Abstract
OBJECTIVE
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) improved multiple proatherogenic risk factors and reduced cardiovascular events in recent clinical trials, suggesting that they may slow progression of atherosclerosis. We tested whether exenatide once weekly reduces carotid plaque progression in individuals with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In a double-blind, pragmatic trial, 163 participants were randomized (2:1) to exenatide (n = 109) or placebo (n = 54). Changes in carotid plaque volume and composition were measured at 9 and 18 months by multicontrast 3 Tesla MRI. Fasting and post–high-fat meal plasma glucose and lipids, and endothelial function responses, were measured at 3, 9, and 18 months.
RESULTS
Exenatide reduced hemoglobin A1c (HbA1c) (estimated difference vs. placebo 0.55%, P = 0.0007) and fasting and postmeal plasma glucose (19 mg/dL, P = 0.0002, and 25 mg/dL, P < 0.0001, respectively). Mean (SD) change in plaque volume in the exenatide group (0.3% [2%]) was not different from that in the placebo group (−2.2% [8%]) (P = 0.4). The change in plaque volume in the exenatide group was associated with changes in HbA1c (r = 0.38, P = 0.0004), body weight, and overall plasma glucose (r = 0.29, P = 0.007 both). There were no differences in changes in plaque composition, body weight, blood pressure, fasting and postmeal plasma triglycerides, and endothelial function between the groups.
CONCLUSIONS
Exenatide once weekly for up to 18 months improved fasting and postprandial glycemic control but did not modify change in carotid plaque volume or composition. This study raises the possibility that short-term antiatherosclerotic effects may not play a central role in the cardiovascular benefits of GLP-1RAs.
Introduction
Intensive glucose lowering, particularly in type 2 diabetes mellitus (T2DM) of longer duration, has only modestly reduced the incidence of cardiovascular disease (CVD) (1). In contrast, targeting multiple cardiovascular risk factors simultaneously has been more successful in preventing CVD (2). Glucagon-like peptide 1 receptor agonists (GLP-1RAs) often improve multiple nonglycemic CVD risk factors, including obesity, dyslipidemia, and hypertension (3–5). In our previous studies, several GLP-1RAs also improved endothelial function (6,7).
Consistent with these effects, several agents from the GLP-1RA class reduced CVD events in recent cardiovascular outcome trials (CVOTs) (8–11). Although data from rodent models of atherosclerosis (12–15) and open-label studies in humans (16,17) suggest that the CVD benefit of GLP-1RAs is due, in whole or in part, to slowed progression of atherosclerosis, this has not been definitively demonstrated in carefully conducted randomized clinical trials.
MRI provides an accurate and highly reproducible measurement of the entire carotid arterial wall and reliably identifies atherosclerotic plaque components such as the lipid-rich necrotic core (LRNC) or plaque calcification that are believed to be related to plaque vulnerability and CVD risk (18–20). When directly compared with ultrasound carotid intima-media thickness (CIMT), MRI carotid plaque volume is more consistently associated with incident CVD, especially strokes (21).
In the current study we tested the hypothesis that exenatide once weekly will retard carotid plaque progression in T2DM using 3 Tesla (3T) carotid MRI. We also explored potential mechanisms by which exenatide could affect changes in plaque volume and composition, including assessment of fasting and postprandial endothelial function. The pragmatic study design was similar to that of the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), which also tested exenatide once weekly (22).
Research Design and Methods
Participants
We conducted an 18-month randomized, double-blind, placebo-controlled, parallel-group, pragmatic study with T2DM patients recruited from the Phoenix Veterans Affairs Health Care System (PVAHCS). The protocol was approved by the PVAHCS Institutional Review Board, and all participants provided informed written consent. The trial was registered at ClinicalTrials.gov (NCT02162550).
The study enrolled individuals with T2DM aged 21–75 years with hemoglobin A1c (HbA1c) 6.5–10.0% [48–96 mmol/mol], on diet management only or stable doses of oral antihyperglycemic agents with or without long-acting insulin, with a primary care provider amenable to their participation. Exclusion criteria included type 1 diabetes, current or recent GLP-1RA use, current use of short-acting insulin, contraindications to MRI, prior or anticipated carotid stenting or endarterectomy, a CVD event within the past 6 months, and other major illness or conditions affecting participation risk (e.g., severe renal disease, multiple endocrine neoplasia syndrome type 2, hypersensitivity to exenatide, severe gastrointestinal disease, or pregnancy). CIMT <0.75 mm in the target vessel, consistent with minimal-to-modest atherosclerosis, was initially among exclusion criteria; however, it was abandoned after this value was exceeded in the first 30 consecutive enrollees. Within ∼4 weeks of the screening exam, qualifying participants underwent a carotid MRI and were invited to a baseline visit.
Outcomes
The primary outcome was change in carotid plaque volume. Secondary outcomes included changes in individual carotid plaque components and changes in fasting and postprandial cardiometabolic measures and endothelial function.
Carotid MRI
Carotid MRI was performed at baseline and 9 and 18 months with a 3T MAGNETOM Skyra scanner (Siemens Healthineers). A custom-built foam head/neck holder limited head mobility and together with a built-in midline laser localizer was used to ensure accurate positioning on repeat scans. An oblique sagittal spin echo image was used to determine flow-divider position of the artery with the greatest plaque thickness (target vessel) on initial scout views (index artery). T1, T2, proton-density (PD) spin echo, and time-of-flight (TOF) gradient echo imaging sequences were performed 12 mm above and below the carotid flow divider at 2-mm slice thickness with a dedicated six-channel carotid coil.
Plaque volume and characteristics were measured by a single trained reader (cardiologist) blind to subject identity, treatment allocation, and image order using standard validated software (MRI-PlaqueView; VP Diagnostics, Seattle, WA). MRI images were analyzed only in participants completing at least 9 months of the study: 126 tests at baseline, 123 tests at 9 months, and 121 tests at 18 months. As described previously (23), during analysis, T1/T2/PD/TOF images of the index artery were simultaneously displayed on the monitor; luminal and adventitial borders were traced, and the volume of plaque was calculated as the area between these borders multiplied by slice thickness (2 mm). Plaque components, including calcified plaque and LRNC, were automatically delineated with validated morphology-enhanced probability mapping (24). The software also delineated fibrous cap area, the region between the LRNC and the vascular lumen, that previously showed high agreement with histologic findings (25). Representative images with plaque component delineation are shown in Supplementary Fig. 1.
Total plaque and plaque component volumes were calculated as the sum of all corresponding slice volumes. For each scan, mean volumes were calculated and used in the statistical analyses. Plaque volume was normalized to the total vessel volume (wall + lumen), while the plaque components volumes were normalized to the plaque volume. Images with poor quality were excluded from the analyses (two at baseline and 9 months and three at 18 months). The same carotid plaque imaging acquisition protocol, software, and measurement methodology have previously been shown to have good to excellent interscan reproducibility in multicenter settings with comparable measurement precision between MRI platforms (26), and with an intrareader coefficient of variability reported as 5.3% (23).
Study Visits
Participants were requested to maintain their normal diet and physical activity patterns and to avoid strenuous exercise for at least 48 h prior to each study visit. On the day of the visit, after fasting overnight (≥10 h) and refraining from morning smoking or alcohol use, participants were admitted to the PVAHCS Clinical Research Centre at ∼8:00 a.m. Upon arrival, they were placed in a hospital bed in a quiet and darkened room in a semirecumbent position.
An antecubital catheter (with blood drawing ports) was placed, and ∼30 min later endothelial function was assessed by peripheral arterial tonometry (EndoPAT 2000; Itamar Medical, Caesarea, Israel) and calculated as reactive hyperemia index (RHI), as previously described (6). A blood sample was then obtained for measurement of fasting plasma lipids, HbA1c, and glucose concentration, followed by ingestion of a standardized high-fat breakfast containing 400 kcal/m2 body surface area (45% fat, 40% carbohydrate, and 15% protein), consumed within 15 min. Blood draws were repeated 2 and 4 h after the meal, and RHI was measured 3 h after the meal.
Randomization
After completing the baseline examinations, participants were randomized in blocks in a 2:1 ratio to receive 2 mg exenatide or matching placebo subcutaneously, once weekly. Standardized meal tests and RHI assessment were repeated at 3, 9, and 18 months. Visit follow-up was augmented by interim phone calls to assess side effects, medication injection techniques and compliance, and current medical history. Diabetes care was maintained by the patient’s primary care team, although participants and providers were informed to avoid use of non–study related GLP-1RAs and limit DPP4 inhibitors, if possible. SGLT2 inhibitor use within the PVAHCS population was minimal during this time frame.
Biochemical Analyses
HbA1c levels were measured on visit days at the PVAHCS clinical laboratory using an automated analyzer (Architect 8000; Abbott, Lake Forest, IL). Plasma samples for lipid measurements were stored at −80°C until assay with an Abbott Architect 8000 automated analyzer. Plasma glucose concentrations during the meal test were measured bedside with an YSI2700-D glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma exenatide levels were evaluated by ELISA at Covance Laboratories Inc. (Chantilly, VA).
Statistical Analyses
Statistical analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, NC). The primary analysis included all participants who completed at least 9 months of the study (i.e., received at least one follow-up MRI). A secondary, per-protocol analysis included those who had at least 80% self-reported compliance with study medication use prior to each MRI test.
Baseline characteristics of the treatment groups were compared by the independent Student t test for continuous data and by χ2 test or Fisher exact test for categorical variables. The effect of treatment on study outcomes over the study was evaluated by mixed models for repeated-measures analysis with adjustment for participant-specific random effects and study visit. Interaction terms were included in the models to test for differential effects of treatment by study visits. High-fat meal–related outcomes, i.e., plasma glucose and triglycerides levels, and RHI, were also adjusted for the measurement time point. Spearman correlation analysis was used to test the correlation of changes in plaque volume with changes in metabolic and cardiovascular measurements. Two-tailed P values <0.05 were considered statistically significant.
Power Analysis
Our previous study showed a mean difference of 6.1% and SD of ∼3% for MRI-measured changes in plaque volume after intensified versus stable statin therapy (23). With use of this SD and two-sample t test sample size calculations, evaluation of 84 patients in the treatment group and 42 patients in the placebo group (total 126 subjects) allowed detection of a more conservative 1.6% mean difference in plaque volume change, i.e., ∼25% of the difference detected with statin intensification, at 80% power and 0.05 significance level.
Data and Resource Availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request as permitted by the Phoenix Veterans Affairs Institutional Review Board and Veterans Affairs research policy.
Results
Participant Study Flow and Characteristics
The study commenced in May 2014, and the last visit occurred in January 2019. Study participant flow is shown in Supplementary Fig. 2. Out of 303 individuals screened in the study, 163 were enrolled and randomized to exenatide (n = 109) or placebo (n = 54). Participants were predominantly white (87%), males (96%) with a high prevalence of obesity (68%), hypertension (91%), and use of lipid-lowering medications (predominantly statins [81%]). Baseline characteristics were not significantly different between the treatment groups, except for higher systolic and diastolic blood pressure in the placebo group (Table 1).
Table 1.
Clinical and demographic characteristics at baseline
| Exenatide (n = 109) | Placebo (n = 54) | P | |
|---|---|---|---|
| Sex (% male) | 95 | 98 | 0.40 |
| Race (% white) | 90 | 80 | 0.09 |
| Age (years) | 63 ± 8 | 64 ± 7 | 0.90 |
| BMI (kg/m2) | 33 ± 6 | 33 ± 6 | 0.50 |
| Obesity (%) | 71 | 63 | 0.40 |
| Diabetes duration (years) | 6 ± 4 | 6 ± 4 | 0.40 |
| Fasting glucose (mg/dL) | 155 ± 45 | 161 ± 53 | 0.30 |
| HbA1c (%) | 7.8 ± 1.2 | 8.1 ± 1.3 | 0.20 |
| HbA1c (mmol/L) | 62 ± 14 | 65 ± 14 | 0.20 |
| Systolic BP (mmHg) | 135 ± 17 | 141 ± 18 | 0.03 |
| Diastolic BP (mmHg) | 81 ± 11 | 85 ± 13 | 0.02 |
| History of hypertension (%) | 94 | 87 | 0.20 |
| History of CVD (%) | 21 | 22 | 0.80 |
| Smoking (%) | 15 | 22 | 0.30 |
| Statins (%) | 80 | 83 | 0.70 |
| Fasting triglycerides (mg/dL) | 170 ± 108 | 166 ± 74 | 0.80 |
| Fasting cholesterol (mg/dL) | 149 ± 32 | 160 ± 39 | 0.11 |
| HDL cholesterol (mg/dL) | 43 ± 10 | 43 ± 10 | 0.90 |
| LDL cholesterol (mg/dL) | 75 ± 24 | 84 ± 34 | 0.10 |
Data are means ± SD unless otherwise indicated. P values indicate between-group comparison by Student t test (for continuous variables) or by χ2 test (for categories). BP, blood pressure.
A total of 131 and 122 participants completed 9 and 18 months of therapy, respectively (Supplementary Fig. 2). Self-withdraw from the study was the most frequent reason for early termination in both groups. Withdrawal rates over the course of the study were slightly lower in those receiving exenatide, but the difference was not statistically significant from that of the placebo group (P = 0.14). Only one major CVD event (a CVD death in the placebo group) occurred during the study. Baseline characteristics and 3-month change in cardiometabolic measures were not significantly different between those who did and who did not complete the 9-month visit, except for lower baseline BMI and fasting triglycerides and less body weight reduction in those who withdrew prior to 9 months (Supplementary Tables 1 and 2).The most common side effect in the exenatide group was nausea (9%), followed by diarrhea, constipation, and minor allergic reaction (all 6%) (Supplementary Table 3).
Treatment Effects on CVD Risk Factors
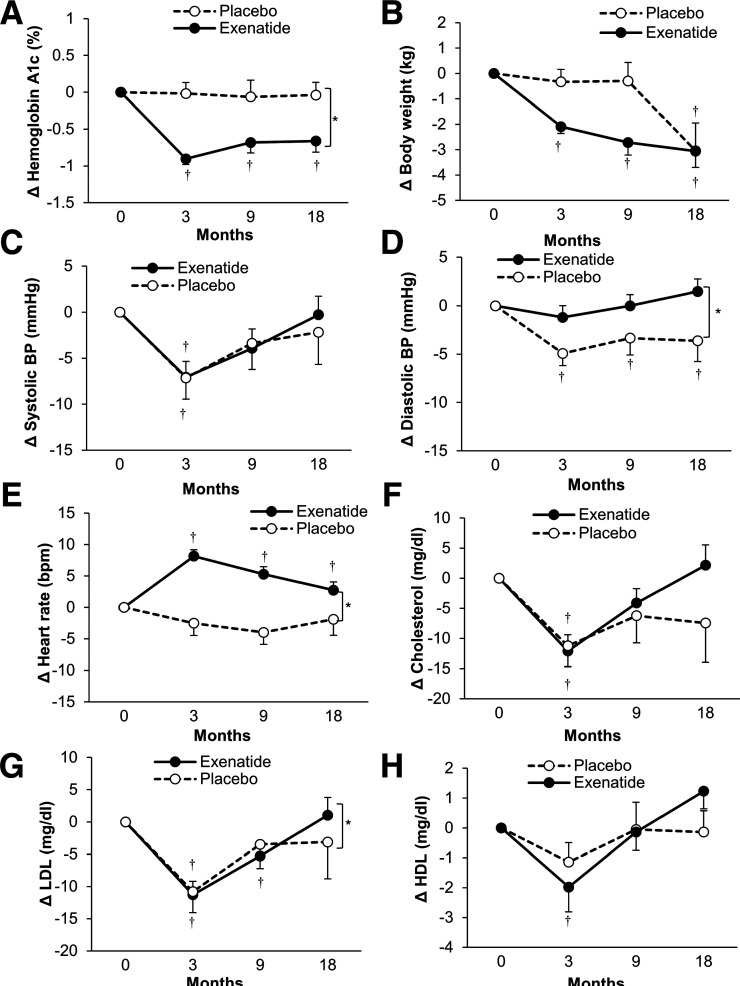
Baseline and on-study use of diabetes, antihypertensive, and statin medications was not significantly different between the treatment groups, except for higher insulin usage in the placebo group at 3 months (Supplementary Table 4). As expected, treatment with exenatide significantly reduced HbA1c (by 0.55% overall vs. placebo, P = 0.0007) (Fig. 1A). Reduction in body weight was significantly greater with exenatide early in the study (3 and 9 months, P = 0.008 at 9 months); however, at 18 months, the reduction in body weight with a placebo group was similar to exenatide (P = 0.9) (Fig. 1B). There was no significant difference between the groups in systolic blood pressure (Fig. 1C), but diastolic blood pressure declined in the placebo group (P = 0.01, baseline vs. month 18), while it remained unchanged with exenatide (Fig. 1D). Heart rate was higher after exenatide (P = 0.01 overall vs. placebo) (Fig. 1E). Plasma LDL decreased more in the placebo group (P = 0.01 overall vs. exenatide), while there were no differences between the groups in plasma total or HDL cholesterol concentrations (Fig. 1F–H).
Figure 1.
The effect of exenatide once weekly on change in cardiometabolic characteristics. HbA1c (mmol/mol) = 10.929 * [HbA1c (%) − 2.15]. Data are means ± SE. *P < 0.05 exenatide vs. placebo over the course of the study, †P < 0.05 vs. baseline (0 months) for each visit. BP, blood pressure.
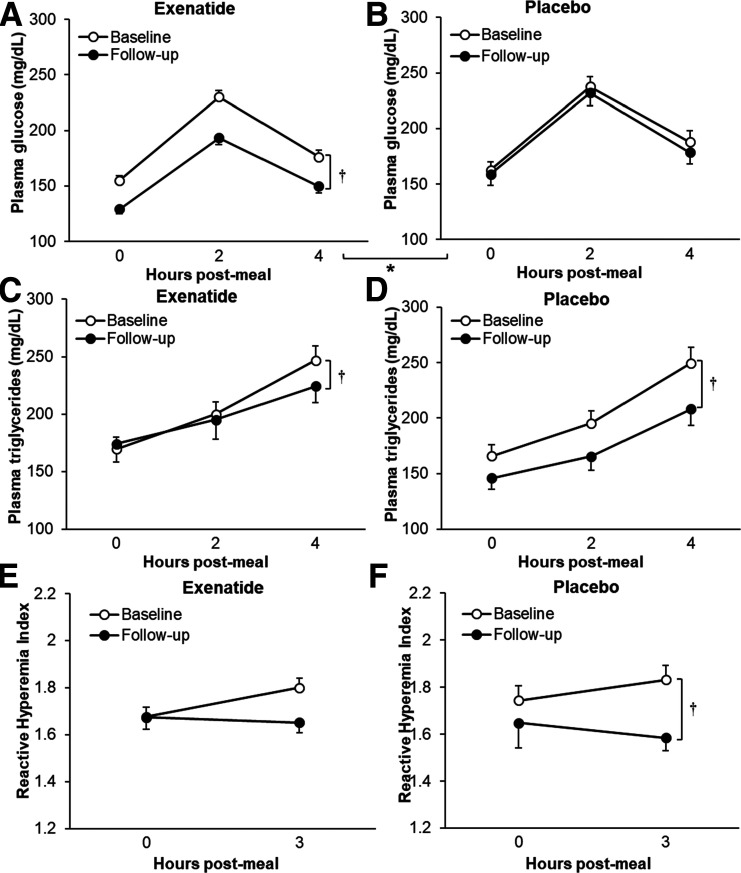
Both fasting and postmeal plasma glucose were reduced in the exenatide group (P = 0.0002 and P < 0.0001 vs. placebo, respectively) (Fig. 2A and B). Plasma triglycerides were reduced with both exenatide and placebo (P < 0.002 and P < 0.0001 vs. baseline, respectively); however, the response was not different between the groups (P = 0.5) (Fig. 2C and D). RHI worsened in the placebo group (P = 0.02 vs. baseline); however, the difference was not significant in comparison with exenatide (P = 0.4) (Fig. 2E and F).
Figure 2.
The effect of exenatide once weekly on metabolic (panels A–D) and vascular (panels E and F) responses to a high-fat meal. Data are means ± SE. *P < 0.05 exenatide vs. placebo, †P < 0.05 follow-up vs. baseline (0 months) overall. The follow-up plot reflects data from the last available visit for each person (9 months, n = 9, or 18 months, n = 122).
Extent and Determinants of Carotid Plaque
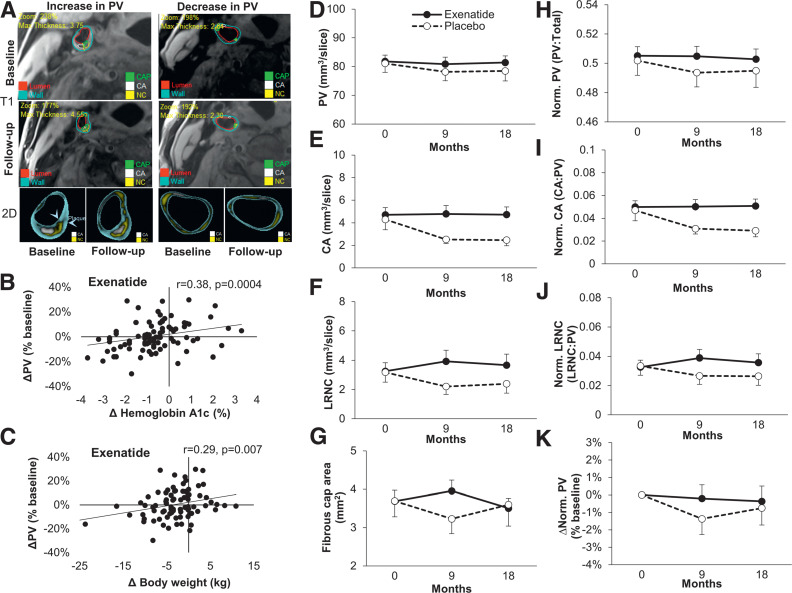
At baseline, mean (SD) total plaque volume was 942 (232) mm3, reflecting 80 (19) mm3 per slice. Among all participants participants, 79% had LRNC plaque and 95% had calcified plaque. In the whole cohort, plaque volume at follow-up (last available reading) was not different from baseline (mean [SD] change 0% [11%]). Figure 3A shows representative images of baseline and follow-up scans of those with an increase versus a decrease in plaque volume. Changes in plaque volume were positively associated with changes in HbA1c, body weight, and overall meal plasma glucose (Supplementary Table 5). After stratification by treatment group, these significant associations of changes in plaque volume and changes in HbA1c, body weight, or glucose levels were evident only in the exenatide group (Fig. 3B and C and Supplementary Table 5).
Figure 3.
Representative images (T1 and two-dimensional [2D]) of carotid wall from individuals with increased and decreased plaque volume (PV) (panel A); the association of changes in HbA1c and body weight with PV in the exenatide group (panels B and C); the effects of exenatide once weekly and placebo on plaque volume (panel D [primary outcome]), plaque components (calcified plaque [panel E] and LRNC [panel F]), fibrous cap overlying LRNC in those with LRNC >0 (panel G), and normalized plaque volume and plaque components (panels H–J); and percent change in normalized plaque volume in those with ≥80% compliance with study medication (per-protocol analysis [panel K, % change from baseline values]). Data are means ± SE. R values are Spearman correlation coefficients. CA, calcified plaque; LRNC, lipid-rich necrotic core; Norm., normalized.
Effects of Treatment on Plaque Volume and Characteristics
Plaque volume and characteristics at baseline were similar between the groups. There were no effects of exenatide on extent or change in plaque volume (P = 0.8 overall effect on plaque volume during the study) (Fig. 3D). The mean (SD) change in plaque volume at the last available MRI follow-up reading was not different between the two groups (−0.4% [12%], exenatide, vs. −2.2% [8%], placebo; P = 0.4 adjusted for the follow-up time). There were also no significant treatment differences in calcified plaque (P = 0.4), LRNC (P = 0.11), or fibrous cap overlying LRNC (P = 0.5) (Fig. 3E–G). Similar results were obtained after normalization of plaque volume to total vessel volume or after normalization of plaque components volumes to the plaque volume (Fig. 3H–J). The change in plaque volume in the exenatide group was also not different in subgroups defined according to baseline characteristics (Supplementary Table 6).
Compliance with study medication was similar in the exenatide and placebo group (Supplementary Fig. 3A). A per-protocol analysis in those who reported injecting ≥80% of study medication showed no differences between the effects of exenatide and placebo on changes in plaque volume (Fig. 3K) and plaque components (Supplementary Fig. 3B–D). Therapeutic levels of exenatide (50 pg/mL) were observed in the last-visit plasma sample from 65 of 84 participants on exenatide. There was no correlation between plasma exenatide levels and changes in metabolic or vascular responses (Supplementary Fig. 4). The mean (SD) change in plaque volume in those with therapeutic exenatide levels (0% [12%]) was similar to that in the whole exenatide group and not different from that in the placebo group (P = 0.4).
Conclusions
To test whether GLP-1RAs slow atherosclerosis progression in T2DM, we conducted an 18-month randomized, double-blind, placebo-controlled study to examine the effect of exenatide once weekly on carotid plaque volume using a highly accurate three-dimensional vascular imaging technique. Although there was a sustained improvement in fasting, postmeal, and overall glycemic control, there was no evidence that exenatide once weekly slowed progression of atherosclerosis or altered plaque composition in comparison with placebo. The negative results of our study are in agreement with the modest and nonsignificant reduction of CVD outcomes in EXCSEL (22).
Previous studies suggested that GLP-1RAs may protect against atherosclerosis and CVD through effects on the risk factors such as hyperglycemia, obesity, hypertension, and dyslipidemia (3–5). In the current study, exenatide once weekly improved fasting and postprandial glycemic control and modestly reduced body weight (early in the study) but had neutral effects on blood pressure and fasting and postprandial plasma lipids. The extent of body weight and HbA1c reduction was in line with that of EXSCEL (22). Although EXSCEL showed a statistically significant reduction in systolic blood pressure and plasma lipids, these effects were rather modest (22). However, CVOTs with GLP-1RAs that showed significant CVD benefits also reported very small or no changes in blood pressure and plasma lipids (8,11). Thus, it is unlikely that improvements in these risk factors were responsible for the observed differences in the CVD benefits between GLP-1RAs. Preclinical and early clinical evidence has also suggested multiple direct protective effects of GLP-1RAs in the vasculature and in the heart, including anti-inflammatory effects and improvements in cardiac and endothelial function (27). Although we did not measure changes in inflammation, we would anticipate these effects to be modest without substantial improvements in body weight or lipids. We also did not see evidence for improvements in endothelial function in the current study.
Whether related to the pragmatic study design or not, there was excellent control of multiple CVD risk factors in both groups in line with current diabetes guidelines. Consistent with this, only one major CVD event occurred during the study. This excellent management of risk factors could have blunted the ability to detect a treatment effect. A high percentage of participants in the current study, especially in the exenatide group, were receiving statins at baseline (90% vs. 74%). As significant reduction of plaque volume and improvement of plaque properties have been previously observed with statin therapy (23,28,29), it is possible that concurrent extensive statin treatment was sufficient to prevent atherosclerosis progression, limiting any effect of exenatide. However, frequent statin usage and very good plasma lipid management were present in CVOTs with GLP-1RAs that reported reduced CVD outcomes.
There was also relatively good reported compliance with study medication, and importantly, similar changes in plaque volume were seen in the secondary per-protocol (compliant participant) analyses. Thus, the lack of significant effect on atherosclerosis progression was unlikely due to noncompliance with exenatide therapy. This is supported by the finding that >75% of the participants randomized to exenatide had plasma exenatide levels over the therapeutic threshold at the end of the study. Although the number of days between the last injection and the final study visit may have varied, it is possible that not all participants received the full benefit of exenatide therapy because of suboptimal drug levels. However, low exenatide levels are unlikely to explain the lack of vascular or metabolic benefits, as no changes in the major study outcomes were related to plasma exenatide levels.
Consistent with previous studies with various GLP-1RAs (8,9,22,26), there was a significant increase in heart rate with exenatide. As increased heart rate is also a risk factor of CVD and carotid artery stiffness (30,31), it is possible that the effects on diastolic blood pressure and heart rate counterbalance benefits of exenatide on other proatherogenic risk factors. However, we found only a weak nonsignificant relationship between changes in heart rate and plaque volume.
We also believe that 9–18 months was long enough to detect intervention-induced change in carotid plaque. Previous open-label studies with liraglutide or exenatide once weekly reported reduction of CIMT after only 8 months of therapy (16,17). Importantly, in a previous study, using 3T MRI, we found significant changes in carotid plaque volume after only 6 months of statin therapy (23).
Despite sustained improvement of glycemic control on average, substantial heterogeneity in glycemic response to therapy was present within the exenatide group, with several participants showing no improvement or rises in HbA1c during the study (Fig. 3B). Interestingly, there was a significant correlation between changes in plaque volume and changes in glycemic response and body weight in the exenatide group. Although the absence of a correlation between changes in plaque volume and HbA1c in the placebo group could simply reflect more modest HbA1c changes, this is not likely an explanation for the correlation of plaque volume change with weight change, as the degree of weight loss was similar in both groups. One may speculate that in the exenatide group these associations reflect the presence of exenatide “responders” and “nonresponders” and that responders with greater reductions in glycemia and body weight with GLP-1RAs may also exhibit greater atherosclerosis inhibition. Polymorphisms in the GLP-1R have been identified and found to be linked to differential metabolic responses (32), but whether they may also account for differences in atherosclerosis has not yet been explored.
The lack of an antiatherosclerotic effect of exenatide does not appear to be explained by insufficient plaque burden, or its location, in the carotid artery in our cohort. The presence of ample atherosclerosis was detected by ultrasound in each of the first 30 patients enrolled in the study. Moreover, baseline MRI estimates of plaque volume were comparable with those reported in individuals at high CVD risk responding to statin therapy (23,29) and LRNC further confirmed the presence of atherosclerotic plaque at baseline in most of the study participants. Additionally, measurements of carotid atherosclerosis typically parallel measures of atherosclerosis in other vascular beds (33), and ultrasound and MRI measures of carotid atherosclerosis have both been shown to predict CVD, including stroke and myocardial infarction (18,21,34).
Recent meta-analysis of four GLP-1RA CVOTs showed significant reduction in overall and cardiovascular mortality but not in stroke or myocardial infarction (35). The lack of significant reduction in these latter two cardiovascular events (which are largely driven by atherosclerosis) suggests that other mechanisms besides slowing of atherosclerosis progression may contribute to the CVD benefit of the GLP-1RAs. Some of these pathways may involve improvement of myocardial energy metabolism and protection against ischemic damage, improved microvascular function, and antithrombotic effects (27,36–39). However, as these putative mechanisms were demonstrated in laboratory models or small clinical studies, their role in cardioprotective action of GLP-1RAs remains speculative.
It must also be noted that CVOTs have demonstrated substantial heterogeneity between the different GLP-1RAs in their effects on individual components of CVD composite outcomes (35), indicating within-class differences in the mechanisms of cardioprotection. Furthermore, it has been hypothesized that exenatide-based compounds may be less protective compared with those derived from human GLP-1 because of less generation of GLP-1 metabolites with alleged cardioprotective actions (27).
A major strength of this study was that changes in carotid plaque volume were evaluated by a highly accurate and reproducible MRI methodology. Unlike ultrasound, carotid MRI captures the entire circumference of the arterial wall and allows detection of plaques that might have remained undetected by ultrasound (18). It is significantly less reader dependent than ultrasound and may allow measurement of additional plaque components that are associated with greater or lower risk of CVD events (20). It has been shown that even simple two-dimensional carotid wall measurement by MRI was more consistently associated with incident CVD, particularly stroke, than were intima-medial thickness measures by ultrasound (21).
The major limitation in this study was a relatively high discontinuation rate, with 80% and 75% of study participants completing 9 and 18 months of therapy, respectively. Of note, the rate of premature discontinuation was driven primarily by patient decision, as it was in EXSCEL (22). However, the withdrawal pattern appeared random, as the numbers and rationale were proportionate between the treatment arms and there were no major differences in baseline characteristics or early treatment responses between those who did or did not withdraw. Although it was surprising to see trends for slightly higher plaque volume, calcified plaque, and LRNC in the exenatide group, these were not statistically significant. The fibrous cap area overlying LRNC, considered a structural morphologic marker of plaque vulnerability (25), was not significantly different, so the above trends in plaque volume and composition differences do not support increased plaque vulnerability in either group. As the current study enrolled typical T2DM patients who would be eligible for therapy with GLP-1RAs, the number of those with known CVD was lower than for those in most CVOTs, including EXCSEL (22).
In conclusion, in this first double-blind placebo-controlled study of precise and comprehensively measured changes in carotid atherosclerosis, we were not able to detect any benefit of 18 months of exenatide once-weekly treatment compared with placebo. It is possible that this may be a medication-specific finding, as the results of recent CVOTs provide evidence for a more favorable effect of several other GLP-1RA compounds on CVD events typically related to atherosclerosis. Our results also raise the possibility that nonatherosclerotic pathways of CVD protection may be more important than previously recognized.
Article Information
Acknowledgments. The authors acknowledge the excellent project assistance provided by Michell Sorley, Linda McDonald, Seth Truran, Douglas Boyd, Frances Keltner, Florentina Lipan, James Carlyle, and staff of the Phoenix Veterans Affairs Radiology and Nutrition Departments.
Duality of Interest. This study was supported by an investigator-initiated grant from AstraZeneca (to P.D.R.). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors reviewed and provided edits and comments on manuscript drafts. P.D.R. was the principal investigator. J.K., R.Q.M., and P.D.R. contributed to the conception and design of the work, analyzed the data and contributed to the interpretation of the data, and wrote the manuscript. K.C.C. and K.C.-C. acquired and developed the radiology data. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. J.K. and R.Q.M. take responsibility for the integrity of the data and the accuracy of the data analysis. P.D.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study was presented in abstract form at the American Heart Association Scientific Sessions, Philadelphia, PA, 16–18 November 2019.
Footnotes
Clinical trial reg. no. NCT02162550, clinicaltrials.gov
See accompanying article, p. 1252.
J.K. and R.Q.M. share equal authorship.
This article contains supplementary material online at https://doi.org/10.2337/figshare.13281389.
References
- 1. Control Group; Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes [published correction appears in Diabetologia 2009;52:2470]. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 2. Dormandy JA, Charbonnel B, Eckland DJ, et al.; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 3. Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013;3:e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bunck MC, Diamant M, Eliasson B, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010;33:1734–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang B, Zhong J, Lin H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab 2013;15:737–749 [DOI] [PubMed] [Google Scholar]
- 6. Koska J, Sands M, Burciu C, et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes 2015;64:2624–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koska J, Lopez L, D’Souza K, et al. Effect of liraglutide on dietary lipid-induced insulin resistance in humans. Diabetes Obes Metab 2018;20:69–76 [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 9. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 11. Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 12. Nagashima M, Watanabe T, Terasaki M, et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011;54:2649–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arakawa M, Mita T, Azuma K, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010;59:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rakipovski G, Rolin B, Nøhr J, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci 2018;3:844–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(-/-) mouse model. Diab Vasc Dis Res 2013;10:353–360 [DOI] [PubMed] [Google Scholar]
- 16. Rizzo M, Rizvi AA, Patti AM, et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovasc Diabetol 2016;15:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patti AM, Nikolic D, Magan-Fernandez A, et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: an 8-month prospective study. Diabetes Res Clin Pract 2019;149:163–169 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler Thromb Vasc Biol 2014;34:1341–1345 [DOI] [PubMed] [Google Scholar]
- 19. Dong L, Wang J, Yarnykh VL, et al. Efficient flow suppressed MRI improves interscan reproducibility of carotid atherosclerosis plaque burden measurements. J Magn Reson Imaging 2010;32:452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368–1373 [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Guallar E, Malhotra S, et al. Carotid artery wall thickness and incident cardiovascular events: a comparison between US and MRI in the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2018;289:649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Migrino RQ, Bowers M, Harmann L, Prost R, LaDisa JF, Jr. Carotid plaque regression following 6-month statin therapy assessed by 3T cardiovascular magnetic resonance: comparison with ultrasound intima media thickness. J Cardiovasc Magn Reson 2011;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerwin W, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging 2007;18:371–378 [DOI] [PubMed] [Google Scholar]
- 25. Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959–964 [DOI] [PubMed] [Google Scholar]
- 26. Boussel L, Arora S, Rapp J, et al.; MAPP Investigators . Atherosclerotic plaque progression in carotid arteries: monitoring with high-spatial-resolution MR imaging--multicenter trial. Radiology 2009;252:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drucker DJ. The ascending GLP-1 road from clinical safety to reduction of cardiovascular complications. Diabetes 2018;67:1710–1719 [DOI] [PubMed] [Google Scholar]
- 28. Corti R, Fayad ZA, Fuster V, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation 2001;104:249–252 [DOI] [PubMed] [Google Scholar]
- 29. Pfeffer MA, Claggett B, Diaz R, et al.; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 30. Fox K, Borer JS, Camm AJ, et al.; Heart Rate Working Group . Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007;50:823–830 [DOI] [PubMed] [Google Scholar]
- 31. Whelton SP, Blankstein R, Al-Mallah MH, et al. Association of resting heart rate with carotid and aortic arterial stiffness: multi-ethnic study of atherosclerosis. Hypertension 2013;62:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Luis DA, Diaz Soto G, Izaola O, Romero E. Evaluation of weight loss and metabolic changes in diabetic patients treated with liraglutide, effect of RS 6923761 gene variant of glucagon-like peptide 1 receptor. J Diabetes Complications 2015;29:595–598 [DOI] [PubMed] [Google Scholar]
- 33. Sillesen H, Muntendam P, Adourian A, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging 2012;5:681–689 [DOI] [PubMed] [Google Scholar]
- 34. Polak JF, Pencina MJ, O’Leary DH, D’Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke 2011;42:3017–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bethel MA, Patel RA, Merrill P, et al.; EXSCEL Study Group . Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–113 [DOI] [PubMed] [Google Scholar]
- 36. Liu H, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol 2009;201:59–66 [DOI] [PubMed] [Google Scholar]
- 37. Lønborg J, Vejlstrup N, Kelbæk H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012;33:1491–1499 [DOI] [PubMed] [Google Scholar]
- 38. Cameron-Vendrig A, Reheman A, Siraj MA, et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 2016;65:1714–1723 [DOI] [PubMed] [Google Scholar]
- 39. Lepore JJ, Olson E, Demopoulos L, et al. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail 2016;4:559–566 [DOI] [PubMed] [Google Scholar]