit (7 h). Participants were supervised while watching TV or working quietly while seated. For minimization of walking distance, they were transported in a wheelchair for bathroom breaks.
SRA3: following the steady-state period, participants interrupted sitting every 30 min to complete a 3-min bout of the SRAs for the 7-h experimental period. The SRAs performed were body weight–resisted half squats, calf-raises, and single leg knee lifts separated by gluteal contractions. This active break protocol has previously been demonstrated to acutely improve postprandial metabolism in those with T2D (20). Exercises were performed on rotation for 20 s each until 3 min of activity were completed. Twelve interruption periods totaling 36 min of activity were performed throughout the experimental period. Activity intensity during the interruptions was monitored with the Borg Rating of Perceived Exertion (RPE) scale (range: minimum–maximum 6–20).
SRA6: as for SRA3, but 6-min bouts of SRAs every 60 min of sitting (i.e., 20-s bouts of each exercise but for double the number of rotations). Six activity interruptions were completed totaling 36 min of activity across the experimental period.
Blood Collection Protocol
Participants arrived at the clinic at 0730 h having fasted from 10:00 p.m. the previous night. A cannula was inserted into a vein in the antecubital fossa (or the most suitable/accessible vein), and a baseline sample was drawn immediately, as illustrated in Fig. 2. In total, 150 mL blood was collected over 16 time points across each experimental period (half hourly).
Figure 2.
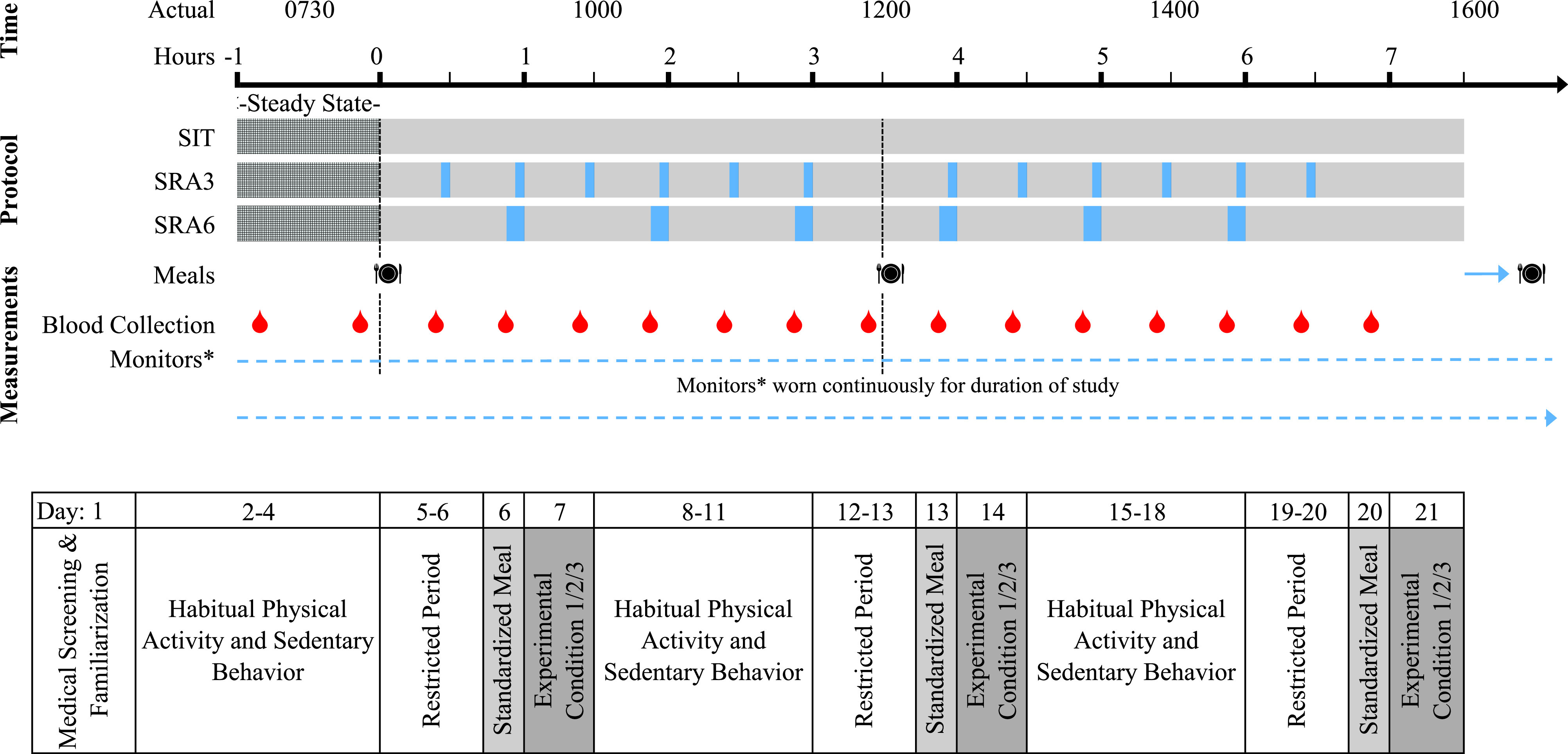
—Experimental protocol and study schedule. Evening meal consumed between 7:00 and 9:00 p.m. the night before and the night of the trial condition. During the restricted period, participants were instructed to avoid MVPA for 48 h and to abstain from any caffeine for 24 h. *Monitors worn: Abbott FreeStyle Libre Flash Glucose Monitor, activPAL4, and ActiGraph GTX3+.
Glucose was analyzed in duplicate immediately from whole venous blood with use of HemoCue Glucose 201+ analyzer (Hemocue AB, Ångelholm, Sweden). HemoCue glucose concentrations were corrected from whole blood to plasma equivalent values with a multiplication factor of 1.1 for normal fasting hematocrit measured in the first blood draw of each trial condition. For abnormal fasting hematocrit (males <36%, >50%; females <32%, >45%), the following formula was used (25):
 |
Samples obtained for triglyceride and full blood examination analysis were sent to Alfred Pathology within 2 h of collection. Triglycerides were analyzed with glycerol phosphate oxidase methods on an Abbott ARCHITECT ci16200 and an Abbott Alinity. The remaining samples obtained were centrifuged in an Allegra X-15R at 2,000 rpm for 15 min at 4°C. The plasma was aliquoted and frozen immediately at –80°C for later analysis. Insulin was analyzed with use of chemiluminescent microparticle immunoassay on an Abbott ARCHITECT ci16200. Insulin concentrations were converted from conventional to Systéme International (SI) units with a conversion factor of 1 μU · mL−1 = 6 pmol · L−1 (26).
Test Meal Protocol
Each participant consumed four standardized meals across each of the three experimental conditions. A take-home meal pack was consumed between 6:00 and 9:00 p.m. the night before the experimental condition. Participants were instructed to consume the meal pack only, with no food after 10:00 p.m. Ad libitum water intake was encouraged over this time. During the experimental conditions, they consumed breakfast and lunch in the clinic, and participants were sent home with a second meal pack to consume that night. Estimated energy requirements were calculated for each participant with Schofield equations and a physical activity adjustment factor of 1.5 (indicative of very sedentary). Daily energy intake was then split into three, with each experimental meal providing 33% of estimated total daily energy requirements. Meals were calculated to provide 55% daily energy requirements from carbohydrates, 30% from fat, and 15% from protein.
Data Handling and Statistical Analyses
Outcomes were net incremental area under the curve (iAUCnet) for glucose, insulin, and triglycerides (trapezoidal method, with the average of two fasting measures taken 1 h apart used as the baseline). iAUCnet was calculated for the experimental period (7 h) and two 3.5-h meal-specific periods: post-breakfast and post-lunch. Total and positive incremental areas under the curve (AUCs) were also calculated and are reported in Supplementary Table 3. A sample size of 24 participants was calculated for the primary outcome measure (vascular function [23]) and was selected based on a previous trial conducted in our laboratory to provide sufficient power (>80%) for the secondary outcomes of postprandial glucose, insulin, and triglyceride concentrations (20). Of the 24 participants who completed all three experimental conditions, one could not be cannulated successfully at two out of three visits and thus was excluded from the analysis. The remaining data from n = 23 participants included one insulin sample that was deemed unsuitable for analysis. Mean imputation was used to substitute the missing data point.
activPAL4-derived MVPA was calculated as the time participants spent stepping with a cadence of >100 steps/min for >1 min. Wrist-worn ActiGraph MVPA was calculated with the R package GGIR (27), and time spent in a state of MVPA was identified with Hildebrand Euclidean Norm Minus One (ENMO) cut points of 100.6mg for moderate and 428.8mg for vigorous physical activity (28).
Mixed-effects linear regression models with random intercepts were used for evaluation of the differential effects of experimental conditions on glucose, insulin, and triglyceride iAUCnet. All models were adjusted for the potential confounders of treatment order, the average of the –1 and 0 h fasting samples, age, sex, and waist circumference. Age, sex, and waist circumference are also controlled for through the use of the crossover study design (29). For accounting for any residual effects of participants’ prior physical activity levels, 7-h iAUCnet models were additionally adjusted for pre–experimental period and pre–restricted period stepping time and MVPA derived from both activPAL4 and wrist-worn ActiGraph. Results of the sensitivity analyses are presented in Supplementary Table 6. Residuals were examined for serial correlation, heteroscedasticity, and normality. Residual data found to not satisfy criteria for normality were log transformed and reported as exponentiated coefficients. All statistical analyses were conducted with Stata 14.1 (StataCorp, College Station, TX). A two-tailed probability level of 0.05 was used.
Results
Of the 25 participants randomized, 23 (10 female; mean ± SD age 62 ± 8 years, BMI 32.7 ± 3.5 kg · m−2, and HbA1c 7.6 ± 0.8%) completed all three study arms and were included in the final analyses. Participant characteristics at the medical screening and familiarization visit are presented in Supplementary Table 1 for the 23 participants. Of these, 7 were on a single diabetes therapy (medication), 10 dual therapy, and 6 triple therapy. Participants were instructed to keep medications unchanged for the course of the study and took these at the same times on trial days. Baseline weight, waist circumference, waist-to-hip ratio, and neck circumference (shown in Supplementary Table 1) were all significantly lower in females. Fasting triglycerides were also significantly lower in females compared with males (1.5 ± 0.5 and 1.8 ± 0.6 mmol/L, respectively; P = 0.029). Pre-experimental data on time spent sitting, standing, stepping, and in a state of MVPA (inferred with use of activPAL4 data from a stepping cadence of >100 steps per minute for >1 min); diet; and glucose, insulin, and triglyceride fasting values are shown in Table 1. Mean pre-experimental MVPA from activPAL4 was 11.8 min/day (range 0.5–41.3). In the 48-h restricted period, total steps and stepping time were significantly greater preceding the SRA3 condition in comparison with SIT, and MVPA was greater prior to both SRA3 and SRA6. No significant differences in sitting time, wrist-worn ActiGraph–derived MVPA, dietary indices, or fasting metabolic values were observed between pre-experimental periods. RPE data were collected for monitoring of activity intensity during the SRAs. During the SRA3 condition, RPE ranged from 6 to 14, average 9 (on a 6–20 scale), and during SRA6 RPE ranged from 6 to 16, average 9.
Table 1.
Pre–experimental period participant demographic information
| SIT | SRA3 | SRA6 | |
|---|---|---|---|
| Weight (kg) | 93.2 (93.0, 93.4) | 93.2 (93.0, 93.5) | 93.4 (93.2, 93.6) |
| Preprandial levels^ | |||
| Glucose (mmol · L−1) | 8.2 (7.8, 8.6) | 8.3 (7.9, 8.7) | 8.0 (7.6, 8.4) |
| Insulin (pmol · L−1) | 74.3 (66.7, 82.0) | 77.7 (70.0, 85.4) | 76.7 (69.0, 84.3) |
| Triglycerides (mmol · L−1) | 1.8 (1.6, 1.9) | 1.8 (1.7, 1.9) | 1.6 (1.5, 1.7) |
| activPAL4 data† | |||
| Sitting time (min/day) | |||
| Habitual | 585 (534, 634) | 609 (559, 658) | 599 (546, 652) |
| Restricted | 633 (564, 703) | 613 (541, 685) | 582 (510, 653) |
| Standing time (min/day) | |||
| Habitual | 227 (185, 269) | 210 (169, 252) | 256 (212, 300) |
| Restricted | 218 (178, 258) | 213 (172, 258) | 218 (178, 259) |
| Total stepping time (min/day) | |||
| Habitual | 86 (71, 102) | 91 (75, 106) | 89 (73, 105) |
| Restricted | 74 (61, 87) | 95 (82, 108)* | 84 (70, 97) |
| Total number of steps | |||
| Habitual | 6,842 (5,593, 8,092) | 7,145 (5,911, 8,378) | 6,974 (5,673, 8,275) |
| Restricted | 5,806 (4,695, 6,917) | 7,605 (6,367, 8,742)* | 6,420 (5,283. 7,557) |
| MVPA (min/day) | |||
| Habitual | 13 (7, 19) | 15 (9, 20) | 11 (5, 18) |
| Restricted | 4 (0, 8) | 18 (13, 22)* | 11 (6, 15)* |
| Wrist-worn ActiGraph data‡ | |||
| MVPA (min/day) | |||
| Habitual | 50 (38, 63) | 56 (44, 68) | 57 (44, 69) |
| Restricted | 45 (31, 59) | 55 (43, 67) | 52 (38, 66) |
| Diet§ | |||
| Total daily energy (kcal/day) | 2,194 (2,049, 2,340) | 2,091 (1,942, 2,241) | 2,164 (2,018, 2,309) |
| Total CHO (g/day) | 227 (213, 241) | 214 (200, 229) | 223 (209, 237) |
| Total fat (g/day) | 90 (82, 99) | 84 (75, 93) | 89 (80, 98) |
| Total protein (g/day) | 99 (89, 110) | 102 (91, 113) | 98 (87, 108) |
Data are means (95% CI). CHO, carbohydrates.
Denotes a statistically significant difference in comparison with the SIT condition, P < 0.05. During the restricted period, participants were instructed to avoid MVPA for 48 h and caffeine for 24 h prior to each experimental condition. ^Preprandial levels based on the average of two time points (–1 and 0 h) immediately before the breakfast meal.
activPAL4 data collected during habitual (free-living) days and the 48-h period preceding the trial condition.
Wrist-worn ActiGraph data were analyzed with the GGIR package (27), and time spent in MVPA was identified with use of Hildebrand ENMO cut points of 100.6mg for moderate and 428.8mg for vigorous physical activity.
Dietary intakes were assessed from 3-day diet records prior to each trial condition and analyzed with FoodWorks dietary analysis software (FoodWorks; Xyris Software, Highgate Hill, Australia).
Trial Period (7-h) Incremental AUC
Figure 3 shows mean glucose, insulin, and triglyceride concentrations during each of the trial conditions. Glucose 7-h iAUCnet during the SRA6 condition was significantly attenuated in comparison with prolonged sitting (SIT mean 21.4 mmol · h · L−1, 95% CI 16.9, 25.8; SRA6 17.0 mmol · h · L−1, 95% CI 12.5, 21.4; P = 0.029) and SRA3 (mean 22.1 mmol · h · L−1, 95% CI 17.7, 26.6; P = 0.011). This remained unchanged after additional adjustment for 48-h restricted MVPA levels. Insulin iAUCnet was also significantly attenuated during SRA6 (1,229 pmol · h · L−1, 95% CI 982, 1,538) in comparison with SIT (1,411 pmol · h · L−1, 95% CI 1,128, 1,767; P = 0.014); however, these attenuations were no longer statistically significant after adjustment for restricted MVPA levels. SRA3 did not attenuate glucose or insulin iAUCnet in comparison with SIT. Triglyceride 7-h iAUCnet was not significantly different between conditions. Comparisons among total AUC, positive incremental AUC (iAUC), iAUCnet, and mean analyte values can be found in Supplementary Table 2. Notably, overall trends are similar; however, in comparison of the difference between glucose AUCs during SRA6 and SRA3, positive iAUC and iAUCnet were statistically significantly different, while total AUC was not.
Figure 3.
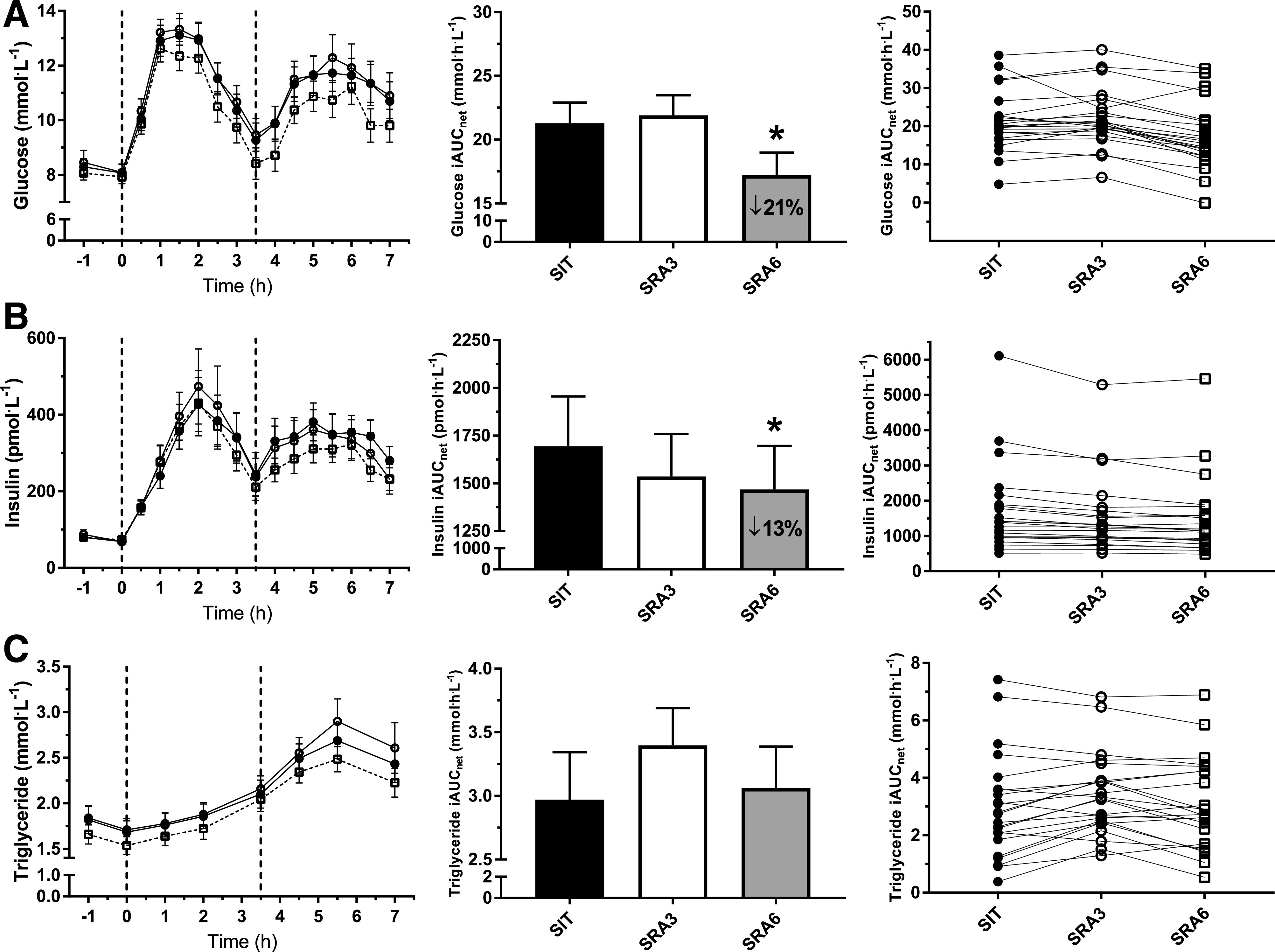
—Postprandial plasma glucose (A), plasma insulin (B), and plasma triglyceride (C) concentrations measured during SIT (●) and sitting interrupted with SRA breaks of 3 min (○) or 6 min (□). Vertical dashed lines indicate timing of the breakfast (0 h) and lunch (3.5 h) meals. Data are presented as means ± SEM. From left to right: mean time course responses, mean iAUCnet, and individual iAUCnet responses. *Difference from uninterrupted sitting (P < 0.05).
Meal-Specific Incremental AUC
Post-breakfast
Meal-specific iAUCnet values are found in Supplementary Table 3. Glucose 3.5-h iAUCnet was lower after breakfast during SRA6 in comparison with SIT but did not reach statistical significance (SIT 11.2 mmol · h · L−1, 95% CI 9.1, 13.3; SRA6 9.6 mmol · h · L−1, 95% CI 7.5, 11.7; P = 0.077). However, SRA6 was significantly lower than SRA3 following breakfast (SRA3 11.6 mmol · h · L−1, 95% CI 9.5, 13.7; P = 0.029). No statistically significant differences were observed for insulin or triglyceride 3.5-h iAUCnet post-breakfast.
Post-lunch
Glucose 3.5-h iAUCnet post-lunch was significantly lower during SRA6 (7.8 mmol · h · L−1, 95% CI 3.6, 12.0) in comparison with SIT (12.0 mmol · h · L−1, 95% CI 7.8, 16.2; P = 0.014) and SRA3 (12.5 mmol · h · L−1, 95% CI 8.3, 16.7; P = 0.006). In comparison with that in SIT (959 pmol · h · L−1, 95% CI 717, 1,282), insulin 3.5-h post-lunch iAUCnet was significantly attenuated during the SRA6 condition (796 pmol · h · L−1, 95% CI 595, 1,064; P = 0.029) and during SRA3 (796 pmol · h · L−1, 95% CI 595, 1,064; P = 0.029). There was no significant effect of condition on post-lunch triglyceride 3.5-h iAUCnet.
Conclusions
In our sample of adults with T2D, interrupting sitting with 6 min of SRAs every 60 min attenuated the whole-day glucose and insulin responses to meals (a decrease of 21% and 13%, respectively) in comparison with uninterrupted sitting. Interrupting sitting with 3-min SRAs every 30 min did not attenuate the postprandial glycemic response to meals in comparison with prolonged sitting. Meal-specific iAUCnet was lower during SRA6 for glucose post-breakfast, and during both SRA6 and SRA3 for insulin post-lunch, in comparison with SIT. No differences were observed for whole-day or meal-specific triglyceride iAUCnet.
Previous studies have examined the effects of reducing and interrupting prolonged sitting in those with T2D. Improvements to whole-day postprandial glucose have been reported when sitting time has been replaced with standing or stepping, as well as in the case of a higher frequency of interruptions throughout the day in free-living settings (16,30). In the laboratory setting, when prolonged sitting was interrupted every 30 min with 3 min of SRA breaks (the same protocol used here), iAUC reductions of 39% and 37% for glucose and insulin were observed (20). In the current study, attenuations were only observed during the SRA6 condition. Other studies have not reported statistically significant findings in examination of effects of different frequencies of interruptions. For instance, in a small study of 14 sedentary and obese but otherwise healthy males (mean age 28 years), no statistically significant differences in postprandial glucose or insulin concentrations were observed when sitting was interrupted every 20, 60, or 120 min with 2, 6, and 12 min of light walking, respectively (31). In sedentary but otherwise healthy women (mean age 34 years), moderate-intensity walking breaks attenuated postprandial insulin concentrations when performed every 30 min but did not do so when performed every 180 min (32).
The evidence on which to base specific quantitative recommendations with respect to how often sitting time should be interrupted in the context of T2D management is inconclusive. Current ADA guidelines state that prolonged sitting should be interrupted at least every 30 min (10). This recommendation was based on limited evidence (Level C, the lowest grade), and it may be premature to suggest explicit frequency targets. From a practical perspective, broader recommendations to reduce and interrupt sitting time, rather than prescriptive guidelines, may be more appropriate given the modest nature of the available evidence. The further insights we have provided here suggest that interrupting sitting, even at a frequency of every 60 min, may be beneficial for glucose metabolism in those with later-stage T2D. Adapting the current recommendation to interrupt sitting every 30 min to incorporate a frequency range (e.g., every 30–60 min) would send a positive message in promotion within real-life scenarios. Recommending interrupting periods of prolonged sitting with activity at least once every hour constitutes a simple, clear message that could be effectively communicated in a variety of settings, in addition to the essential promotion of structured MVPA. However, care should be taken in also communicating that interrupting sitting at a lesser frequency should involve longer-duration activity, for the metabolic benefits. Pragmatically, each of the frequencies and durations of interruptions were well tolerated, with a high degree of acceptance by our T2D patients (based on subjective feedback given alongside RPE [data not shown]), and participants reported being confident that they could integrate these activities into various contexts in their daily lives. In free-living situations, people very rarely sit all day but naturally interrupt sitting on an irregular basis. Understanding the impact of altering the frequency of these interruptions in day-to-day living, over the long term, would be a relevant goal for future studies.
There are several possible explanations as to why our study did not observe reductions in postprandial glucose and insulin during the SRA3 condition. Most notably, our participants were taking up to three antihyperglycemic agents. As T2D is a progressive disease, combination therapy is often necessary (33). Thus, it is not surprising that a wide range in duration of diabetes was present for our study participants. The medication use and duration of diabetes may be a key explanation for the discrepancy in the findings from those of a previous study with T2D participants (20) that used a similar active-interruption protocol. While our trial is underpowered to examine the effect of the different medications on the outcomes for subsets of participants, it is plausible that the medication’s mechanisms of action may differentially interact with the effects of the SRAs and glucose clearance. In our study, 22 of our 23 participants were on metformin, which is widely used in diabetes management to enhance insulin sensitivity. GLP-1 agonists, sulfonylureas, and dipeptidyl peptidase 4 inhibitors stimulate insulin secretion, which were taken by 16 of 23 participants. Sodium–glucose cotransporter 2 inhibitors limit glucose reabsorption and were taken by eight of the study participants. In addition, the combinations of these medications varied between individuals (listed in Supplementary Table 4). Previous studies have reported that metformin and exercise do not have an additive effect on the glycemic response to meals but, rather, that metformin may attenuate the glycemic-lowering effects of exercise (34,35). Information on medications for common diabetes comorbidities including statin and antihypertensive use can be found in Supplementary Table 4. Briefly, 14 (61%) of participants were using a statin, 12 (52%) were using an ACE inhibitor or angiotensin receptor blocker, 3 (13%) used calcium channel blockers, 3 used a β-blocker, and 2 used a diuretic. While it is likely that the different classes of medications and combinations used by participants would increase the variability in individual postprandial responses to SRA breaks, the study was not designed to directly or robustly test this.
During the SRA3 condition, a small, non-significant increase in postprandial glucose responses was observed. This may be partly explained by an acute glycemic response to the SRAs itself. Studies have reported transient increases in blood glucose levels immediately following a bout of resistance-based exercise in adults with insulin resistance or T2D (35–37). A speculative explanation of our findings could be that because more frequent interruptions require the participant to get up out of their chair twice as often, this may result in some hepatic glucose release—and/or the shorter duration may result in less upregulation of GLUT4 to the cell surface (and thus less clearance of glucose into peripheral tissues). Indeed, among subjects with T2D medicated with metformin, compared with those not medicated, Boulé et al. (34) reported increased levels of the hormone glucagon (responsible for increasing glucose levels) following exercise in the fed state. The exercise performed in the Boulé study was high intensity and over an extended duration; however, this provides some potential insight into the broader implications of the interactions of metformin with physical activity. A previous study in adults with T2D, which used the SRA protocol, reported that this type of break elicited a greater activity response as measured by intensity and energy expenditure in comparison with the same duration of light walking (20). By nature, in comparison with walking, SRAs evoke greater contractile activity of skeletal muscle by recruitment of large muscle groups (quadriceps, gluteals, hamstrings, and calves) and placing them under body weighted resistance. The longer duration of the less frequent SRA breaks reported here may provide the stimulus required for GLUT4 translocation, resulting in greater clearance and/or less hepatic glucose release (38,39).
No significant effect of interrupting sitting at any frequency was observed on postprandial triglyceride responses. During the SRA3 condition, triglyceride iAUCnet, particularly during the post-lunch period, appeared to be larger, albeit not significantly so. In T2D, due to insulin-resistant tissues being less able to absorb fatty acids released in the bloodstream following a meal, postprandial triglyceride responses are often elevated (40). Diabetes medications also play a role in lipid metabolism, and indeed their interaction with activity should be a focus for future research (35). The individual triglyceride response in our study varied greatly between study participants, as illustrated in Fig. 3C, possibly due in part to the different medications, including statins, taken by participants (61% of our sample). Previous studies in T2D where participants have been restricted to those only taking metformin for glycemic management, but where 67% of participants also used statins, have reported significant improvements to postprandial triglyceride response when sitting is interrupted with active breaks (20). In healthy individuals, improvements in postprandial triglyceride response have also been reported when sitting has been interrupted with active breaks on the day before (41). Therefore, our study findings may be the result of a combination of factors, such as varied number and combination of medications, not enough muscle activity stimulus, or a delayed reduction in triglycerides that was not captured within our 7-h trial day.
Our study showed different effects on postprandial metabolism when iAUCnet was calculated for the breakfast and lunch meals separately. During the SRA6 condition, post-breakfast glucose iAUCnet was reduced, but not significantly so, in comparison with SIT. However, in comparison with SRA3, there was a 17% reduction in glucose iAUCnet during SRA6. If transient increases in blood glucose concentrations did occur as a result of the shorter, more frequent interruptions to sitting, this may have resulted in the significantly greater post-breakfast iAUC observed. No significant differences were observed post-breakfast for either insulin or triglycerides. By contrast, following lunch, insulin 3.5-h iAUC was 33% lower during both SRA3 and SRA6 in comparison with SIT. The relative efficiency of insulin may be improved in the post-lunch period of the active conditions, resulting in less insulin being needed to effectively clear the same amount of glucose from the circulation (38,39).
Standardized lead-in periods where device-measured free-living activity and dietary intake data were collected to ensure adherence to study protocols showed differences in the pre-experimental activity level of the participants. Specifically, number of steps and time spent stepping were significantly higher preceding the SRA3 condition compared with SIT, and activPAL4-derived MVPA was significantly greater in the restricted period preceding both SRA3 and SRA6 in comparison with SIT. Participants were instructed to abstain from as much MVPA as possible in the 48-h period prior to the experimental conditions. As shown in Table 1, participants completed 4–18 min MVPA/day in the restricted period. Residual effects of activity, particularly on insulin sensitivity, may have confounded the results. This may be especially true during the SRA3 condition given there was a higher level of pre-experimental physical activity, and no significant differences were observed during the study condition). Sensitivity analyses provided as supplementary material indicate that inclusion of restricted-period activity (stepping time and MVPA) strengthened the effect of the SRA6 breaks for glucose, but weakened the effect for insulin, which is a significant limitation of the study. Of note, it is well accepted that wrist-worn accelerometry, despite having higher compliance, has limitations for the assessment of physical activity (42–45). Specifically, the wrist-worn ActiGraph captures isolated movement of the upper limbs, which results in higher activity counts. In comparison with hip-worn accelerometry, the cut points used to characterize the wrist-worn data (28) tend to give higher estimates of the amount of whole-body physical activity performed (42–45). As such, the level of confidence with which we report pre–experimental activity data (Table 1) and results of the sensitivity analyses (Supplementary Table 6) is low.
The crossover design is a key strength of the study, as it controls for person-to-person differences and affords smaller sample sizes. During trial condition days, participants were strictly supervised and fed standardized meals. However, to facilitate faster recruitment rates, the initial eligibility criteria of the study were widened to include multiple medications and a wider age and HbA1c inclusion range. This introduced greater heterogeneity in the disease stage of the participants. Although this increases the external validity of our findings (as we included a wider T2D population), in proof-of-concept studies such as ours, internal validity is more relevant, and thus a greater sample size may have been required. As original sample size calculations were based on a more homogenous sample (shorter diabetes duration and metformin only), the internal validity is compromised. Additionally, 7 h of uninterrupted sitting may be an exaggerated simulation of a usual working day. Thus, the ability to extrapolate findings to free-living and longer-term scenarios is also limited.
In conclusion, our findings provide further evidence to inform T2D management guidelines: interrupting sitting, even at a frequency of every 60 min, may be beneficial for glycemic control in those with later-stage T2D. This supports the current recommendation to reduce overall time spent in sedentary behaviors and interrupt periods of prolonged sitting, while broadening the frequency (every 30–60 min) at which this needs to occur. Future studies are required for understanding of the impact of free-living situations in which sitting is interrupted at irregular intervals and the influence this has on longer-term glycemic control.
Article Information
Acknowledgments. The authors are grateful for the excellent technical assistance from Kym Rickards, Melanie Townsend, and Louise Hammond (research nurses) from Baker Institute and Sarah Green (sample analysis) from Alfred Pathology. Most importantly, the authors thank the study participants for their time and commitment to the study protocol; this study would not have been possible without them.
Funding. This research was supported by a Heart Foundation Australia Vanguard Grant (award no. 101449), a National Health and Medical Research Council (NHMRC) Centre of Research Excellence grant (1057608), and the Victorian Government OIS scheme. A.R.H. and F.C.T. are supported by Research Training Program awards. P.C.D., D.J.G., N.O., B.A.K., and D.W.D. are supported by the NHMRC Fellowships scheme.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.R.H. conducted the study, analyzed and interpreted data, and wrote the manuscript. F.C.T. and M.K.T. assisted with the conduct of the study. P.C.D., M.J.W., P.S., M.S.G., D.J.G., N.D.C., R.N.L., B.A.K., N.O., and D.W.D. assisted in the concept and design of the study and participated in critical revision of the manuscript for intellectual content. P.C.D. and P.S. assisted with data cleaning or management and statistical analyses or interpretation. N.D.C. provided clinical support during data collection. All authors approved the final version of the manuscript. A.R.H. and D.W.D. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 67th Annual Scientific Meeting of the American College of Sports Medicine, San Francisco, CA, 26–30 May 2020.
Footnotes
Clinical trial reg. no. ACTRN12617000392369, www.isrctn.org
This article contains supplementary material online at https://doi.org/10.2337/figshare.14096582.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 2012;55:2895–2905 [DOI] [PubMed] [Google Scholar]
- 2. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 2018;33:811–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brocklebank LA, Falconer CL, Page AS, Perry R, Cooper AR. Accelerometer-measured sedentary time and cardiometabolic biomarkers: a systematic review. Prev Med 2015;76:92–102 [DOI] [PubMed] [Google Scholar]
- 4. Swindell N, Mackintosh K, McNarry M, et al. Objectively measured physical activity and sedentary time are associated with cardiometabolic risk factors in adults with prediabetes: the PREVIEW study. Diabetes Care 2018;41:562–569 [DOI] [PubMed] [Google Scholar]
- 5. Edwardson CL, Gorely T, Davies MJ, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One 2012;7:e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Berg JD, Stehouwer CD, Bosma H, et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: the Maastricht Study. Diabetologia 2016;59:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Rooij BH, van der Berg JD, van der Kallen CJ, et al. Physical activity and sedentary behavior in metabolically healthy versus unhealthy obese and non-obese individuals - the Maastricht Study. PLoS One 2016;11:e0154358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathe N, Boyle T, Al Sayah F, et al. Correlates of accelerometer-assessed physical activity and sedentary time among adults with type 2 diabetes. Can J Public Health 2017;108:e355–e361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenzweig JL, Bakris GL, Berglund LF, et al. Primary prevention of ASCVD and T2DM in patients at metabolic risk: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2019;104:3939–3985 [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association . 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S48–S65 [DOI] [PubMed] [Google Scholar]
- 11. Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 2008;31:661–666 [DOI] [PubMed] [Google Scholar]
- 12. Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J 2011;32:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellettiere J, Winkler EAH, Chastin SFM, et al. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS One 2017;12:e0180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chastin SF, Egerton T, Leask C, Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity (Silver Spring) 2015;23:1800–1810 [DOI] [PubMed] [Google Scholar]
- 15. Duvivier BMFM, Bolijn JE, Koster A, Schalkwijk CG, Savelberg HHCM, Schaper NC. Reducing sitting time versus adding exercise: differential effects on biomarkers of endothelial dysfunction and metabolic risk. Sci Rep 2018;8:8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duvivier BM, Schaper NC, Hesselink MK, et al. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 2017;60:490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012;35:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen RN, Kingwell BA, Robinson C, et al. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin Sci (Lond) 2015;129:117–127 [DOI] [PubMed] [Google Scholar]
- 19. Larsen R, Ali H, Dempsey PC, et al. Interrupting sitting time with simple resistance activities lowers postprandial insulinemia in adults with overweight or obesity. Obesity (Silver Spring) 2019;27:1428–1433 [DOI] [PubMed] [Google Scholar]
- 20. Dempsey PC, Larsen RN, Sethi P, et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care 2016;39:964–972 [DOI] [PubMed] [Google Scholar]
- 21. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paing AC, McMillan KA, Kirk AF, Collier A, Hewitt A, Chastin SFM. Dose-response between frequency of breaks in sedentary time and glucose control in type 2 diabetes: a proof of concept study. J Sci Med Sport 2019;22:808–813 [DOI] [PubMed] [Google Scholar]
- 23. Taylor FC, Dunstan DW, Homer AR, et al. Acute effects of interrupting prolonged sitting on vascular function in type 2 diabetes. Am J Physiol Heart Circ Physiol 2021;320:H393–H403 [DOI] [PubMed] [Google Scholar]
- 24. Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR. Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms 2015;13:172–180 [Google Scholar]
- 25. Hagvik J. HemoCue Technical Letter, No 5. HemoCue AB, Angleholm, Sweden, 2004 [Google Scholar]
- 26. Vølund A. Conversion of insulin units to SI units. Am J Clin Nutr 1993;58:714–715 [DOI] [PubMed] [Google Scholar]
- 27. van Hees VT. GGIR: raw accelerometer data analysis, 2015. Accessed 1 December 2020. Available from https://CRAN.R-project.org/package=GGIR
- 28. Hildebrand M, VAN Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816–1824 [DOI] [PubMed] [Google Scholar]
- 29. Kenward MG, Roger JH. The use of baseline covariates in crossover studies. Biostatistics 2010;11:1–17 [DOI] [PubMed] [Google Scholar]
- 30. Paing AC, McMillan KA, Kirk AF, Collier A, Hewitt A, Chastin SFM. Impact of free-living pattern of sedentary behaviour on intra-day glucose regulation in type 2 diabetes. Eur J Appl Physiol 2020;120:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thorsen IK, Johansen MY, Pilmark NS, et al. The effect of frequency of activity interruptions in prolonged sitting on postprandial glucose metabolism: a randomized crossover trial. Metabolism 2019;96:1–7 [DOI] [PubMed] [Google Scholar]
- 32. Maylor BD, Zakrzewski-Fruer JK, Stensel DJ, Orton CJ, Bailey DP. Effects of frequency and duration of interrupting sitting on cardiometabolic risk markers. Int J Sports Med 2019;40:818–824 [DOI] [PubMed] [Google Scholar]
- 33. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S98–S110 [DOI] [PubMed] [Google Scholar]
- 34. Boulé NG, Robert C, Bell GJ, et al. Metformin and exercise in type 2 diabetes: examining treatment modality interactions. Diabetes Care 2011;34:1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharoff CG, Hagobian TA, Malin SK, et al. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab 2010;298:E815–E823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gordon BA, Bird SR, Macisaac RJ, Benson AC. Glycemic response varies between resistance and aerobic exercise in inactive males with long-term type 2 diabetes. Appl Physiol Nutr Metab 2013;38:900–904 [DOI] [PubMed] [Google Scholar]
- 37. Boulé NG, Weisnagel SJ, Lakka TA, et al. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care 2005;28:108–114 [DOI] [PubMed] [Google Scholar]
- 38. Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 2013;93:993–1017 [DOI] [PubMed] [Google Scholar]
- 39. Bergouignan A, Latouche C, Heywood S, et al. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci Rep 2016;6:32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol 1999;83:25F–29F [DOI] [PubMed] [Google Scholar]
- 41. Homer AR, Fenemor SP, Perry TL, et al. Regular activity breaks combined with physical activity improve postprandial plasma triglyceride, nonesterified fatty acid, and insulin responses in healthy, normal weight adults: a randomized crossover trial. J Clin Lipidol 2017;11:1268–1279.e1 [DOI] [PubMed] [Google Scholar]
- 42. Montoye AHK, Clevenger KA, Pfeiffer KA, et al. Development of cut-points for determining activity intensity from a wrist-worn ActiGraph accelerometer in free-living adults. J Sports Sci 2020;38:2569–2578 [DOI] [PubMed] [Google Scholar]
- 43. Kingsley MIC, Nawaratne R, O’Halloran PD, et al. Wrist-specific accelerometry methods for estimating free-living physical activity. J Sci Med Sport 2019;22:677–683 [DOI] [PubMed] [Google Scholar]
- 44. Kerr J, Marinac CR, Ellis K, et al. Comparison of accelerometry methods for estimating physical activity. Med Sci Sports Exerc 2017;49:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ellingson LD, Hibbing PR, Kim Y, Frey-Law LA, Saint-Maurice PF, Welk GJ. Lab-based validation of different data processing methods for wrist-worn ActiGraph accelerometers in young adults. Physiol Meas 2017;38:1045–1060 [DOI] [PubMed] [Google Scholar]


