Abstract
OBJECTIVE
The RELIEF study assessed rates of hospitalization for acute diabetes complications in France before and after initiation of the FreeStyle Libre system.
RESEARCH DESIGN AND METHODS
A total of 74,011 patients with type 1 diabetes or type 2 diabetes who initiated the FreeStyle Libre system were identified from the French national claims database with use of ICD-10 codes, from hospitalizations with diabetes as a contributing diagnosis, or the prescription of insulin. Patients were subclassified based on self-monitoring of blood glucose (SMBG) strip acquisition prior to starting FreeStyle Libre. Hospitalizations for diabetic ketoacidosis (DKA), severe hypoglycemia, diabetes-related coma, and hyperglycemia were recorded for the 12 months before and after initiation.
RESULTS
Hospitalizations for acute diabetes complications fell in type 1 diabetes (−49.0%) and in type 2 diabetes (−39.4%) following FreeStyle Libre initiation. DKA fell in type 1 diabetes (−56.2%) and in type 2 diabetes (−52.1%), as did diabetes-related comas in type 1 diabetes (−39.6%) and in type 2 diabetes (−31.9%). Hospitalizations for hypoglycemia and hyperglycemia decreased in type 2 diabetes (−10.8% and −26.5%, respectively). Before initiation, hospitalizations were most marked for people noncompliant with SMBG and for those with highest acquisition of SMBG, which fell by 54.0% and 51.2%, respectively, following FreeStyle Libre initiation. Persistence with FreeStyle Libre at 12 months was at 98.1%.
CONCLUSIONS
This large retrospective study on hospitalizations for acute diabetes complications shows that a significantly lower incidence of admissions for DKA and for diabetes-related coma is associated with use of flash glucose monitoring. This study has significant implications for patient-centered diabetes care and potentially for long-term health economic outcomes.
Introduction
For people with type 1 diabetes or type 2 diabetes on insulin therapy, regular monitoring of blood glucose levels is an essential part of diabetes care (1). This can be done by periodic self-monitoring of blood glucose (SMBG) using finger prick testing, or by the use of continuous glucose monitoring (CGM), which involves a sensor that measures glucose in the interstitial fluid and provides a wealth of information on daily glucose levels that the user can access using a reader or a smartphone app (2). In so-called intermittently scanned CGM, also called flash glucose monitoring, glucose levels are measured continuously but are available to the user only when the sensor is scanned with the reading device or smartphone app (2). Flash glucose monitoring is currently only supported by the FreeStyle Libre system (Abbott Diabetes Care, Witney, Oxon, U.K.).
Poor glucose control in either type 1 diabetes or type 2 diabetes can lead to episodes of uncontrolled hyperglycemia or hypoglycemia requiring hospitalization. Such acute events include severe hypoglycemia, severe hyperglycemia, and diabetic ketoacidosis (DKA), each of which may also result in coma (3–9). Although these health care emergencies can be prevented by frequent glucose monitoring, the burden of emergency room attendance and hospital admissions for these acute events is increasing and is associated with significant health care use and expenditure (3–9).
Data on the efficacy of the FreeStyle Libre system in acute diabetes complications are now starting to emerge. The Flash Glucose Monitoring Study for Diabetes (FUTURE) with 1,913 people with type 1 diabetes across three specialized centers in Belgium did find a significant fall in rates of hospitalization for acute diabetes complications following access to the FreeStyle Libre system (10). However, specific reductions in individual rates of hospitalization for DKA, severe hypoglycemia, or coma related to diabetes were not evident in this study. A study of 900 adults with type 1 diabetes indicated a reduction in hospital admissions for DKA at two centers in Edinburgh in the 6 months following national reimbursement for the FreeStyle Libre system in Scotland (11). The number of DKA cases reported was small, but the fall in admissions was significant (P = 0.043).
The FreeStyle Libre flash glucose monitoring system was approved for reimbursement by the National Sickness Fund in France on 1 June 2017 for people treated with insulin (at least three injections per day or continuous subcutaneous insulin infusion [CSII]) >4 years old with diabetes—not limited to type 1 diabetes. The FreeStyle Libre system efficacy had been demonstrated in the Randomised Controlled Study to Evaluate the Impact of Novel Glucose Sensing Technology on Hypoglycaemia in Type 1 Diabetes (IMPACT) and Randomised Controlled Study to Evaluate the Impact of Novel Glucose Sensing Technology on HbA1c in Type 2 Diabetes (REPLACE) (12,13) in adults with type 1 diabetes or type 2 diabetes on insulin to reduce time in hypoglycemia <70 mg/dL by 38% (IMPACT) and 43% (REPLACE) over 26 weeks in comparison with SMBG testing. However, the efficacy of FreeStyle Libre system in the case of acute diabetes complications remains to be demonstrated.
The overall objective of the Real-World Evidence of FreeStyle Libre Analysis of the SNDS Database in France (RELIEF) study was to use the French national health claims database, Système National des Données de Santé (SNDS), to define how prescription and use of the FreeStyle Libre device is being implemented for people with diabetes and to understand the impact on acute health care outcomes and associated costs in standard practice in France after 1 and 2 years of use. SNDS (14) covers the entire French population (∼66 million people) and includes extensive information on all health care resource use, including outpatient visits, dispensed medication, procedures, and chronic conditions, as well as hospital admission diagnoses and procedures, and date of death, on an individual level.
The aims of this current analysis were to assess the risk of hospitalization related to acute diabetes complications in the 12 months before and after access to the FreeStyle Libre system in France in type 1 diabetes or in type 2 diabetes. Acute events considered were hospitalizations for DKA, hypoglycemia, diabetes-related coma, and hyperglycemia.
Research Design and Methods
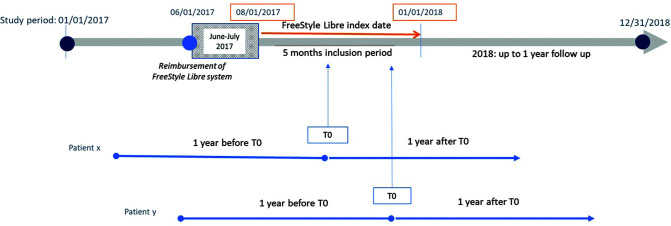
This longitudinal retrospective cohort study was carried out with use of data extracted from the national French database SNDS covering the period between 1 January 2015 and 31 December 2018. Costs for the FreeStyle Libre system have been reimbursed in France since 1 June 2017, but the FreeStyle Libre system was marketed before this date without reimbursement. In our study, initiation of the FreeStyle Libre system was defined as a first reimbursement of sensor or reader in the SNDS over the period after 1 August 2017 without any reimbursement of reader or sensors in the previous months of 2017. We chose to consider in our study only individuals with diabetes who initiated the FreeStyle Libre system during the period between 1 August 2017 and 31 December 2017. These individuals did not purchase FreeStyle Libre sensors or readers between 1 June 2017 and 1 August 2017 and so were considered new users of the FreeStyle Libre system. We then analyzed SNDS data from these patients over the 12 months prior to their initiation of the FreeStyle Libre system and in the 12 months following initiation (Fig. 1).
Figure 1.
Patient selection in the RELIEF study. Initiation of the FreeStyle Libre system was defined as a first reimbursement (T0) of sensor or reader in the SNDS during the period between 1 August 2017 and 31 December 2017. SNDS data on these patients were then analyzed for the 12 months before T0 and in the 12 months after T0.
Patient Selection
An algorithm was used to define and qualify any patient who used the FreeStyle Libre system as having diabetes only if they had received at least three reimbursements for antidiabetes drugs (oral or insulin) over 1 year or within the 2 years preceding or 1 year after FreeStyle Libre initiation (or at least two reimbursements if one large pack size was dispensed) or, alternatively, if the patient had been hospitalized at least once with a diagnostic ICD-10 code (15)—E10 (type 1 diabetes), E11 (type 2 diabetes), or E14 (unspecified diabetes)—or was identified in the French health care system as having a long-standing condition with the same ICD-10 codes during the same period. Exclusion criteria included the following: patients with only one FreeStyle Libre reader reimbursement without any further reimbursements for FreeStyle Libre sensors over the study period, patients deceased before the first sensor reimbursement, and patients <18 years old without insulin therapy 6 months before and 6 months after FreeStyle Libre initiation.
Eligible patients enrolled in the study were then classified according to their diabetes type and their use of SMBG test strips in the 12-month period before initiation of the FreeStyle Libre system. A previously published decision algorithm (Charbonnel et al. [16]) was used to distinguish patients with type 1 diabetes from those with type 2 diabetes, with the latter considered the diagnosis by default. This algorithm was based on the ICD-10 codes associated with Long Standing Condition (LSC) status or associated with hospitalizations, and on the prescription of insulin.
Quantities of strips supplied as documented by pharmacy claims were used for calculation of SMBG strip usage (e.g., 100 test strips dispensed = 100 tests assumed). SMBG buyers were distinguished according to the average daily number of blood glucose test strips (none, 1–3, 4–5, and >5) estimated over a 12-month period based on the reported number of strips delivered.
Hospital Episode Statistics for Acute Diabetes Complications
Hospitalizations for DKA were recorded in the 12 months prior to initiation of the FreeStyle Libre system and in the 12 months following initiation with use of ICD-10 code E10.1 (type 1 diabetes with ketoacidosis) and E11.1 (type 2 diabetes with ketoacidosis) as the main or related diagnosis. It was not possible to exclude hospitalizations for DKA at the point of diabetes diagnosis.
Hospitalizations for severe hypoglycemia were recorded in the 12 months prior to initiation of the FreeStyle Libre system and in the 12 months following initiation with ICD-10 codes E16.0 (drug-induced hypoglycemia without coma), E16.1 (other hypoglycemia), E16.2 (hypoglycemia, unspecified), and T38.3 (poisoning by adverse effects of insulin and oral hypoglycemic [antidiabetes] drugs).
Hospitalizations for diabetes-related comas were identified with ICD-10 codes E10.0 (type 1 diabetes with coma), E11.0 (type 2 diabetes with coma), and E14.0 (diabetes unspecified with coma) and hyperglycemia-related stays with ICD-10 code R739 (hyperglycemia, unspecified).
Persistence With Use of the FreeStyle Libre System
Persistence with the FreeStyle Libre system was defined between first delivery of a FreeStyle Libre sensor and discontinuation of the FreeStyle Libre system. Discontinuation was assumed when no sensors were delivered to the patient over a consecutive 6-month period. The date of discontinuation was calculated by noting of the last recorded date of sensor delivery and then addition of the associated 28-day sensor-wear period for two sensors (i.e., if two sensors were delivered in the last recorded claim, the date of discontinuation was the date of the last claim plus 1 month for taking into account that two sensors will last 28 days).
Statistical Analysis
As this study was conducted on a single nationwide database, no statistical probability tests were performed to compare the frequency of events before and after FreeStyle Libre system initiation. We first compared crude rates of patients with at least one diabetes-related acute event without any adjustment until the end of the study period. We did not recode missing values.
We also conducted a multivariable analysis using a linear model to examine the separate factors that may be associated with hospitalization for any acute diabetes-related events before and after initiation of the FreeStyle Libre system, considering age, sex, diabetes type, universal health coverage for people with low socioeconomic status, treatment, and SMBG acquisition level before the FreeStyle Libre system initiation.
Results
With application of the protocol we identified 74,011 individuals with diabetes with first reimbursement for FreeStyle Libre sensors during the period between 1 August 2017 and 31 December 2017. Among them, we identified 33,165 and 40,846 people with type 1 diabetes or type 2 diabetes, respectively (Supplementary Table 1), initiating the FreeStyle Libre system during the selection period. Of these patients, 88% were treated with multiple daily injections of insulin (MDI) (n = 46,828) or CSII (n = 18,593). The remaining 12% were mainly people with type 2 diabetes treated with a single basal insulin injection or with oral agents only.
Impact of Starting the FreeStyle Libre System on Rates of Hospitalization for Acute Diabetes Complications
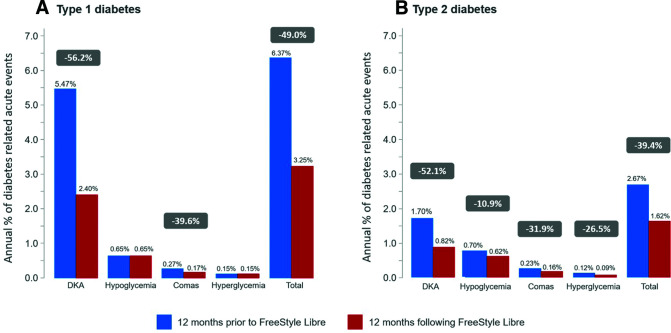
There was a highly significant reduction in the proportion of people with diabetes who were hospitalized for at least one acute diabetes complication in the 12 months following their start with the FreeStyle Libre system in comparison with the 12 months prior to starting (Fig. 2). Amongst the whole study population, hospitalization for acute diabetes complications fell by 45.7%, from 3,204 patients in the year prior to starting FreeStyle Libre to 1,740 in the year after starting. The fall was most marked in type 1 diabetes (Fig. 2A), with a reduction of 49.0%, from 2,114 patients to 1,079. The reduction in acute diabetes complications in type 2 diabetes (Fig. 2B) was also significant, from 1,090 to 661, in the year after starting FreeStyle Libre in comparison with the year before, a drop of 39.4%.
Figure 2.
Annual percentage of patients with type 1 diabetes–related (A) and type 2 diabetes–related (B) acute events before and after initiation of the FreeStyle Libre system. Data show percentage of patients with at least one acute diabetes complication in the 12 months before and after FreeStyle Libre initiation.
Hospitalization Classified According to the Type of Acute Diabetes Complications
The fall in DKA hospitalization rates was more marked than for other acute complications in both types of diabetes. Hospitalization rates for DKA fell by 55.0%, from 2,508 patients in the year prior to starting FreeStyle Libre to 1,128 in the year after starting (−56.2% [type 1 diabetes] and −52.1% [type 2 diabetes]) (Fig. 2). A slight but significant decrease was also observed for diabetes-related comas. Overall, the percentage of patients with at least one hospitalization for a diabetes-related coma fell by 35.7%. The fall was by 39.6% for type 1 diabetes and by 31.9% for type 2 diabetes (Fig. 2). Amongst the whole study population, hospitalizations rates for hypoglycemia and hyperglycemia fell by 6.4% and 13%, respectively, in each case, with a decrease mainly observed in type 2 diabetes (−10.8% and −26.5%) (Fig. 2B). The data broken down by age and sex of the study population are provided in Supplementary Table 3, and the frequency of events per 1,000 patient-years is presented in Supplementary Table 2.
Interestingly, we conducted separate analyses comparing the frequency of acute events per 1,000 patient-years over 3, 6, and 12 months after starting the FreeStyle Libre system (Supplementary Table 4). In comparison with the overall frequency of events prior to starting FreeStyle Libre in Supplementary Table 2, a marked decrease in the frequency of DKA was observed over the first months after starting FreeStyle Libre, whereas the effect on hypoglycemia is somewhat delayed in time.
The before and after results were similar in the subgroup of 65,183 patients (88%) treated with MDI (n = 43,041) or CSII (n = 18,282) therapy and therefore aligned with the conditions of reimbursement of the FreeStyle Libre system in France (see Supplementary Table 5).
Hospitalizations for Acute Diabetes Complications Classified by Use of SMBG Prior to Starting the FreeStyle Libre System
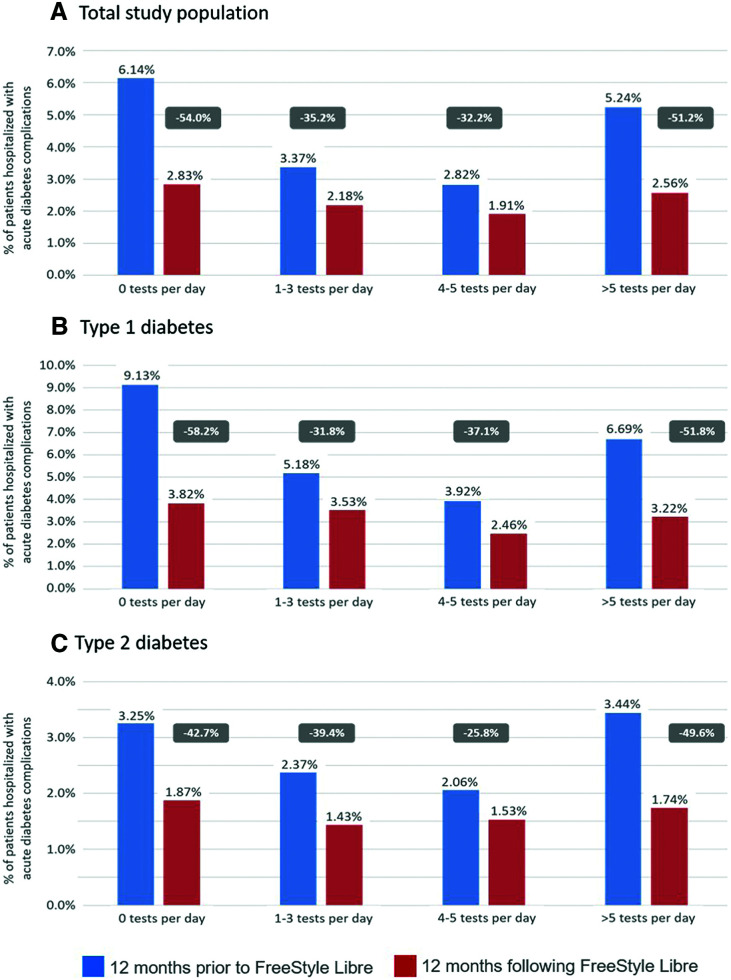
We analyzed hospitalization rates among study individuals who had been subcategorized based on their assumed average daily frequency of SMBG usage prior to their first FreeStyle Libre sensor use. The assumed average daily frequency of SMBG categories were no strips, 1–3 strips, 4–5 strips, >5 strips, as described in Table 1. Both the incidence and the fall in rates of hospitalizations for acute diabetes complications were most marked for the 19,396 people who were not compliant with SMBG testing, as indicated by no consumption of test strips, but also for people with the most regular use of SMBG testing (>5 strips) (Fig. 3A). A reduction in hospitalizations of −54.0% and −51.2%, respectively, was observed in these two groups. Patients with acute diabetes complications in the subgroups using one to three or four to five SMBG test strips per day showed a significant reduction of −35.2% and −32.2%, respectively, in each case in the year after starting the FreeStyle Libre system (Fig. 3A). These patterns of reductions were observed both in type 1 diabetes (Fig. 3B) and in type 2 diabetes (Fig. 3C).
Table 1.
Number of patients with categorization by diabetes type and SMBG acquisition prior to starting the FreeStyle Libre system
| 0 test strips per day | 1–3 test strips per day | 4–5 test strips per day | >5 test strips per day | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Type 1 diabetes | 9,531 | 28.7 | 6,311 | 19.0 | 8,731 | 26.3 | 8,592 | 25.9 |
| Type 2 diabetes | 9,865 | 24.2 | 11,371 | 27.8 | 12,643 | 31.0 | 6,967 | 17.1 |
| Totals | 19,396 | 26.2 | 17,682 | 23.9 | 21,374 | 28.9 | 15,559 | 21.0 |
Data show SMBG buyers categorized based on their predicted average daily number of blood glucose test strips estimated over a 12-month period based on the reported number of strips delivered.
Figure 3.
Hospitalizations for diabetes acute complications classified by use of SMBG tests in the 12 months prior to FreeStyle Libre initiation for the total study population (A) and by patients with type 1 (B) and type 2 (C) diabetes separately. Data show rates of hospitalizations with acute diabetes complications in the 12 months before and after FreeStyle Libre initiation. For numbers of patients in each SMBG test subgroup, see Table 1. The percentage reduction in rates of hospitalization in the 12 months following FreeStyle Libre initiation is indicated.
Multivariable Analysis of Acute Diabetes Complications in the Hospital Before and After FreeStyle Libre Initiation in the Population of Reimbursement
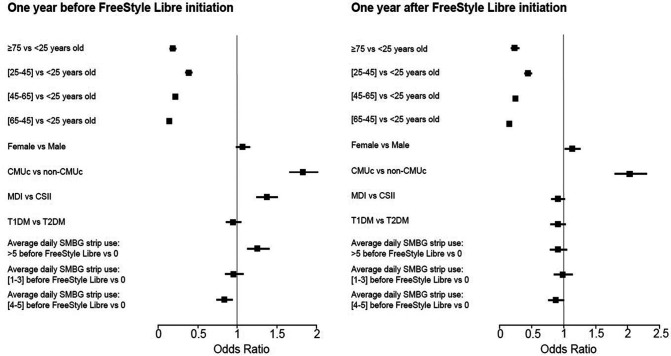
Before initiation, hospitalization rates were mainly related to age (<25 years old as compared to older age classes), universal health coverage for people with low socioeconomic status (odds ratio 1.83 [95% CI 1.65–2.02]), and use of MDI as compared with CSII therapy (odds ratio 1.37 [95% CI 1.24–1.51]) (Fig. 4). After initiation of the FreeStyle Libre system, if no changes were observed with regard to the impact of age and universal health coverage for people with low socioeconomic status, use of MDI as compared with CSII therapy was no more a risk factor for hospitalization related to acute diabetes complications.
Figure 4.
Multivariable analysis of hospitalizations for acute events related to diabetes before and after FreeStyle Libre initiation. T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; CMUc, couverture maladie universelle complémentaire (universal health coverage).
Persistence With the FreeStyle Libre System
Based on the definition outlined in the methods section, persistence with the FreeStyle Libre system at 12 months after initiation (i.e., proportion of people still purchasing the device) was 98.1% (95% CI 98.0–98.2) across all users (Supplementary Table 6). Importantly, persistence with FreeStyle Libre was consistently high across all categories of SMBG test buyers, with no difference between FreeStyle Libre users who were not SMBG compliant (0 strip use, 97.3%) and those at all levels of average daily SMBG test strip acquisition (Supplementary Table 6). These results were observed both in MDI and in CSII subgroups.
Conclusions
Results from our retrospective observational real-world nationwide cohort study show that access to the FreeStyle Libre system for people with intensively insulin-treated diabetes is associated with a subsequent drop in rates of hospitalizations for acute diabetes complications, mainly DKA and diabetes-related comas; these results were observed in both MDI and CSII subgroups (Supplementary Table 5). For 74,011 people in this group who had their first FreeStyle Libre sensor prescribed between 1 August and 31 December 2017, rates of hospitalization for acute complications of diabetes fell by 45.7% in the year after starting. The fall was most marked in type 1 diabetes, with a −49.0% reduction in hospitalizations. The reduction in hospitalizations for acute complications in type 2 diabetes was also significant, with a drop of −39.4%. Results were particularly notable for hospitalizations related to DKA and diabetes-related comas (overall drop of −52.1% and −31.9%, respectively), but falls were also observed for hypoglycemia and hyperglycemia (−10.9% and −26.5%). These data from a large nationwide sample size support and extend the outcomes from the smaller studies with inconclusive findings (10,11). The generalizability of our results is worthy of comment. Although our sample corresponds to the whole population of French patients starting the FreeStyle Libre system over a 5-month period, they represent 25% of the theoretical full target population estimated as benefiting every year from this device by the French Health Authorities (n = 300,000 patients) (17). Further studies based on new data extraction will complement this current work.
We hypothesized that a patient who was not reimbursed for any sensors/readers in the months before starting the FreeStyle Libre system (over a period of 2–7 months) did not use the system over the full year before the index date used as the device starting date. This could lead to an underestimation of the outcomes, as some patients may have experienced the system previously (with a rather long interruption). We also agree that individuals could have issues learning how to use the system or incorrectly use the system at initiation, and this could also lead to a decrease in the potential impact of FreeStyle Libre in comparison of the first year of use with the previous year.
When the study individuals were categorized by frequency of SMBG acquisition prior to their first sensor use, the fall in hospitalization for acute diabetes complications was most marked for people who were not compliant with SMBG testing, as evidenced by lack of test strip acquisition. This is understandable given that poor awareness of glucose control may induce the occurrence of acute diabetes complications. The problem of noncompliance in type 1 diabetes has previously been raised (18), with concern about outcomes for poorly compliant patients. In that small-scale study of 19 adults with type 1 diabetes, the use of CSII in conjunction with the FreeStyle Libre system was seen to significantly reduce HbA1c as well as the reported incidence of severe hypoglycemia and DKA. Our nationwide analysis on 74,011 people with type 1 diabetes or type 2 diabetes shows that important reductions in hospitalization for acute diabetes complications in this poorly compliant group are associated with application of flash glucose monitoring.
We hypothesized that the number of strips supplied was directly related to the number of strips used by the patients, although we accept that is a potential limitation. Interestingly, the data also show that the people with the highest SMBG test strip acquisition had the second-highest incidence of hospitalization for acute diabetes complications in the year prior to starting FreeStyle Libre and that this fell markedly in the 12 months after their first sensor prescription. The rate of hospitalization for acute diabetes complications in this high-testing group may be explained by the fact that they use SMBG more frequently as a consequence of the inherent instability of their blood glucose, so called brittle diabetes, which ultimately puts them at higher risk of acute diabetes complications, especially DKA (19). Presumably, access to the FreeStyle Libre system allows them to scan their sensors with an even higher daily frequency, which then provides them with awareness of trends in their glucose control that helps them to manage their risk of acute complications better. Another hypothesis may be that some patients had intensified their insulin therapy with FreeStyle Libre initiation, including a possible switch from MDI to CSII. This hypothesis was not supported by a subgroup analysis that considered only patients who maintained either MDI or CSII treatment before and after FreeStyle Libre system initiation (data not shown). Furthermore, the reduction in hospitalization rates seen following FreeStyle Libre initiation (Supplementary Table 5) and the multivariable analysis (Fig. 4) was also observed with independent consideration of whether the patients were treated with MDI or CSII.
Given the high numbers of patients in the study cohort who were noncompliant with SMBG testing or with low strip usage prior to starting the FreeStyle Libre system, persistence with the FreeStyle Libre system was high, with 98.1% of starters still dispensed FreeStyle Libre sensors at 12 months. Importantly, persistence with FreeStyle Libre was consistently high across all categories of SMBG test buyers, with no difference between FreeStyle Libre users who were noncompliant with SMBG testing (0 strip use, 97.3%) and those at all levels of average daily SMBG test strip acquisition (Supplementary Table 6). This suggests that noncompliant and low-frequency SMBG users are concerned about their glycemic control and risk of complications but the burden of finger pricking is particularly high for them. Persistence with FreeStyle Libre was also consistent across the MDI and CSII subgroups (Supplementary Table 6). This compares well with the limited data on discontinuation rates for the FreeStyle Libre system, which have been reported to be as high as 17% (20). However, this last observation was reported in a small-scale study on 130 patients with type 1 diabetes, all of whom were self-funding their use of the FreeStyle Libre system.
Another important observation from this analysis is that hospitalizations for severe hypoglycemia were only marginally reduced in the year following access of patients to the FreeStyle Libre system. At first glance, this contrasts with the data from clinical trials in patients with type 1 diabetes and type 2 diabetes on insulin that flash glucose monitoring significantly reduces the frequency and incidence of hypoglycemia <70 mg/dL and clinically relevant hypoglycemia <54 mg/dL (12,13). However, since most severe hypoglycemia does not result in hospitalization, direct comparison with the clinical trial data is not informative here. Also, it is worth pointing out that the pre–FreeStyle Libre rates of hospitalization for severe hypoglycemia in France revealed in this study are much lower than other reported frequencies (21). In the U.K., Zhong et al. (3) reported an annual frequency of hospitalizations of 14.1 events per 1,000 person-years in 2013 in patients with type 1 diabetes in comparison with the 7.1 events per 1,000 person-years observed here (Supplementary Table 2). Also, other studies have shown that rates of hypoglycemia do not necessarily change following introduction of the FreeStyle Libre system in children with type 1 diabetes (22) or adults with type 2 diabetes on insulin therapy (23), and at least one prospective observational study on 900 people with type 1 diabetes reported an increase in asymptomatic and symptomatic hypoglycemia following introduction of FreeStyle Libre (11). The data in our analysis are only focused on hospitalization events, which is an outcome different from the outcomes assessed in these cited reports, but the data are not inconsistent with their findings. Of note in this context, the FUTURE study in 1,913 people with type 1 diabetes also reported no change in hospitalizations for hypoglycemia in the 12 months following introduction of flash glucose monitoring (10).
In this nationwide survey, the observed rates of hospitalization for DKA are high compared with those reported in other published studies in type 1 diabetes (7,20) and especially in type 2 diabetes (7,24). A possible explanation for this is that the analysis covers people who have been provided with access to flash glucose monitoring soon after the date of approval of reimbursement. This may indicate a selection bias toward more motivated patients with acknowledged poor glycemic control and at higher risk of diabetes complications.
It is important to point out that our study has limitations. The lack of a control group is a limitation, since a control group would also have highlighted the impact of other factors on acute diabetes complications over the study period. Furthermore, it is possible that previous experience of DKA would be a factor in the decision to initiate the FreeStyle Libre system. This could contribute to the DKA frequency reduction after FreeStyle Libre system initiation. Because it is a longitudinal retrospective cohort study with a 24-month study window, it is possible that confounding factors, other than use of the FreeStyle Libre system, had an impact on the outcomes. For example, initiation of the FreeStyle Libre system could have been accompanied by device training or diabetes education, as well as more focused time with health care professionals during the initiation process. Patient empowerment may have ensued. Each of these can result in improved diabetes self-care behavior that may affect the frequency of acute complications. This possibility for enhanced self-care cannot be controlled for in this retrospective cohort analysis. Interestingly, changes to treatment with insulin that may have been implemented after starting FreeStyle Libre did not alter rates of DKA or hypoglycemia. As previously mentioned, our data cannot exclude DKA at the point of diagnosis, which may have occurred during the 12 months prior to initiation. A potential calendar effect could not be excluded without use of a control group. However, an ancillary analysis of the French National Drug-Related Group hospital payments database did not show any change over the period 2015–2018 in the proportion of hospital stays with DKA as a main reason for hospitalization among patients with type 1 diabetes and showed only a slight increase among patients with type 2 diabetes (data not shown). These results are not in favor of such a calendar effect.
A further limitation is the fact that other real-time CGM systems could not be included in the request for data submitted to SNDS. This has implications for the generalizability of our data as previously discussed. Another limitation is the lack of clinical or biological data, such as HbA1c measurements, due to the nature of this administrative database. Similarly, the data give no indication as to daily sensor-scan rates in the 12 months after starting FreeStyle Libre. We know these rates can have an impact on overall glycemic control (25), and their lack in our study limits detailed interpretation. As such, it is complementary to other analyses, such as the FUTURE study (10), which on the other hand was on a much smaller scale. The method of classification of types of diabetes may also be challenged. However, the algorithm used here has previously been used in several published studies (21,25,26). Lastly, the accuracy of the outcomes relies on the quality of coding, which was not assessable in the work. However, the consistency with other studies with a different design is reassuring, and it is not expected that outcomes like DKA would be miscoded frequently. Strengths of the study include its large size and a nationwide recruitment scope in the context of a very generous reimbursement scheme.
In conclusion, this is the largest study of its kind to date, using health episode statistics of people with diabetes from a nationwide database, that quantifies the association of intervention with flash glucose monitoring with the incidence of hospitalization for acute diabetes complications in France, both in type 1 diabetes and in type 2 diabetes. This analysis has significant implications for patient-centered clinical care in diabetes and supports the further investigation of the long-term health economic benefits at a national level.
Article Information
Duality of Interest. The authors acknowledge funding support from Abbott Diabetes Care. R.R. is an advisory panel member for AstraZeneca, Sanofi, Merck Sharp & Dohme (MSD), Eli Lilly, Boehringer Ingelheim, Janssen, Mundipharma, Novo Nordisk, and Physiogenex and has received research funding from and provided research support to Amgen, Diabnext, Sanofi, and Novo Nordisk. J.-P.R. is an advisory panel member for Sanofi, MSD, Eli Lilly, Novo Nordisk, Abbott, and Medtronic and has received research funding and provided research support to Abbott, Air Liquide, Sanofi, and Novo Nordisk. G.d.P. is a health economist and an advisory board member for the following companies: Abbott, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb (BMS), Boehringer Ingelheim, Janssen, MSD, Novartis, Novo Nordisk, Roche, and Sanofi. He has received research funding from Amgen, BMS, Boehringer Ingelheim, MSD, Novartis, Roche, and Sanofi. He has received honoraria for his participation on the RELIEF study advisory board. E.V. is a consultant specializing in the methodology and statistical analysis of clinical trials for Abbott, BMS, Celgene, Edwards, Novartis, Pfizer, and Sanofi. He has received honoraria for his participation. B.D. and C.E. are employees of CEMKA, a French heath care Clinical Research Organization. B.D. has also received advisory board and lecture fees from Sanofi, MSD, Eli Lilly, Novo Nordisk, Pfizer, and Janssen. F.L.-G. is employed by Abbott Diabetes Care. B.G. is an advisory board member for Sanofi, Eli Lilly, Novo Nordisk, Novartis, GSK, MSD, Boehringer Ingelheim, AstraZeneca, Abbott, Medtronic, and Roche Diagnostics. He is a clinical investigator for Sanofi, Eli Lilly, Novo Nordisk, GSK, BMS, AstraZeneca, Medtronic, Abbott, Roche Diagnostics, MSD, Novartis, Janssen, and Boehringer Ingelheim. He receives research support from Medtronic, Vitalaire, Sanofi, Eli Lilly, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
The funding support from Abbott Diabetes Care did not affect the collection, analysis, or presentation of the data.
Author Contributions. J.-P.R. contributed to the methodology, the analyses, and the manuscript writing. E.V. contributed to the methodology, the analyses, and the manuscript writing. G.d.P. contributed to the methodology, the analyses, and the manuscript writing. B.D. and C.E. contributed to the methodology, conducted the statistical analyses, and contributed to the manuscript writing. F.L.-G. contributed to the methodology, the analyses, and the manuscript writing. B.G. contributed to the methodology, the analyses, and the manuscript writing. R.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14143874.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S66–S76 [DOI] [PubMed] [Google Scholar]
- 2. Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetologia 2017;60:2319–2328 [DOI] [PubMed] [Google Scholar]
- 3. Zhong VW, Juhaeri J, Cole SR, et al. Incidence and trends in hypoglycemia hospitalization in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care 2017;40:1651–1660 [DOI] [PubMed] [Google Scholar]
- 4. Parekh WA, Ashley D, Chubb B, Gillies H, Evans M. Approach to assessing the economic impact of insulin-related hypoglycaemia using the novel Local Impact of Hypoglycaemia Tool. Diabet Med 2015;32:1156–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: a nationwide analysis. Diabetes Care 2018;41:1631–1638 [DOI] [PubMed] [Google Scholar]
- 6. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care 2018;41:1870–1877 [DOI] [PubMed] [Google Scholar]
- 8. Dhatariya KK, Skedgel C, Fordham R. The cost of treating diabetic ketoacidosis in the UK: a national survey of hospital resource use. Diabet Med 2017;34:1361–1366 [DOI] [PubMed] [Google Scholar]
- 9. Benoit SR, Hora I, Pasquel FJ, Gregg EW, Albright AL, Imperatore G. Trends in emergency department visits and inpatient admissions for hyperglycemic crises in adults with diabetes in the U.S., 2006–2015. Diabetes Care 2020;43:1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care 2020;43:389–397 [DOI] [PubMed] [Google Scholar]
- 11. Tyndall V, Stimson RH, Zammitt NN, et al. Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia 2019;62:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254–2263 [DOI] [PubMed] [Google Scholar]
- 13. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017;26:954–962 [DOI] [PubMed] [Google Scholar]
- 15. International Statistical Classification of Diseases and Related Health Problems: 10th Revision. Vol. 2. Geneva, World Health Org., 2010 [Google Scholar]
- 16. Charbonnel B, Simon D, Dallongeville J, et al. Direct medical costs of type 2 diabetes in France: an insurance claims database analysis. Pharmacoeconom Open 2018;2:209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haute Autorité de Santé (HAS) . FreeStyle Libre. Avis du 12 Juillet 2016. Commission Nationale d’Evaluation des Dispositifs Medicaux et des Technologies de Santé. Available from https://www.has-sante.fr/jcms/c_2657325/fr/freestyle-libre
- 18. Halbron M, Bourron O, Andreelli F, et al. Insulin pump combined with flash glucose monitoring: a therapeutic option to improve glycemic control in severely nonadherent patients with type 1 diabetes. Diabetes Technol Ther 2019;21:409–412 [DOI] [PubMed] [Google Scholar]
- 19. Vantyghem MC, Press M. Management strategies for brittle diabetes. Ann Endocrinol (Paris) 2006;67:287–296 [DOI] [PubMed] [Google Scholar]
- 20. Landau Z, Abiri S, Gruber N, et al. Use of flash glucose-sensing technology (FreeStyle Libre) in youth with type 1 diabetes: AWeSoMe study group real-life observational experience. Acta Diabetol 2018;55:1303–1310 [DOI] [PubMed] [Google Scholar]
- 21. Detournay B, Halimi S, Robert J, Deschaseaux C, Dejager S. Hypoglycemia hospitalization frequency in patients with type 2 diabetes mellitus: a comparison of dipeptidyl peptidase 4 inhibitors and insulin secretagogues using the French health insurance database. Vasc Health Risk Manag 2015;11:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campbell FM, Murphy NP, Stewart C, Biester T, Kordonouri O. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr Diabetes 2018;19:1294–1301 [DOI] [PubMed] [Google Scholar]
- 23. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 2019;42:1178–1184 [DOI] [PubMed] [Google Scholar]
- 24. Thomas M, Harjutsalo V, Feodoroff M, Forsblom C, Gordin D, Groop PH. The Long-Term Incidence of Hospitalization for Ketoacidosis in Adults with Established T1D-A Prospective Cohort Study. J Clin Endocrinol Metab 2020;105:dgz003. [DOI] [PubMed] [Google Scholar]
- 25. Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: A European analysis of over 60 million glucose tests. Diabetes Res Clin Pract 2018;137:37–46 [DOI] [PubMed] [Google Scholar]
- 26. Roussel R, Charbonnel B, Behar M, Gourmelen J, Emery C, Detournay B. Persistence with insulin therapy in patients with type 2 diabetes in France: an insurance claims study. Diabetes Ther 2016;7:537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]