Abstract
OBJECTIVE
Epidemiological studies have shown contradictory results regarding the time trend of end-stage renal disease (ESRD) in people with diabetes. This study aims to analyze the incidence of ESRD, defined as chronic renal replacement therapy (RRT), to investigate time trends among people with and without diabetes in Germany and to examine whether these patterns differ by age and sex.
RESEARCH DESIGN AND METHODS
The data were sourced from nationwide data pooled from two German branches of statutory health insurances covering ∼25 million inhabitants. We estimated age- and sex-standardized incidence rates (IRs) for chronic RRT among people with and without diabetes in 2010–2016 and the corresponding relative risks. Time trends were analyzed using Poisson regression.
RESULTS
We identified 73,638 people with a first chronic RRT (male 60.0%, diabetes 60.6%, mean age 71.3 years). The IR of chronic RRT among people with diabetes (114.1 per 100,000 person-years [95% CI 110.0–117.2]) was almost six times higher than among people without diabetes (19.6 [19.4–19.8]). A consistent decline in IR was observed among people with diabetes (3% annual reduction, P < 0.0001) for both sexes and all age classes. In contrast, no consistent change of IR was identified in people without diabetes. Only among women aged <40 years (P = 0.0003) and people aged ≥80 years (P < 0.0001) did this IR decrease significantly.
CONCLUSIONS
Incidence of chronic RRT remained significantly higher among people with diabetes. The IR decreased significantly in people with diabetes independent of age and sex. Time trends were inconsistent in people without diabetes.
Introduction
End-stage renal disease (ESRD) is a life-threatening complication in patients with diabetes, resulting in reduced quality of life, high mortality, and increased medical costs (1–5). A number of epidemiological studies estimated that one-half of patients with ESRD have diabetes when starting chronic renal replacement therapy (RRT) (6–8). Nevertheless, data comparing the RRT incidence in people with diabetes with those without diabetes are limited (9) and show wide variation, with incidence rates (IRs) among people with diabetes ranging from 59 per 100,000 person-years (PY) (10) to 678 per 100,000 PY (11). The relative risk (RR) comparing IRs in people with and without diabetes ranged from 4 (12) to 8 (11). However, different methodological approaches among the studies reduce the comparability of results. In particular, a high number of studies referred the RRT incidence in people with diabetes to the entire population (6,13–15). Other studies solely analyzed diabetes-associated ESRD with diabetic nephropathy as the primary reason for RRT, which is the cause of ESRD in only one-half of people with type 2 diabetes (7,10). The results of those population-based studies, which analyzed the time trend of RRT IRs among people with diabetes irrespective of the underlying reason for RRT in the diabetic population, were contradictory. A decrease in incidence was seen in Hong Kong (16), whereas a stable time trend was found in Italy (17) and, indeed, an increasing trend found in Australia when solely considering type 2 diabetes (18). Moreover, some studies reported significant age differences regarding the time trend of RRT incidence (18,19). A U.S. study observed an increased incidence of ESRD as a result of diabetic nephropathy among people aged 18–45 years since 2010 but a plateaued trend among people aged ≥45 years (19). These results demonstrate the relevance of age- and sex-specific analysis for a correct understanding and interpretation of the temporal development of RRT incidence in people with and without diabetes.
In another recent study, we analyzed the RRT incidence in people with and without diabetes in 2002–2016, using data from one regional German dialysis center (8). In this study, the IR did not change during the observation period either in the population with or in the population without diabetes. The incidence was ∼4.5 times higher among people with diabetes than among those without. However, the study population was too small to analyze age- and sex-specific time trends. Furthermore, the generalizability of those results to a nationwide population was limited. The aim of the current study, therefore, was 1) to analyze the IR of RRT in Germany among people with and without diabetes as well as the corresponding RR and attributable risk to diabetes and 2) to investigate time trends for the period 2010–2016 and analyze whether these patterns differ by age class and sex.
Research Design and Methods
Study Design, Study Population, and Data Assessment
We pooled anonymized nationwide data of people who were insured at the two German branches of statutory health insurance companies—Allgemeine Ortskrankenkasse (AOK) (87% of the study population) and Betriebskrankenkasse (13%)—between 1 January 2009 and 31 December 2017. These data cover ∼25 million inhabitants (i.e., 30% of the German population) who were continuously insured in this period (i.e., ≤90-day gap) for at least 1 year, a prerequisite for defining the insured person’s diabetes status. In Germany, health insurance is mandatory. Approximately 90% of the population in Germany are insured by statutory health insurance funds, while the remaining 10% are privately insured. Although there are several differences between the statutory and private system, both provide full-coverage health insurance, and German citizens have the same access to medical services, such as RRT.
Using an established algorithm (20), all people included in the study were classified as having diabetes if at least one of the following criteria was met: 1) diagnosis of diabetes (ICD-10 codes E10–E14) in at least three of four consecutive quarters, 2) at least two prescriptions of antihyperglycemic medications (Anatomical Therapeutic Chemical code A10) within 1 year, or 3) at least one diagnosis of diabetes and prescription of an antihyperglycemic medication and one measurement of blood glucose or HbA1c in the same quarter (to avoid false-positive cases as a result of data errors). We also included people with new-onset diabetes. These people were classified as having diabetes from the first quarter in which the diabetes criterion was fulfilled and retained their status throughout the study.
We identified all people with a first RRT between 1 January 2010 and 31 December 2016. Data from the years 2009 and 2017 were used only for the definition of RRT and diabetes (see below).
All cases of RRT among people with and without diabetes were recorded independently of the underlying reason for RRT. Chronic RRT was defined as chronic dialysis or preemptive kidney transplantation as indicators for treated ESRD. In line with a previous study (5), occurrence of dialysis was defined as at least one relevant physician service (i.e., hemodialysis, hemofiltration, peritoneal dialysis, hemodiafiltration) or related consultation fee arising from hospital or outpatient treatment. Dialysis was recorded as chronic dialysis if one of the following criteria was fulfilled: 1) dialysis claims were documented at least once per week over a period of 12 consecutive weeks; 2) dialysis < 12 weeks was documented before a person died with an ESRD-relevant diagnosis in at least three subsequent quarters.
People with a “condition after transplantation” diagnosis were excluded if there had been no documented kidney transplantation during the observation period. Furthermore, we excluded people with RRT in 2009 or in the first year of their insurance period since only incident RRT in 2010–2016 was assessed.
Statistical Analysis
We conducted all analyses for the entire population as well as stratified by sex and age class using the age strata 0–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years. IRs for chronic RRT were estimated by taking the number of first chronic RRT per person for each year of the observation period as the numerator and dividing by the cumulative PYs at risk from all insurance quarters of all insured people in the respective year minus those with a prevalent chronic RRT as the denominator. Stratum-specific and age- and sex-standardized IRs of chronic RRT were calculated with a 95% CI in the population with and without diabetes for each calendar year, using the German population of 2013 as a standard population with the aforementioned age strata. Furthermore, the standardized IR of the population with and without diabetes was divided to calculate the IR ratios (IRRs) for each calendar year. We also calculated attributable risk among the population with diabetes and the population-attributable risk as a result of diabetes for each year to determine the percentage of people in whom RRT could theoretically be avoided if there were no exposure (i.e., diabetes).
To examine time trends, Poisson regression models were fitted with the IR of chronic RRT as the dependent variable for people with and without diabetes, both in the population as a whole and in the age and sex strata. Year of RRT (difference from 2010) was used as an independent variable to estimate the effect of calendar time. All models that were not stratified for age and/or sex were adjusted for these variables using the youngest age-group (<40 years) and female sex as a reference group. Furthermore, analogous Poisson models were fitted for the entire population, including a variable presence of diabetes (yes vs. no) and an interaction term “diabetes ∗ year of RRT” to ascertain whether time trends differed significantly between the populations with and without diabetes.
To account for overdispersion of the outcome, we adjusted all models for descale on the basis of cumulated data on the covariate strata. We performed all analyses using SAS 9.4 TS1M1 for Windows (SAS Institute, Cary, NC).
Ethics
Neither individual written consent by patients nor ethical approval was required because the data were anonymous and no link to primary data was intended (21).
Results
Study Population
The description of all insured people is presented in Table 1. Diabetes prevalence remained nearly constant (12.6% in 2010, 12.4% in 2016), with a somewhat higher proportion in women (13.2% vs. 12.5%).
Table 1.
Description of all people with first RRT and the background population, Germany, 2010–2016
| Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total | Men | Women | Diabetes | No diabetes | Diabetes | No diabetes | Diabetes | No diabetes |
| All years combined | |||||||||
| People with RRT | 73,638 | 44,196 | 29,442 | 44,607 (60.6) | 29,031 (39.4) | 26,187 (59.3) | 18,009 (40.7) | 18,420 (62.6) | 11,022 (37.4) |
| Age* (years) | 71.3 (13.0) | 70.1 (12.9) | 73.0 (13.0) | 73.0 (10.3) | 68.6 (15.8) | 71.7 (10.3) | 67.8 (15.6) | 74.9 (10.1) | 69.8 (16.1) |
| <40 | 2,062 | 1,265 | 797 | 373 (18.1) | 1,689 (81.9) | 205 (16.2) | 1,060 (83.8) | 168 (21.1) | 629 (78.9) |
| 40–49 | 2,990 | 1,986 | 1,004 | 999 (33.4) | 1,991 (66.6) | 707 (35.6) | 1,279 (64.4) | 292 (29.1) | 712 (70.9) |
| 50–59 | 6,969 | 4,772 | 2,197 | 3,391 (48.7) | 3,578 (51.3) | 2,376 (49.8) | 2,396 (50.2) | 1,015 (46.2) | 1,182 (53.8) |
| 60–69 | 12,942 | 8,753 | 4,189 | 8,244 (63.7) | 4,698 (36.3) | 5,610 (64.1) | 3,143 (35.9) | 2,634 (62.9) | 1,555 (37.1) |
| 70–79 | 28,372 | 17,226 | 11,146 | 19,125 (67.4) | 9,247 (32.6) | 11,397 (66.2) | 5,829 (33.8) | 7,728 (69.3) | 3,418 (30.7) |
| ≥80 | 20,303 | 10,194 | 10,109 | 12,475 (61.4) | 7,828 (38.6) | 5,892 (57.8) | 4,302 (42.2) | 6,583 (65.1) | 3,526 (34.9) |
| Year RRT started: 2010 | |||||||||
| People with RRT | 11,055 | 6,492 | 4,563 | 6,591 (59.6) | 4,464 (40.4) | 3,779 (58.2) | 2,713 (41.8) | 2,812 (61.6) | 1,751 (38.4) |
| Age* (years) | 71.2 (12.6) | 69.8 (12.5) | 73.2 (12.5) | 72.6 (10.1) | 69.2 (15.3) | 71.0 (10.1) | 68.2 (15.0) | 74.7 (9.7) | 70.7 (15.8) |
| Population at risk | 23,662,600 | 11,218,907 | 12,443,693 | 2,985,436 (12.6) | 20,677,165 (87.4) | 1,372,354 (12.2) | 9,846,553 (87.8) | 1,613,082 (13.0) | 10,830,611 (87.0) |
| Year RRT started: 2011 | |||||||||
| People with RRT | 10,854 | 6,474 | 4,380 | 6,619 (61.0) | 4,235 (39.0) | 3,864 (59.7) | 2,610 (40.3) | 2,755 (62.9) | 1,625 (37.1) |
| Age* (years) | 71.1 (12.9) | 69.8 (12.9) | 73.0 (12.7) | 72.8 (10.2) | 68.5 (15.9) | 71.4 (10.2) | 67.6 (15.8) | 74.9 (9.9) | 69.9 (15.9) |
| Population at risk | 24,284,929 | 11,564,010 | 12,720,919 | 3,128,277 (12.9) | 21,156,652 (87.1) | 1,448,121 (12.5) | 10,115,889 (87.5) | 1,680,156 (13.2) | 11,040,763 (86.8) |
| Year RRT started: 2012 | |||||||||
| People with RRT | 10,465 | 6,241 | 4,224 | 6,452 (61.7) | 4,013 (38.3) | 3,751 (60.1) | 2,490 (39.9) | 2,701 (63.9) | 1,523 (36.1) |
| Age* (years) | 71.6 (12.7) | 70.5 (12.5) | 73.2 (12.8) | 73.1 (10.2) | 69.2 (15.6) | 71.8 (10.2) | 68.6 (15.2) | 74.9 (10.0) | 70.2 (16.2) |
| Population at risk | 24,606,706 | 11,765,762 | 12,840,944 | 3,220,715 (13.1) | 21,385,991 (86.9) | 1,498,549 (12.7) | 10,267,214 (87.3) | 1,722,167 (13.4) | 11,118,777 (86.6) |
| Year RRT started: 2013 | |||||||||
| People with RRT | 10,075 | 6,032 | 4,043 | 6,213 (61.7) | 3,862 (38.3) | 3,656 (60.6) | 2,376 (39.4) | 2,557 (63.2) | 1,486 (36.8) |
| Age* (years) | 71.5 (12.9) | 70.3 (12.7) | 73.2 (13.1) | 73.2 (10.4) | 68.8 (15.9) | 71.8 (10.3) | 68.0 (15.5) | 75.1 (10.2) | 70.1 (16.4) |
| Population at risk | 24,899,932 | 11,952,605 | 12,947,327 | 3,280,425 (13.2) | 21,619,507 (86.8) | 1,534,545 (12.8) | 10,418,060 (87.2) | 1,745,881 (13.5) | 11,201,447 (86.5) |
| Year RRT started: 2014 | |||||||||
| People with RRT | 10,132 | 6,090 | 4,042 | 6,144 (60.6) | 3,988 (39.4) | 3,596 (59.0) | 2,494 (41.0) | 2,548 (63.0) | 1,494 (37.0) |
| Age* (years) | 71.3 (13.1) | 70.3 (13.0) | 72.8 (13.2) | 73.1 (10.6) | 68.7 (16.0) | 71.9 (10.4) | 68.1 (15.8) | 74.7 (10.6) | 69.6 (16.3) |
| Population at risk | 25,475,526 | 12,257,432 | 13,218,094 | 3,335,648 (13.1) | 22,139,878 (86.9) | 1,566,635 (12.8) | 10,690,797 (87.2) | 1,769,013 (13.4) | 11,449,081 (86.6) |
| Year RRT started: 2015 | |||||||||
| People with RRT | 10,495 | 6,398 | 4,097 | 6,278 (59.8) | 4,217 (40.2) | 3,793 (59.3) | 2,605 (40.7) | 2,485 (60.7) | 1,612 (39.3) |
| Age* (years) | 71.1 (13.2) | 70.1 (13.1) | 72.6 (13.1) | 73.1 (10.4) | 68.0 (16.0) | 72.0 (10.4) | 67.3 (15.9) | 74.7 (10.2) | 69.2 (16.0) |
| Population at risk | 26,378,852 | 12,771,182 | 13,607,671 | 3,367,044 (12.8) | 23,011,808 (87.2) | 1,587,856 (12.4) | 11,183,326 (87.6) | 1,779,188 (13.1) | 11,828,483 (86.9) |
| Year RRT started: 2016 | |||||||||
| People with RRT | 10,562 | 6,469 | 4,093 | 6,310 (59.7) | 4,252 (40.3) | 3,748 (57.9) | 2,721 (42.1) | 2,562 (62.6) | 1,531 (37.4) |
| Age* (years) | 71.1 (13.4) | 70.0 (13.4) | 72.9 (13.3) | 73.5 (10.5) | 67.6 (16.1) | 72.2 (10.5) | 66.8 (15.9) | 75.3 (10.3) | 68.9 (16.4) |
| Population at risk | 27,476,848 | 13,384,263 | 14,092,585 | 3,394,440 (12.4) | 24,082,409 (87.6) | 1,606,163 (12.0) | 11,778,100 (88.0) | 1,788,276 (12.7) | 12,304,309 (87.3) |
Data are n, n (%), or, for age, mean (SD).
Measured at the start of RRT.
We identified 73,638 people (44,196 men, 29,442 women) with a first chronic RRT in the period 2010–2016. About three-fifths of these people (men 59.3%, women 62.6%) had diabetes at the start of first chronic RRT, with proportions remaining stable throughout the study period.
The mean age at the start of chronic RRT was 71.3 years. People with diabetes were markedly older at the start of chronic RRT (73.0 years) than those without diabetes (68.6 years). The age at the start of chronic RRT increased slightly in people with diabetes from 72.6 to 73.5 years between 2010 and 2016 but decreased in people without diabetes from 69.2 to 67.6 years.
IR, RR, and Attributable Risk
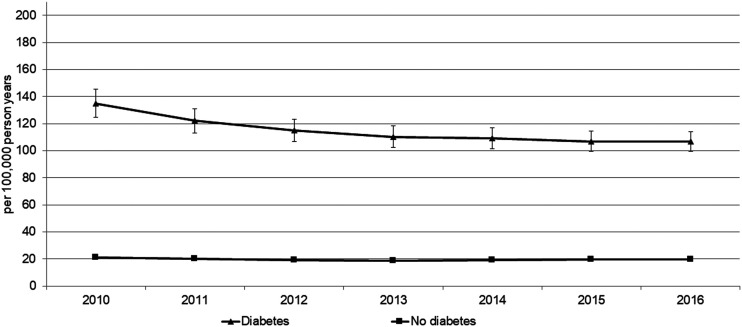
Age- and sex-standardized IR, IRR, and attributable risks are shown for each calendar year in Table 2 and Fig. 1. The IR (per 100,000 PY) of chronic RRT in the population with diabetes was 135.0 (95% CI 124.7–145.3) in 2010, 110.2 (102.1–118.4) in 2013, and 106.7 (99.4–113.9) in 2016. We observed a substantially lower IR in the population without diabetes of 21.3 (20.7–21.9) in 2010, 18.4 (17.9–19.0) in 2013, and 19.7 (19.1–20.3) in 2016. The RR (IRR) comparing the RRT incidence between people with and without diabetes was 6.3 (95% CI 5.8–6.9) in 2010, 6.0 (5.5–6.5) in 2013, and 5.4 (5.0–5.8) in 2016. More than four-fifths of the incidence of chronic RRT in people with diabetes was attributable to diabetes. In the total population, almost one-half of chronic RRT incidence was attributable to diabetes. The IR was twice as high in men than in women, with greater differences in the subpopulation without diabetes than with diabetes. In contrast, the RR and attributable risk were higher in women. However, all observed trends were quite similar in both sexes.
Table 2.
RRT incidence Germany, 2010–2016
| IR* (95% CI) per 100,000 PY | ||||||
|---|---|---|---|---|---|---|
| Variable | IRt | IRd | IRn | IRR | ARE | PAR |
| All years combined | ||||||
| Total | 35.5 (35.2–35.7) | 114.1 (111.0–117.2) | 19.6 (19.4–19.8) | 5.8 (5.6–6.0) | 0.83 (0.82–0.83) | 0.45 (0.44–0.45) |
| Men | 49.9 (49.4–50.4) | 147.6 (142.5–152.7) | 28.9 (28.5–29.3) | 5.1 (4.9–5.3) | 0.80 (0.80–0.81) | 0.42 (0.41–0.43) |
| Women | 24.2 (23.9–24.4) | 84.7 (81.4–88.1) | 12.6 (12.4–12.8) | 6.7 (6.4–7.0) | 0.85 (0.84–0.86) | 0.48 (0.47–0.49) |
| Men and women stratified by year | ||||||
| 2010 | 38.3 (37.6–39.0) | 135.0 (124.7–145.3) | 21.3 (20.7–21.9) | 6.3 (5.8–6.9) | 0.84 (0.83–0.85) | 0.44 (0.43–0.46) |
| 2011 | 36.9 (36.2–37.6) | 122.1 (113.2–130.9) | 20.0 (19.4–20.6) | 6.1 (5.6–6.6) | 0.84 (0.82–0.85) | 0.46 (0.45–0.46) |
| 2012 | 35.2 (34.6–35.9) | 114.9 (106.5–123.4) | 19.0 (18.4–19.6) | 6.0 (5.6–6.5) | 0.83 (0.82–0.85) | 0.46 (0.45–0.47) |
| 2013 | 34.0 (33.3–34.6) | 110.2 (102.1–118.4) | 18.4 (17.9–19.0) | 6.0 (5.5–6.5) | 0.83 (0.82–0.85) | 0.46 (0.45–0.47) |
| 2014 | 34.0 (33.3–34.7) | 109.1 (101.2–117.0) | 19.0 (18.4–19.5) | 5.8 (5.3–6.2) | 0.83 (0.81–0.84) | 0.44 (0.43–0.45) |
| 2015 | 35.1 (34.4–35.8) | 107.0 (99.7–114.2) | 19.8 (19.2–20.5) | 5.4 (5.0–5.8) | 0.81 (0.80–0.83) | 0.43 (0.43–0.44) |
| 2016 | 34.9 (34.2–35.6) | 106.7 (99.4–113.9) | 19.7 (19.1–20.3) | 5.4 (5.0–5.8) | 0.82 (0.80–0.83) | 0.43 (0.43–0.44) |
| Men | ||||||
| 2010 | 53.5 (52.1–54.8) | 166.8 (151.6–182.0) | 31.1 (29.9–32.2) | 5.4 (4.9–5.9) | 0.81 (0.79–0.83) | 0.42 (0.40–0.43) |
| 2011 | 52.1 (50.8–53.4) | 156.3 (142.3–170.3) | 29.5 (28.3–30.6) | 5.3 (4.8–5.8) | 0.81 (0.79–0.83) | 0.43 (0.43–0.44) |
| 2012 | 49.6 (48.4–50.9) | 149.1 (135.2–163.1) | 28.3 (27.2–29.5) | 5.3 (4.8–5.8) | 0.81 (0.79–0.83) | 0.43 (0.42–0.44) |
| 2013 | 47.9 (46.6–49.1) | 142.2 (128.9–155.4) | 27.1 (26.0–28.2) | 5.3 (4.7–5.8) | 0.81 (0.79–0.83) | 0.43 (0.42–0.45) |
| 2014 | 47.7 (46.5–48.9) | 139.9 (126.6–153.2) | 28.1 (27.0–29.2) | 5.0 (4.5–5.5) | 0.80 (0.78–0.82) | 0.41 (0.40–0.42) |
| 2015 | 49.5 (48.3–50.7) | 139.7 (127.5–151.9) | 28.8 (27.7–29.9) | 4.9 (4.4–5.3) | 0.79 (0.77–0.81) | 0.42 (0.41–0.43) |
| 2016 | 49.4 (48.2–50.6) | 142.0 (129.2–154.7) | 29.4 (28.3–30.5) | 4.8 (4.4–5.3) | 0.79 (0.77–0.81) | 0.41 (0.39–0.42) |
| Women | ||||||
| 2010 | 26.3 (25.5–27.1) | 107.8 (93.9–121.6) | 13.8 (13.2–14.5) | 7.8 (6.8–8.9) | 0.87 (0.85–0.89) | 0.47 (0.46–0.49) |
| 2011 | 24.9 (24.2–25.7) | 92.4 (81.8–103.0) | 12.9 (12.2–13.5) | 7.2 (6.3–8.1) | 0.86 (0.84–0.88) | 0.48 (0.47–0.49) |
| 2012 | 24.1 (23.4–24.9) | 85.1 (75.9–94.3) | 12.1 (11.5–12.7) | 7.0 (6.2–7.9) | 0.86 (0.84–0.87) | 0.50 (0.49–0.51) |
| 2013 | 23.1 (22.4–23.9) | 82.4 (73.2–91.6) | 11.9 (11.3–12.5) | 6.9 (6.1–7.8) | 0.86 (0.84–0.87) | 0.48 (0.47–0.50) |
| 2014 | 23.3 (22.5–24.0) | 81.8 (73.6–90.1) | 12.1 (11.5–12.7) | 6.8 (6.0–7.6) | 0.85 (0.83–0.87) | 0.48 (0.47–0.49) |
| 2015 | 23.9 (23.1–24.6) | 78.4 (70.7–86.2) | 13.1 (12.4–13.7) | 6.0 (5.4–6.7) | 0.83 (0.81–0.85) | 0.45 (0.44–0.46) |
| 2016 | 23.5 (22.7–24.2) | 75.1 (68.4–81.8) | 12.3 (11.7–12.9) | 6.1 (5.5–6.8) | 0.84 (0.82–0.85) | 0.48 (0.46–0.49) |
IRR = IRd / IRn, where IRd is the cases of RRT in individuals with diabetes in the population with diabetes and IRn is the cases of RRT in individuals without diabetes in the population without diabetes. ARE, attributable risk of RRT because of diabetes among the population with diabetes ([IRd − IRn] / IRd); PAR, attributable risk of RRT because of diabetes in the total population ([IRt − IRn] / IRt), where IRt is all cases of RRT in the total population.
Standardized to the German population, 2013.
Figure 1.
Time trend of age- and sex-standardized IRs of RRT, men and women.
Analysis of Time Trend in the Entire Population and Stratified by Age and Sex
The results of the incidence time trend from the fully adjusted Poisson models are shown in Table 3. The effect of calendar year in the population with and without diabetes is shown in models 1 and 2. We found a significant decrease in chronic RRT incidence in the population with diabetes during the observation period of 3% per year (RR per calendar year 0.972 [95% CI 0.965–0.979], P < 0.0001). We observed this trend in both sexes in almost all age classes (except men aged <40 years) (RR 0.998 [0.971–1.026], P = 0.91), although this decrease was not statistically significant in some age-groups.
Table 3.
Results of Poisson models for people with and without diabetes: RRs for RRT, Germany, 2010–2016
| Both sexes | Men | Women | ||||
|---|---|---|---|---|---|---|
| RR† (95% CI) | P value | RR† (95% CI) | P value | RR† (95% CI) | P value | |
| Model 1 (population with diabetes) | ||||||
| Year | 0.972 (0.965–0.979) | <0.0001 | 0.973 (0.964–0.981) | <0.0001 | 0.971 (0.961–0.980) | <0.0001 |
| Men vs. women | 1.772 (1.720–1.825) | <0.0001 | — | — | — | — |
| Age (years)* | ||||||
| ≥80 | 3.814 (3.252–4.472) | <0.0001 | 4.071 (3.355–4.939) | <0.0001 | 3.591 (2.926–4.407) | <0.0001 |
| 70–79 | 3.969 (3.387–4.650) | <0.0001 | 3.920 (3.235–4.749) | <0.0001 | 4.055 (3.305–4.974) | <0.0001 |
| 60–69 | 2.506 (2.135–2.942) | <0.0001 | 2.538 (2.091–3.080) | <0.0001 | 2.463 (2.000–3.034) | <0.0001 |
| 50–59 | 1.629 (1.381–1.922) | <0.0001 | 1.612 (1.322–1.965) | <0.0001 | 1.693 (1.361–2.105) | <0.0001 |
| 40–49 | 1.235 (1.028–1.485) | 0.024 | 1.247 (1.004–1.547) | 0.046 | 1.223 (0.949–1.577) | 0.12 |
| People aged <40 years only | ||||||
| Year | 0.945 (0.904–0.989) | 0.016 | 0.998 (0.971–1.026) | 0.91 | 0.884 (0.838–0.933) | <0.0001 |
| Men vs. women | 1.718 (1.432–2.061) | <0.0001 | — | — | — | — |
| People aged 40–49 years only | ||||||
| Year | 0.933 (0.905–0.963) | <0.0001 | 0.917 (0.890–0.944) | <0.0001 | 0.975 (0.921–1.032) | 0.38 |
| Men vs. women | 1.754 (1.530–2.012) | <0.0001 | — | — | — | — |
| People aged 50–59 years only | ||||||
| Year | 0.970 (0.941–0.999) | 0.046 | 0.973 (0.929–1.020) | 0.26 | 0.962 (0.927–0.999) | 0.042 |
| Men vs. women | 1.647 (1.446–1.875) | <0.0001 | — | — | — | — |
| People aged 60–69 years only | ||||||
| Year | 0.972 (0.959–0.984) | <0.0001 | 0.966 (0.951–0.981) | <0.0001 | 0.983 (0.962–1.005) | 0.13 |
| Men vs women | 1.782 (1.685–1.883) | <0.0001 | — | — | — | — |
| People aged 70–79 years only | ||||||
| Year | 0.974 (0.962–0.986) | <0.0001 | 0.979 (0.966–0.992) | 0.0018 | 0.967 (0.946–0.988) | 0.0022 |
| Men vs. women | 1.671 (1.591–1.755) | <0.0001 | — | — | — | — |
| People aged ≥80 years only | ||||||
| Year | 0.973 (0.962–0.985) | <0.0001 | 0.972 (0.958–0.986) | 0.0001 | 0.974 (0.955–0.994) | 0.012 |
| Men vs. women | 1.960 (1.868–2.056) | <0.0001 | — | — | — | — |
| Model 2 (population without diabetes) | ||||||
| Year | 0.991 (0.980–1.001) | 0.091 | 0.993 (0.983–1.002) | 0.14 | 0.987 (0.977–0.997) | 0.009 |
| Men vs. women | 2.313 (2.210–2.420) | <0.0001 | — | — | — | — |
| Age (years)* | ||||||
| ≥80 | 39.492 (35.742–43.637) | <0.0001 | 48.587 (44.411–53.156) | <0.0001 | 28.279 (25.781–31.020) | <0.0001 |
| 70–79 | 27.160 (24.622–29.960) | <0.0001 | 30.524 (27.965–33.317) | <0.0001 | 21.499 (19.595–23.589) | <0.0001 |
| 60–69 | 12.505 (11.258–13.891) | <0.0001 | 13.954 (12.713–15.315) | <0.0001 | 10.045 (9.080–11.112) | <0.0001 |
| 50–59 | 6.355 (5.697–7.089) | <0.0001 | 6.845 (6.214–7.539) | <0.0001 | 5.516 (4.964–6.129) | <0.0001 |
| 40–49 | 3.485 (3.083–3.939) | <0.0001 | 3.578 (3.209–3.989) | <0.0001 | 3.325 (2.958–3.737) | <0.0001 |
| People aged <40 years only | ||||||
| Year | 0.979 (0.960–0.998) | 0.028 | 0.980 (0.946–1.014) | 0.24 | 0.977 (0.965–0.989) | 0.0003 |
| Men vs. women | 1.633 (1.508–1.768) | <0.0001 | — | — | — | — |
| People aged 40–49 years only | ||||||
| Year | 1.011 (0.993–1.029) | 0.22 | 1.016 (0.986–1.047) | 0.29 | 1.002 (0.986–1.019) | 0.78 |
| Men vs. women | 1.754 (1.629–1.890) | <0.0001 | — | — | — | — |
| People aged 50–59 years only | ||||||
| Year | 1.005 (0.984–1.027) | 0.64 | 1.006 (0.974–1.040) | 0.70 | 1.003 (0.973–1.033) | 0.86 |
| Men vs. women | 2.025 (1.848–2.220) | <0.0001 | — | — | — | — |
| People aged 60–69 years only | ||||||
| Year | 0.995 (0.975–1.014) | 0.58 | 0.996 (0.976–1.018) | 0.73 | 0.991 (0.952–1.031) | 0.65 |
| Men vs. women | 2.266 (2.085–2.464) | <0.0001 | — | — | — | — |
| People aged 70–79 years only | ||||||
| Year | 0.987 (0.968–1.006) | 0.17 | 0.987 (0.961–1.013) | 0.31 | 0.987 (0.958–1.018) | 0.42 |
| Men vs. women | 2.313 (2.142–2.498) | <0.0001 | — | — | — | — |
| People aged ≥80 years only | ||||||
| Year | 0.982 (0.976–0.989) | <0.0001 | 0.987 (0.978–0.995) | 0.0022 | 0.977 (0.968–0.986) | <0.0001 |
| Men vs. women | 2.805 (2.732–2.880) | <0.0001 | — | — | — | — |
Baseline: <40 years.
RR per 1-year increment.
No consistent temporal change was observed among the entire population without diabetes (RR 0.991 [95% CI 0.980–1.001], P = 0.091). The age- and sex-stratified analysis only revealed a significant decrease in incidence among women aged <40 years (0.977 [0.965– 0.989], P = 0.0003) and in both sexes aged >80 years (0.982 [0.976–0.989], P < 0.0001).
Across the whole population (Supplementary Table 1, model 1), incidence of chronic RRT increased significantly with age, male sex, and diabetes (all P < 0.0001), the latter being true in all age and sex classes. No consistent changes were observed in the RRs between populations with and without diabetes as evidenced by the nonsignificant (P = 0.29) diabetes ∗ calendar year interaction (Supplementary Table 1, model 2).
Conclusions
Main Findings
This study is one of only few large population-based studies to analyze the time trend of chronic RRT incidence in the population with and without diabetes, using the population with diabetes as a population at risk and recording all cases of chronic RRT irrespective of the underlying reason. IRs among people with diabetes were almost six times higher than in people without diabetes. A significant decrease in the incidence of chronic RRT was found during the observation period in people with diabetes independent of age and sex. However, no consistent time trend was observed in people without diabetes regarding age and sex.
Comparison With National Studies
In the current study, the IR in the populations with and without diabetes is in line with and somewhat higher, respectively, than the findings of another German study that analyzed claims data of one small insurance company in 2005/2006–2008 (5). Compared with our recent regional study analyzing data from one regional German dialysis center (8) where no change of time trend was found, the IR of RRT in the current study is ∼1.3 times higher in both populations with and without diabetes, and thus, the RRs were very comparable. One possible explanation for the different findings between the two studies could be disparities between German health insurance funds and German regions with regard to insurant structures, health behaviors, and prevalence of diseases such as cardiovascular disease and diabetes (22–24). In particular, the increased RRT incidence in our study compared with the two previous German studies is due to the large proportion of study participants insured by the AOK. AOK is known to have a high proportion of insured persons with cardiovascular diseases and diabetes, a high number of people with migratory backgrounds, and a high number of people who smoke (24), all of which are known factors for the development of kidney disease and ESRD.
Comparison With International Studies
An international comparison with other studies is difficult since different methodological approaches were used among the studies. We found only a few epidemiological population-based studies with comparable study design regarding 1) outcome (all cases of RRT and not only diabetic nephropathy as a primary reason for RRT) and 2) denominator (population with diabetes as a population at risk [i.e., diabetes prevalence known or at least estimable]).
The age- and sex-standardized IR of chronic RRT in people with diabetes included in our study (114.1 per 100,000 PY) was fairly comparable with findings from Australia (93 per 100,000 PY) (18) and with results from Italy (17). Likewise, the age-adjusted RR comparing people with and without diabetes in our study was well in line with those observed in Italy (17).
The crude IR of chronic RRT among people with diabetes in our study was considerably lower (i.e., three to seven times) than those estimated in studies from Taiwan (11,25) and Hong Kong (16). However, comparability was limited by different definitions of outcomes and study populations. Interestingly, unlike our study, neither of the Asian studies identified a sex difference regarding incidence of chronic RRT (11,25).
Three studies reported time trend with contradictory results. The decrease in chronic RRT incidence in people with diabetes of 4% per year observed in the Hong Kong study between 2000 and 2012 was very comparable with our findings (16). In contrast, the 2004– 2013 Italian study observed stable IRs in both populations with and without diabetes (17). Likewise, a study from Australia covering the years 2002–2013 reported a stable time trend in people with type 1 diabetes but a 4.5% increase per year in people with type 2 diabetes (18). This increase in people with type 2 diabetes may be explained by extended access to RRT or a greater willingness among elderly people with several comorbidities to start RRT.
Despite only having analyzed diabetic nephropathy as a reason for chronic RRT among people with diabetes, the findings of a large U.S. study are worthy of mention (7). Although diabetic nephropathy is the reason for chronic RRT in only 50% of people with type 2 diabetes, the age- and sex-adjusted IRs in the U.S. study were considerably higher than in the current study (260.2 per 100,000 PY in 2000, 173.9 per 100,000 PY in 2014). The reported decrease in IRs during the study period (2.8% reduction per year) was well in line with the declining incidence in the population with diabetes found in our study. It is remarkable that another U.S. study analyzing time trends of incidence of hospitalizations for ESRD found an increasing trend among young people with diabetes aged 18–45 years while the incidence has been plateauing in the age-groups >45 years since 2010 (19). An Australian study analyzing the age-specific time trend of chronic RRT incidence found a strong annual increase of 4.2% in the <50 and >80 age-groups among nonindigenous people, with no consistent time trend observed in the interim age-groups (18). In contrast, the decrease in incidence among people with diabetes identified in our study was more prominent in the younger age-groups, with the steepest decrease in women <40 years (12% reduction per year) and men aged 40–49 years (8% reduction per year). The observed decrease of chronic RRT incidence among people with diabetes in all age classes might partially be apportioned to improvements in diabetes care: better control of blood glucose, among others, therapy with sodium–glucose cotransporter 2 inhibitors, as well as early, adequate, and consistent therapy of hypertension with renin-angiotensin-aldosterone system blockers, and early diagnosis and treatment of kidney disease at an early stage in people with diabetes. This suggestion is confirmed by increasing age at the start of RRT during the study period (72.6 years in 2010, 73.5 years in 2016). Moreover, the increased number of people with diabetes participating in the disease management programs for type 1 and type 2 diabetes, which aim to prevent complications of diabetes, including ESRD, could also contribute to this favorable trend. A further explanation of this decline could be that more people with diabetes were detected at earlier stages of disease and, thus, had a lower risk of late complications of diabetes, including ESRD. In contrast, the time trend of incidence in the population without diabetes was age and sex dependent, with a significant decrease only in women aged <40 years and in men and women aged >80 years. The decrease observed among younger women could be a result of better compliance and regulation of blood pressure than in young men. The inconsistent time trend of RRT incidence in the middle-age-group might be explained by a late and insufficient treatment of hypertension, which leads to deterioration of renal function and, as a consequence, to vascular nephropathy. The declining rates among the elderly population could be explained by improvements in medical care for nephrological disorders. Another explanation could be that older patients with ESRD, who are often multimorbid, are treated without dialysis. Indirect support for the latter could be the decreasing age of patients without diabetes at the start of dialysis from 69.2 to 67.6 years during the study period.
Limitations and Strengths
Our study has some limitations. First, the claims data used potentially did not clearly distinguish between acute and chronic dialysis, particularly among people who died within 3 months of starting dialysis. To account for this, we developed an algorithm using a combination of physician services data for dialysis and clinical diagnosis relevant to chronic terminal renal disease. Second, we were unable to analyze important clinical variables, such as diabetes duration; clinical markers, such as glomerular filtration rate and blood pressure; and lifestyle factors, such as smoking. These variables are known prognostic factors for the development of ESRD among people with diabetes. Because of the highly sensitive nature of these personal data and current data protection legislation, physicians are not permitted to transfer such data to insurance companies. However, this data source does offer the advantage of providing a large number of cases, which allows for a population-based approach. Besides, the investigation of potentially explanatory factors was not the main objective of this study. Third, we were unable to distinguish between type 1 and type 2 diabetes with the current data set. However, since the majority of people with diabetes starting chronic RRT can be assumed to be people with type 2 diabetes, our findings are primarily true for a population with type 2 diabetes. Fourth, our study population is confined to two large statutory health insurance branches constituting ∼30% of the German population. Because of sociodemographic and health-related differences between health insurance companies, the insured people included in the study may vary from those of other public and especially private health insurance companies (22–24). Therefore, the results can only be partially generalized to the entire German population. However, the estimated IRs in the populations both with and without diabetes and the corresponding RRs were comparable with those of both previous German studies (5,8). Finally, we analyzed the incidence of chronic RRT, which only counts cases of treated ESRD. It cannot be ruled out that some patients did not receive dialysis because of severe comorbidities, such as a threat of heart failure, or because of decisions against dialysis for religious or other personal reasons. However, all patients in Germany with an existing medical indication for dialysis have a statutory entitlement to dialysis.
A number of strengths should also be considered. First, our study is the first nationwide population-based study in Germany, covering almost one-third of the German population, to analyze the time trend of chronic RRT incidence among people with and without diabetes. Second, we were able to record all cases of chronic RRT in people with diabetes independently of their primary cause (i.e., not only diabetic nephropathy as a primary cause for chronic RRT). This is of note because especially in patients with type 2 diabetes, it is not always easy to distinguish between diabetic nephropathy as a main reason for ESRD and diabetes as a comorbidity when people with diabetes have coexisting diseases (e.g., hypertension or renal disease with nondiabetic pathogenesis) (26,27). Finally, we were able to estimate diabetes prevalence in the study population using an established algorithm. This methodological approach considers the increasing prevalence of diabetes in the population at risk (in contrast to IRs calculated in the general population) and, thus, allows a correct interpretation of results concerning the time trend.
In conclusion, the IR of RRT was six times higher in people with diabetes than in those without diabetes during the study period. The incidence of chronic RRT significantly decreased during the observation period in people with diabetes in all age and sex classes. In contrast, no consistent time trend was seen in people without diabetes with divergent age- and sex-specific results.
Article Information
Acknowledgments. The authors thank Jeremy Groves (conference interpreter, freelance) for editing the manuscript and revising the English language used in this article.
Funding. The project was supported by the German Federal Ministry of Health (grant number 1368-1511). The project on which this report is based was funded by the Robert Koch Institute as part of the National Diabetes Surveillance project with funds from the German Federal Ministry of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.C. and M.N. analyzed and interpreted the findings and drafted the first version of the manuscript. M.N., T.K., and F.H. contributed to the overall coordination, data collection, and research data. A.W., H.F., and C.G. collected and provided data. M.K. and K.J.-D. provided clinical expertise. A.I. designed the study and codrafted the first version of the manuscript. All authors interpreted the analysis, reviewed and provided input to the final manuscript, and gave final approval of the version to be published. A.I. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Virtual 56th Annual Meeting of the European Association for the Study of Diabetes, 21–25 September 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14055110.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
H.C. and M.N. are joint first authors and contributed equally.
References
- 1. Kähm K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R. Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care 2018;41:971–978 [DOI] [PubMed] [Google Scholar]
- 2. Icks A, Haastert B, Gandjour A, et al. Costs of dialysis--a regional population-based analysis. Nephrol Dial Transplant 2010;25:1647–1652 [DOI] [PubMed] [Google Scholar]
- 3. Zhao X, Wang M, Zuo L. Early mortality risk in incident Chinese hemodialysis patients: a retrospective cohort study. Ren Fail 2017;39:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sattar A, Argyropoulos C, Weissfeld L, et al. All-cause and cause-specific mortality associated with diabetes in prevalent hemodialysis patients. BMC Nephrol 2012;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann F, Haastert B, Koch M, Giani G, Glaeske G, Icks A. The effect of diabetes on incidence and mortality in end-stage renal disease in Germany. Nephrol Dial Transplant 2011;26:1634–1640 [DOI] [PubMed] [Google Scholar]
- 6. Assogba FG, Couchoud C, Hannedouche T, et al.; French Renal Epidemiology and Information Network Registry . Trends in the epidemiology and care of diabetes mellitus-related end-stage renal disease in France, 2007-2011. Diabetologia 2014;57:718–728 [DOI] [PubMed] [Google Scholar]
- 7. Burrows NR, Hora I, Geiss LS, Gregg EW, Albright A. Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes - United States and Puerto Rico, 2000-2014. MMWR Morb Mortal Wkly Rep 2017;66:1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Narres M, Claessen H, Kvitkina T, et al. Incidence and relative risk of renal replacement therapy in people with and without diabetes between 2002 and 2016 in a German region. Diabetologia 2020;63:648–658 [DOI] [PubMed] [Google Scholar]
- 9. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia 2019;62:3–16 [DOI] [PubMed] [Google Scholar]
- 10. Comas J, Arcos E, Castell C, et al. Evolution of the incidence of chronic kidney disease stage 5 requiring renal replacement therapy in the diabetic population of Catalonia. Nephrol Dial Transplant 2013;28:1191–1198 [DOI] [PubMed] [Google Scholar]
- 11. Chu YW, Wu WS, Hsu CF, Wang JJ, Weng SF, Chien CC. Bidirectional association between ESRD dialysis and diabetes: national cohort study. PLoS One 2017;12:e0173785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narres M, Claessen H, Droste S, et al. The incidence of end-stage renal disease in the diabetic (compared to the non-diabetic) population: a systematic review. PLoS One 2016;11:e0147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Dijk PR, Kramer A, Logtenberg SJ, et al. Incidence of renal replacement therapy for diabetic nephropathy in the Netherlands: Dutch Diabetes Estimates (DUDE)-3. BMJ Open 2015;5:e005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prischl FC, Auinger M, Säemann M, et al.; Austrian Dialysis and Transplant Registry . Diabetes-related end-stage renal disease in Austria 1965-2013. Nephrol Dial Transplant 2015;30:1920–1927 [DOI] [PubMed] [Google Scholar]
- 15. Sesso RC, Lopes AA, Thomé FS, Lugon JR, Martins CT. Brazilian chronic dialysis census 2014. J Bras Nefrol 2016;38:54–61 [DOI] [PubMed] [Google Scholar]
- 16. Luk AOY, Hui EMT, Sin MC, et al. Declining trends of cardiovascular-renal complications and mortality in type 2 diabetes: the Hong Kong Diabetes Database. Diabetes Care 2017;40:928–935 [DOI] [PubMed] [Google Scholar]
- 17. Giorda CB, Carnà P, Salomone M, et al. Ten-year comparative analysis of incidence, prognosis, and associated factors for dialysis and renal transplantation in type 1 and type 2 diabetes versus non-diabetes. Acta Diabetol 2018;55:733–740 [DOI] [PubMed] [Google Scholar]
- 18. Koye DN, Magliano DJ, Reid CM, et al. Trends in incidence of ESKD in people with type 1 and type 2 diabetes in Australia, 2002-2013. Am J Kidney Dis 2019;73:300–308 [DOI] [PubMed] [Google Scholar]
- 19. Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321:1867–1868 [DOI] [PubMed] [Google Scholar]
- 20. Köster I, von Ferber L, Ihle P, Schubert I, Hauner H. The cost burden of diabetes mellitus: the evidence from Germany--the CoDiM study. Diabetologia 2006;49:1498–1504 [DOI] [PubMed] [Google Scholar]
- 21. Swart E, Gothe H, Geyer S, et al.; German Society for Social Medicine and Prevention; German Society for Epidemiology . Good Practice of Secondary Data Analysis (GPS): guidelines and recommendations. Gesundheitswesen 2015;77:120–126 [in German] [DOI] [PubMed] [Google Scholar]
- 22. Hoffmann F, Icks A. Diabetes prevalence based on health insurance claims: large differences between companies. Diabet Med 2011;28:919–923 [DOI] [PubMed] [Google Scholar]
- 23. Hoffmann F, Icks A. Structural differences between health insurance funds and their impact on health services research: results from the Bertelsmann Health-Care Monitor. Gesundheitswesen 2012;74:291–297 [in German] [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann F, Koller D. Different regions, differently insured populations? socio-demographic and health-related differences between insurance funds. Gesundheitswesen 2017;79:e1–e9 [in German] [DOI] [PubMed] [Google Scholar]
- 25. Lin CC, Li CI, Liu CS, et al. Development and validation of a risk prediction model for end-stage renal disease in patients with type 2 diabetes. Sci Rep 2017;7:10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villar E, McDonald SP, Couchoud C. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline: response to Burrows, Li, and Geiss. Diabetes Care 2010;33:e69; author reply e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan CM, Nee R, Ceckowski KA, Knight KR, Abbott KC. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin Kidney J 2017;10:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]