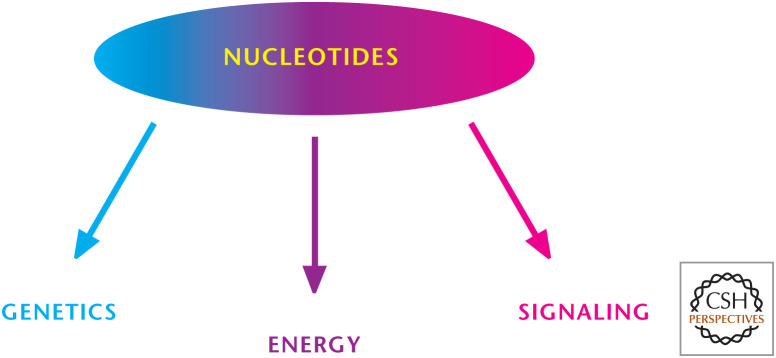
The single most important experiment of the 20th century was elucidation of the structure of DNA by James D. Watson and Francis H.C. Crick, along with Maurice Wilkins and Rosalind Franklin. Watson, Crick, and Wilkins were awarded the 1962 Nobel Prize in Physiology or Medicine “for their discoveries concerning the molecular structure of nucleic acids and its significance for information transfer in living material.” (Franklin had died in 1958 and thus was not eligible for the award.) The discovery that there exists a heritable genetic code captured the imagination of the public and continues to have profound biological and social implications. In 1963, the surrealist artist Salvador Dali paid homage to the discovery in his painting, Galacidalacidesoxyribonucleicacid. Today, the discovery is celebrated and remembered in many ways, including my favorite, a plaque at The Eagle pub in Cambridge, United Kingdom, where, in 1953, Watson and Crick first publicly announced the discovery to their colleagues. Many decades later, DNA continues to be the center of biology. Furthermore, the discoveries of microRNA, noncoding RNA, and RNA interference have transformed biological science in the past two decades. Beyond genetics, nucleotides have important roles at the cellular level, including providing energy (ATP is a nucleotide!) and as signaling molecules (Fig. 1).
Figure 1.
Overview of nucleotide metabolism. Nucleotides are key components of DNA and RNA (genetics), provide energy (ATP), and can activate signaling pathways.
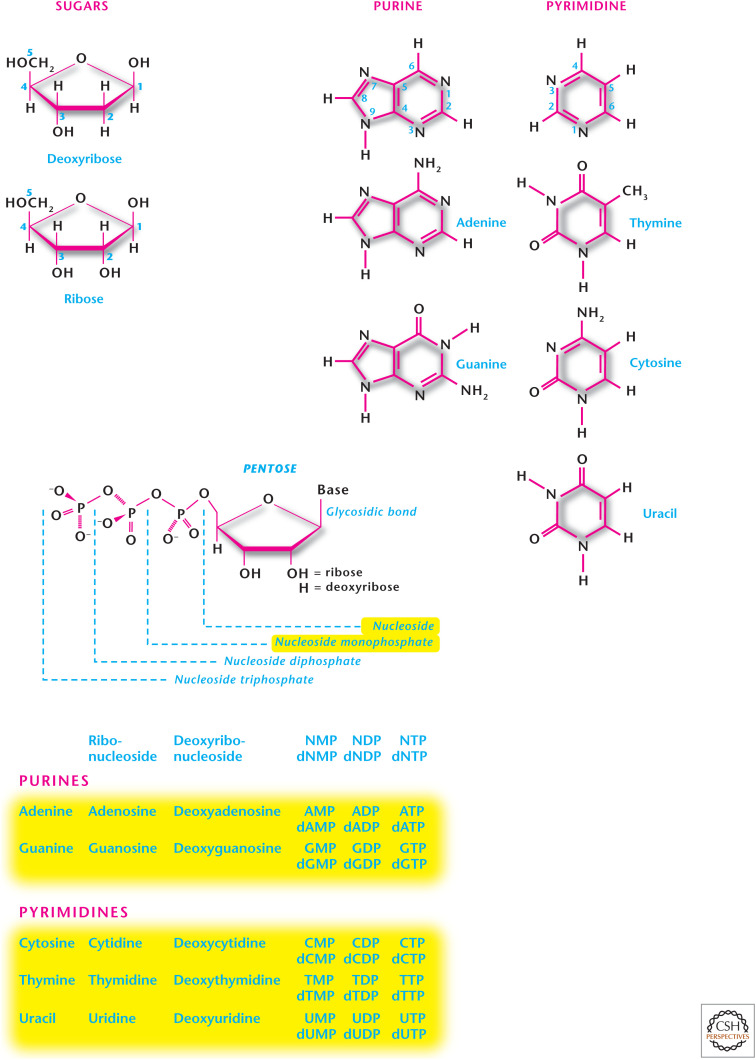
QUICK GUIDE TO NUCLEOTIDES
Nucleosides have either a ribose or 2-deoxyribose bound to purine or pyrimidine. The addition of one or more phosphates to a nucleoside results in a nucleotide.
Purines (adenine and guanine) are comprised of attached six-membered and five-membered nitrogen-containing rings.
Pyrimidines (uracil, thymine, and cytosine) have only a six-membered nitrogen-containing ring.
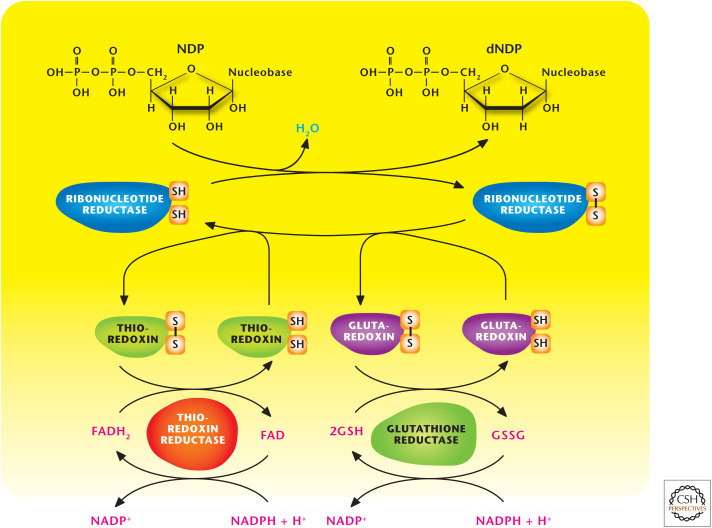
Ribonucleotide reductase (RR) generates deoxynucleoside diphosphate (dNDP) from ribonucleoside diphosphate (rNDPs). Nucleoside diphosphate (NDP) kinases use ATP to phosphorylate dNDP to produce deoxynucleoside triphosphates (dNTPs).
Purine nucleotides synthesis begins with 5-phosphoribosyl-1-pyrophosphate (PRPP), which, through a series of reactions, generates the nucleotide inosine 5′-monophosphate (IMP). Subsequently, IMP can be converted into either AMP or GMP through distinct reactions. AMP or GMP can be converted to ADP or GDP, respectively.
Pyrimidine nucleotides synthesis begins with carbamoyl phosphate and aspartate generating the pyrimidine base orotate. Succeeding steps attach PRPP to orotate to generate orotate monophosphate (OMP), which is then decarboxylated to UMP. UMP generates UDP and UTP, which can generate CTP.
Humans cannot break down the purine ring. The catabolism of purine nucleotides results in a uric acid. In contrast, the pyrimidine ring can be completely degraded. Catabolism of the pyrimidine nucleotides leads, ultimately, to β-alanine or β-aminoisobutyrate production, as well as NH3 and CO2.
Nucletodies are signaling molecules that regulate multiple physiological processes, including neurotransmission and inflammation.
ATP activates a family of ionotropic receptors (P2X) and metabotropic receptors (P2Y) Extracellular adenosine activates G-protein-coupled cell-surface receptors, which are divided into four subtypes: A1, A2A, A2B, and A3.
NITROGEN BASES, NUCLEOSIDES, AND NUCLEOTIDES
There are two kinds of nitrogen-containing bases: purines and pyrimidines. Purines are comprised of attached six-membered and a five-membered nitrogen-containing rings (Fig. 2). The major purines are adenine, guanine, hypoxathine, and xanthine. Adenine and guanine are present in both DNA and RNA. Hypoxanthine and xanthine are not incorporated into the nucleic acids, but they are important intermediates in the synthesis and degradation of the purine nucleotides. Pyrimidines have only a six-membered nitrogen-containing ring (Fig. 2). The major pyrimidines include uracil, thymine, and cytosine. Cytosine and thymine are present in both DNA and RNA, whereas uracil is found only in RNA. Nucleosides have a sugar—either ribose or 2-deoxyribose—added to a nitrogen group (Fig. 3). Purines bond to the C1′ carbon of the sugar at their N9 atoms and pyrimidines bond to the C1′ carbon of the sugar at their N1 atoms. Purine and pyrimidine nucleosides end in -osine and -idine, respectively. The addition of one or more phosphates to the sugar portion of a nucleoside results in a nucleotide (Fig. 2).
Figure 2.
Structure of purines and pyrimidines. Nucleosides have either a ribose or 2-deoxyribose bound to purine or pyrimidine. The addition of one or more phosphates to a nucleoside results in a nucleotide. Purines (adenine and guanine) are compromised of attached six-membered and five-membered nitrogen-containing rings. Pyrimidines (uracil, thymine, and cytosine) have only a six-membered nitrogen-containing ring.
Figure 3.
Ribonucleotide reductase (RR) produces dNDPs. RR generates dNDP from rNDPs. NDP kinases use ATP to phosphorylate dNDP to produce dNTPs. GSH, reduced glutathione; GSSG, oxidized glutathione.
DEOXYRIBONUCLEOTIDES
In 1959, the Nobel Prize in Physiology or Medicine was awarded to Severo Ochoa and Arthur Kornberg “for their discovery of the mechanisms in the biological synthesis of ribonucleic acid and deoxyribonucleic acid.” DNA and RNA polymerases use nucleotides to build DNA and RNA molecules. Most cells contain more RNA—messenger, ribosomal, and transfer RNAs—than DNA. Thus, nucleotide biosynthesis generates ample ribonucleotides. Because proliferating cells need to replicate their genomes, the production of deoxynucleotides is also necessary and begins with the reduction of rNDPs to generate dNDP by RR (Fig. 3). RR has a large R1 subunit that is a dimer of monomeric molecular mass 90 kDa, whereas the smaller R2 subunit is a dimer of monomeric molecular mass 45 kDa. This multifunctional enzyme contains redox-active thiol groups that participate in the transfer of electrons during the reduction reactions. RR becomes oxidized as rNDP and is reduced to dNDP. RR is reduced to its original state by either thioredoxin or glutaredoxin, which in turn become oxidized and are regenerated to the reduced form by the enzymes thioredoxin reductase and glutathione reductase, respectively. The ultimate source of the electrons driving these reactions is NADPH (see also Chandel 2020).
RR is the only enzyme used in the generation of all the deoxyribonucleotides, and so its activity is tightly regulated to ensure stable production of all four of the dNTPs required for DNA replication. Each R1 monomer contains two distinct binding sites for deoxynucleoside triphosphates, which act as allosteric regulators of the enzymatic activity. The binding of dATP to allosteric sites, known as the activity sites, on the enzyme inhibits the overall catalytic activity of the enzyme. This prevents the reduction of any of the four NDPs to effectively block DNA synthesis. In contrast, ATP bound to these sites activates the enzyme. Thus, the ATP/dATP ratio modulates the activity of the enzyme to ensure that sufficient dNTPs are produced for DNA synthesis when needed. In the absence of this regulatory mode, ribonucleotides needed for RNA synthesis would be depleted by needlessly being converted into deoxynucleotides.
Another allosteric site, known as a substrate-specific site, is responsible for maintaining balanced nucleotide pools, for optimal fidelity of DNA replication. Deoxythymidine triphosphate (dTTP) binding at the specificity sites causes a conformational change that allows reduction of GDP to dGDP at the catalytic site. The binding of ATP or dATP induces reduction of cytidine 5′-diphosphate (CDP) and uridine 5′-diphosphate (UDP) to dCDP and dUDP, respectively. The binding of 2′-deoxyguanosine 5′-triphosphate (dGTP) causes reduction of adenosine 5′-diphosphate (ADP) to dADP. Once a dNDP is generated by RR, that particular dNDP can undergo phosphorylation to produce dNTPs, a reaction catalyzed by the NDP kinases using ATP as the phosphate donor. There are also nucleoside monophosphate (NMP) kinases that catalyze ATP-dependent reactions of the type (d)NMP + ATP ↔ (d)NDP + ADP. The activity of the NDP kinases is higher than that of the NMP kinases to maintain a high intracellular level of (d)NTPs relative to that of (d)NDPs.
PURINE NUCLEOTIDE BIOSYNTHESIS
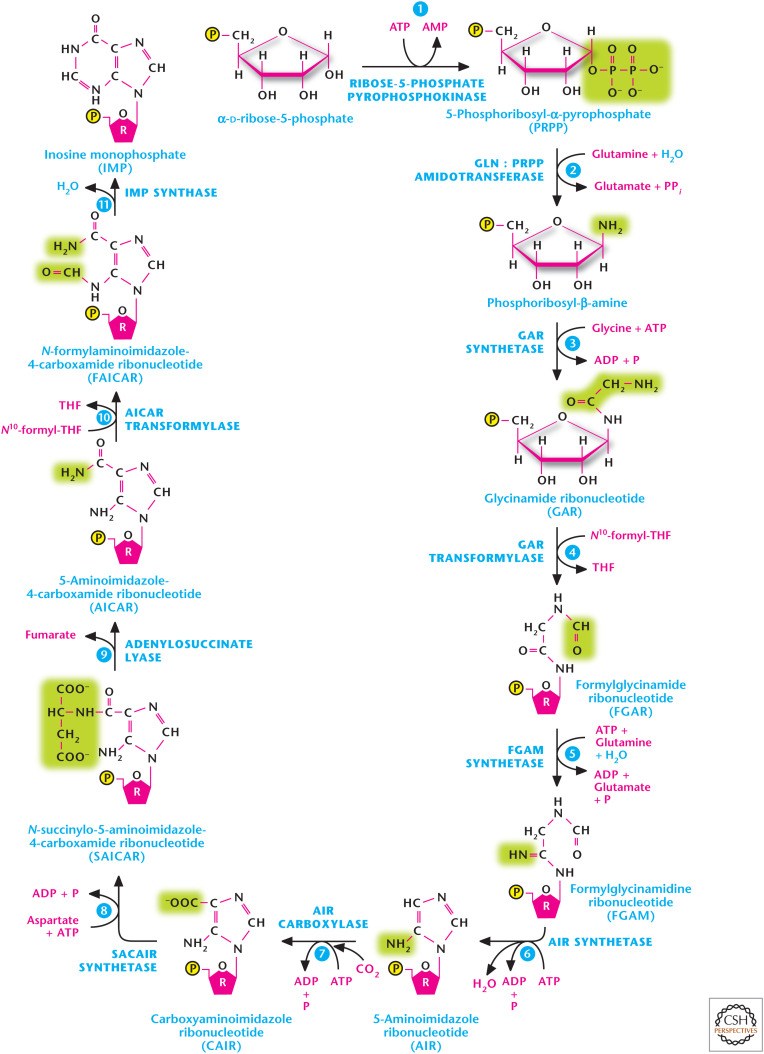
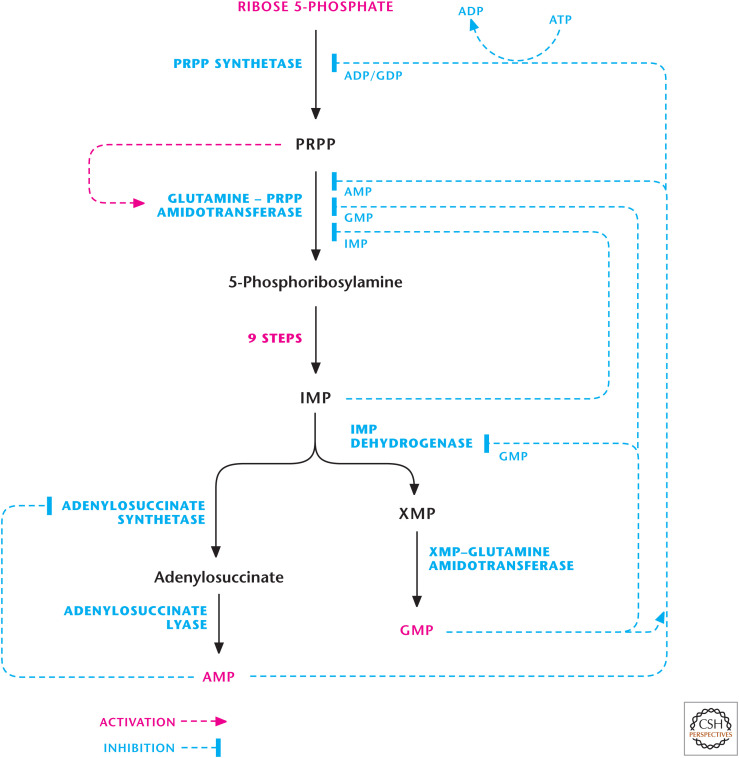
Purine nucleotides synthesis begins with PRPP, which, through a series of reactions, generates the nucleotide IMP. The first reaction is catalyzed by ribose 5-phosphate pyrophosphokinase to generate PRPP from ribose 5-phosphate and the two high-energy phosphates of ATP. Ribose 5-phosphate serves as a platform for the construction of the purine ring structure. PRPP is not committed just to purine synthesis; it is also used for other processes, including pyrimidine and histidine biosynthesis. The second step, catalyzed by PRPP glutamylamidotransferase, commits PRPP to purine synthesis and is tightly regulated. In this step, there is a loss of the PRPP pyrophosphate and the addition of an amino group from glutamine. Subsequently, there are nine additional enzymatic reactions that result in the formation of IMP, which contains the nitrogen base hypoxanthine. The pathway first builds the five-membered ring and then the six-membered ring (Fig. 4). The amino acids glutamine, aspartate, and glycine serve as nitrogen donors, as ammonium (NH4+) is not a nitrogen donor in biosynthetic reactions. The synthesis of IMP requires five moles of ATP, two moles of glutamine and formate, and one mole of glycine, CO2, and aspartate. The formyl moieties are transported on tetrahydrofolate (THF) in the form of N10-formyl-THF. The rate-limiting steps in purine biosynthesis are the first two steps of the pathway (Fig. 5). The first step catalyzed by ribose-5-pyrophosphokinase is a feedback-inhibited reaction by ADP and GDP. The second reaction is catalyzed by PRPP amidotransferase. This reaction is also feedback inhibited allosterically by binding of AMP, GMP, and IMP. Conversely, the activity of PRPP amidotransferase is stimulated by PRPP.
Figure 4.
The purine synthesis pathway. The purine ring is built on the ribose sugar 5-phosphoribosyl-1-pyrophosphate (PRPP) as the base. Purine nucleotides synthesis begins with PRPP, which, through a series of reactions, generates the nucleotide, inosine 5′-monophosphate (IMP). (Adapted from Garrett and Grisham 1999.)
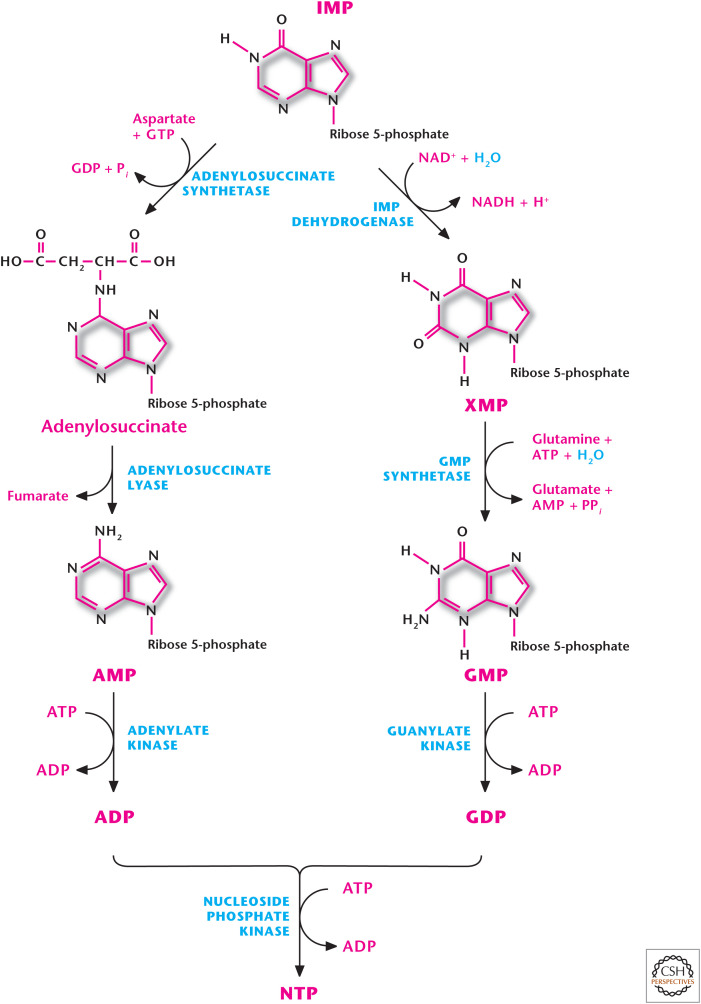
Figure 5.
Purine nucleotide production. IMP can be converted into either AMP or GMP through distinct reactions. AMP or GMP can be converted to ADP or GDP by adenylate kinase or guanylate kinase, respectively.
IMP can be converted into either AMP or GMP through distinct reactions (Fig. 6). The formation of AMP requires GTP and production of GMP requires ATP. This allows for control of AMP or GMP depending on the availability of GTP or ATP, respectively. The accumulation of GTP results in AMP synthesis from IMP at the cost of GMP production. Conversely, excess ATP favors GMP production at the expense of AMP synthesis. Furthermore, AMP or GMP accumulation inhibits adenylosuccinate synthetase or IMP dehydrogenase to prevent further production of AMP or GMP, respectively (Fig. 6). Subsequently, AMP or GMP can be converted to ADP or GDP by adenylate kinase or guanylate kinase using ATP, respectively (Fig. 5). Because free ATP is present in higher levels than any other nucleotide, it is usually used as the phosphate donor in these reactions.
Figure 6.
Regulation of purine biosynthesis. The first two reactions of the purine biosynthesis pathway are rate-limiting steps, which are regulated. (Adapted, with permission, from Nelson and Cox 2013, © W.H. Freeman and Company.)
PURINE NUCLEOTIDE DEGRADATION
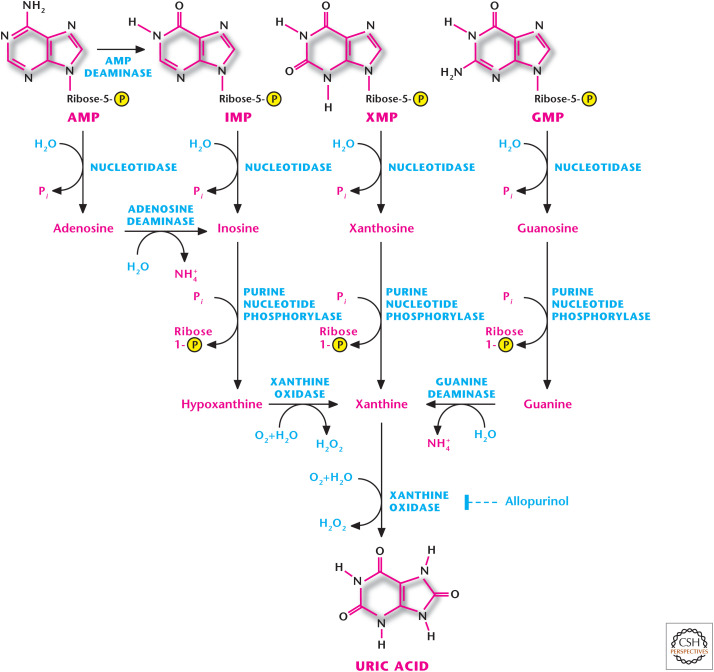
Humans cannot break down the purine ring. The catabolism of purine nucleotides results in uric acid, which has low solubility in water and is excreted as sodium urate crystals in the urine (Fig. 7). Purine catabolism does not alter the basic purine structure. The primary pathway is initiated by conversion of AMP to IMP or adenosine to generate inosine, which subsequently makes hypoxanthine. GMP can be converted to guanosine and, subsequently, into guanine. Both guanine and hypoxanthine generate xanthine. The final step involves converting xanthine to uric acid by xanthine oxidase, a step that produces hydrogen peroxide (Box 1).
Figure 7.
Catabolism of purines. Humans cannot break down the purine ring of purine nucleotides. The catabolism of purine nucleotides results in a uric acid, which has low solubility in water and is excreted as sodium urate crystals in the urine. Allopurinol inhibits xanthine oxidase and is used to treat gout.
PURINE NUCLEOTIDE SALVAGE
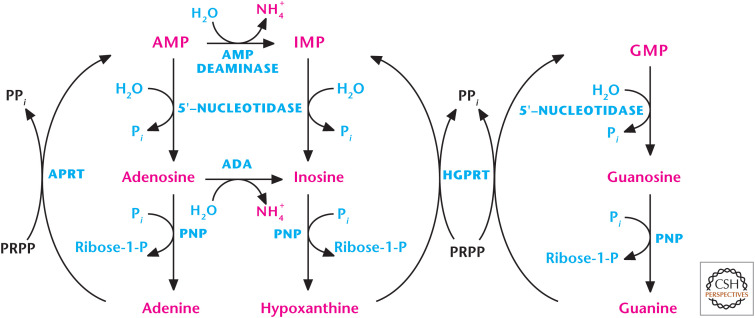
Nucleotides can also be synthesized from the purine bases and purine nucleosides in a series of steps referred to as salvage pathways. Phosphoribosylation converts the free purine bases adenine, guanine, and hypoxanthine to their corresponding nucleotides. Two key transferase enzymes are involved in the salvage of purines are adenosine phosphoribosyltransferase (APRT), which converts adenine to AMP, and hypoxanthine-guanine phosphoribosyltransferase (HGPRT), which converts hypoxanthine to IMP or guanine to GMP by using PRPP as a substrate (Fig. 8). By using PRPP as a substrate, the salvage pathway decreases the levels of PRPP, and therefore diminishes the rate of de novo purine synthesis. The de novo purine synthesis and salvage pathways are tightly coupled based on the availability of PRPP.
Figure 8.
Purine salvage pathway. Two key transferase enzymes involved in the salvage of purines are adenosine phosphoribosyltransferase (APRT), which converts adenine to AMP, and hypoxanthine-guanine phosphoribosyltransferase (HGPRT), which converts hypoxanthine to IMP or guanine to GMP by using 5-phosphoribosyl-1-pyrophosphate (PRPP) as a substrate.
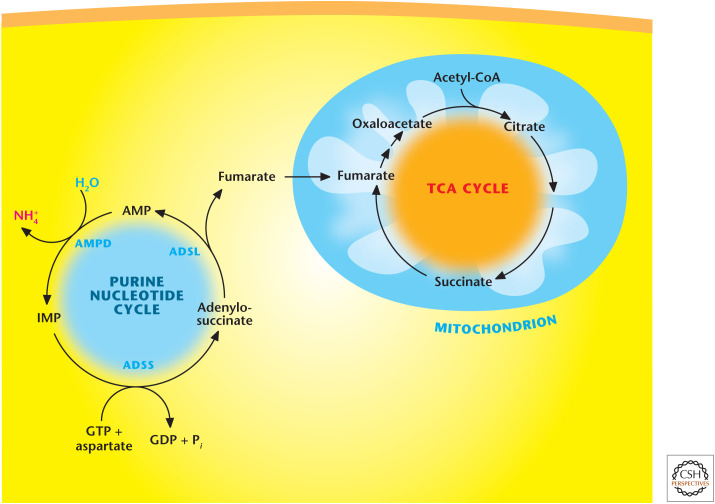
One important salvage pathway is the purine nucleotide cycle, which produces the TCA-cycle intermediate fumarate as a mechanism to boost the TCA cycle's ability to generate more NADH for the production of ATP (Fig. 9). An increase in muscle activity creates a high-metabolic demand for ATP. An increase in AMP levels is a signal that energy generation by the TCA cycle and electron transport chain is insufficient to keep up with metabolic demand. This cycle is very important in muscle cells because they lack most of the enzymes of the major anapleurotic reactions (i.e., those that replenish pathway intermediates). Thus, muscle replenishes TCA-cycle intermediates through fumarate generated by the purine nucleotide cycle. Myoadenylate deaminase is the muscle-specific isoenzyme of AMP deaminase, and deficiencies in myoadenylate deaminase lead to postexercise fatigue and cramping.
Figure 9.
Purine nucleotide cycle. Purine nucleotide pathway converts AMP into IMP via AMP deaminase (AMPD). Next, adenylosuccinate synthetase (ADSS) converts IMP into adenylosuccinate using aspartate and GTP as substrates. Subsequently, adenylosuccinate lyase (ADSL) converts IMP into fumarate and AMP. Fumarate enters the TCA cycle.
PYRIMIDINE NUCLEOTIDE BIOSYNTHESIS
Pyrimidine synthesis differs from purine synthesis in that the pyrimidine ring is fully synthesized before being attached to the ribose sugar, whereas the purine ring is built on the ribose sugar (PRPP) as the base. Overall synthesis of pyrimidines is a less-complex process than purines primarily because of the simpler six-membered ring base structure (Fig. 10). Carbamoyl phosphate and aspartate contribute to the ring structure. The carbamoyl phosphate is derived from 1 mole of glutamine, 2 moles of ATP, and 1 mole of bicarbonate within the cytosol. Note that the urea cycle also uses carbamoyl phosphate, but it is derived from ammonia and bicarbonate in the mitochondrion. The urea cycle reaction is catalyzed by carbamoyl phosphate synthetase I, whereas the pyrimidine nucleotide precursor is generated by carbamoyl phosphate synthetase II (CPS-II). Subsequently, carbamoyl phosphate funnels into the pyrimidine nucleotide biosynthesis pathway through the action of aspartate transcarbamoylase, which is the rate-limiting step in pyrimidine biosynthesis. The next steps build the first fully formed pyrimidine base orotate. PRPP attaches to oroate to generate OMP, which is subsequently decarboxylated to UMP. UDP is formed from UMP via an ATP-dependent NMP kinase (UMP + ATP → UDP + ADP), whereas UTP is formed by the NDP kinase (UDP + ATP → UTP + ADP) (Fig. 10). UTP can be used to generate CTP using ATP and glutamine, a reaction catalyzed by CTP synthetase.
Figure 10.
Pyrimidine ring is fully synthesized before being attached to the ribose sugar. Pyrimidine nucleotides synthesis begins with carbamoyl phosphate and aspartate generating the pyrimidine base orotate. Next, steps attach PRPP to oroate to generate orotate monophosphate (OMP), which is subsequently decarboxylated to UMP. UMP generates UDP and UTP, which can generate CTP. (Adapted from Garrett and Grisham 1999.)
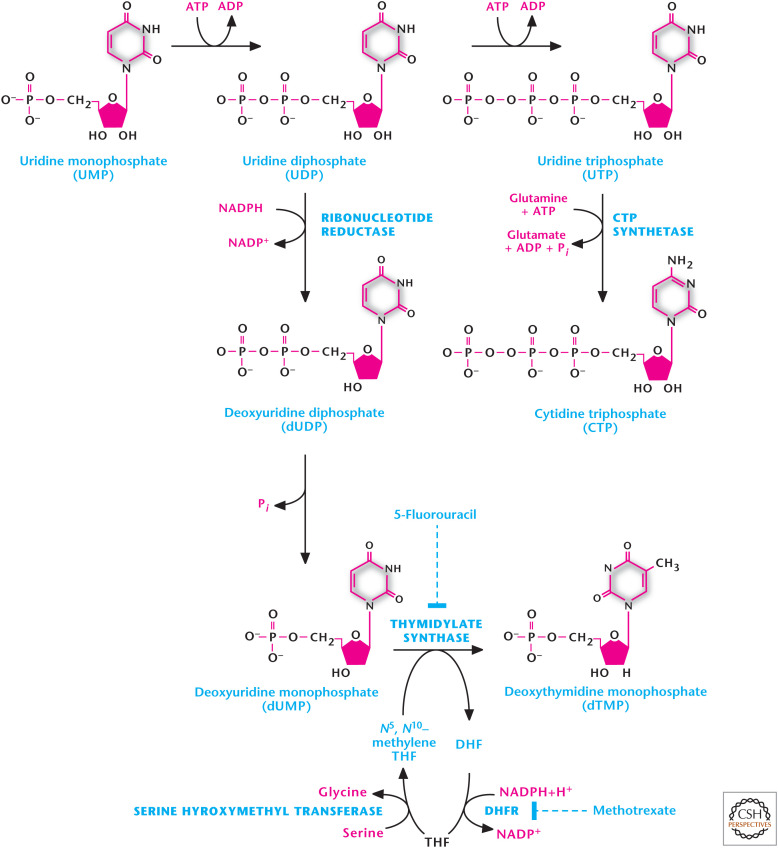
Uridine nucleotides are also the precursors for de novo synthesis of the thymine nucleotides. However, thymidine is only present in DNA, so the first step is the conversion of UDP to dUDP catalyzed by RR, which removes the 2′-hydroxyl, to create the deoxynucleotide. Subsequently, dUDP is converted to dUMP, a precursor for dTMP generation (Fig. 11). Any dUDP that is phosphorylated to dUTP is rapidly converted into dUMP by UTP diphosphatase, thus, reducing availability of dUTP and preventing erroneous incorporation of uracil into DNA. The nitrogen base thymine is 5-methyl uracil. Thus, methylation of dUMP to generate dTMP is catalyzed by thymidylate synthase using N5,N10-methylene THF as methyl donor (Fig. 11). The N5,N10-methylene THF is converted to dihydrofolate (DHF) in this reaction. To continue the thymidylate synthase reaction, THF is regenerated from DHF by dihydrofolate reductase (DHFR) coupling to conversion of NADPH to NADP+. THF is then converted to N5,N10-methylene THF by serine hydroxymethyl transferase using serine as substrate. The essential role of DHFR in thymidine nucleotide biosynthesis makes it a suitable target for cancer therapy because rapidly dividing cancer cells require thymidine nucleotides for DNA synthesis.
Figure 11.
Uridine nucleotides are also the precursors for de novo synthesis of the thymine nucleotides. UDP to dUDP is catalyzed by ribonucleotide reductase. Subsequently, dUDP is converted to dUMP. The methylation of dUMP to generate dTMP is catalyzed by thymidylate synthase using N5,N10-methylene THF as methyl donor.
Cells that are unable to regenerate THF suffer defective DNA synthesis and, eventually, death. Thus, it is therapeutically possible to target rapidly proliferating cancer cells through inhibitors of thymidylate synthase, such as 5-fluorouracil (Fig. 11), which is metabolically converted to 5-fluorodeoxyuridine monophosphate and becomes irreversibly bound to thymidylate synthase. The enzyme DHFR is reversibly inhibited by folate analogs, such as methotrexate (Fig. 11), thereby decreasing the supply of THF to diminish purine synthesis, as well as methylation of dUMP to dTMP.
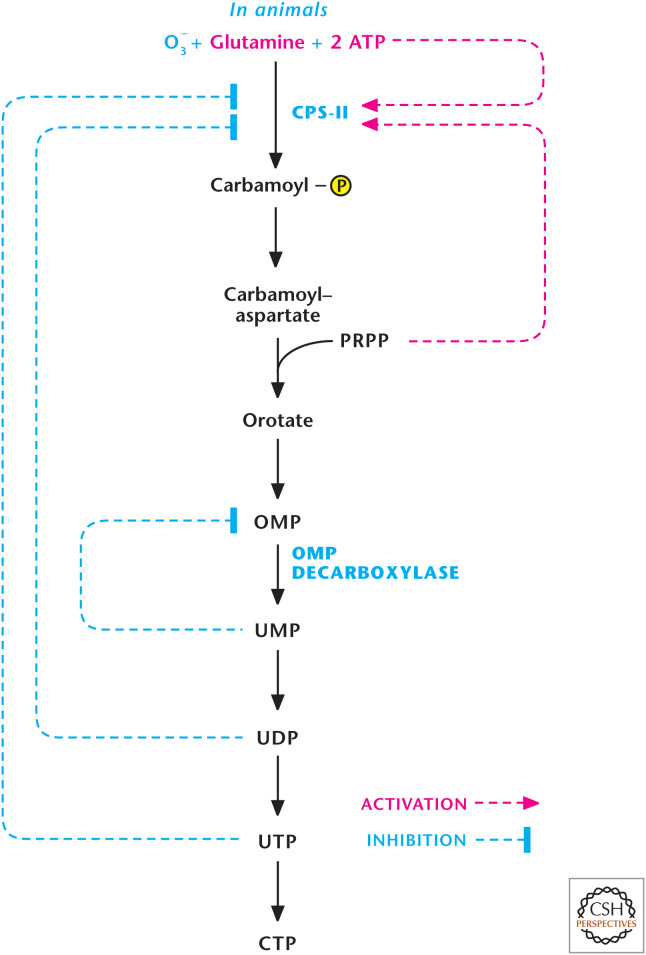
In animals, the regulation of pyrimidine synthesis occurs mainly at the first step catalyzed by CPS-II. It is activated by ATP and feedback inhibited by UDP and UTP (Fig. 12). It is also activated allosterically by PRPP, a UMP synthase substrate. PRPP is a feed-forward activator that helps coordinate pyrimidine and purine production because PRPP is also a substrate in the first step of purine biosynthesis. There is also regulation of orotidine 5′-phosphate decarboxylase, which is competitively inhibited by UMP and, to a lesser degree, CMP. Finally, CTP synthase is feedback-inhibited by CTP.
Figure 12.
Regulation of pyrimidine synthesis occurs at multiple steps. In animals, the regulation of pyrimidine synthesis occurs mainly at steps catalyzed by CPS-II and orotidine 5′-phosphate decarboxylase (OMP decarboxylase). (Adapted from Garrett and Grisham 1999.)
CATABOLISM OF PYRIMIDINE NUCLEOTIDES
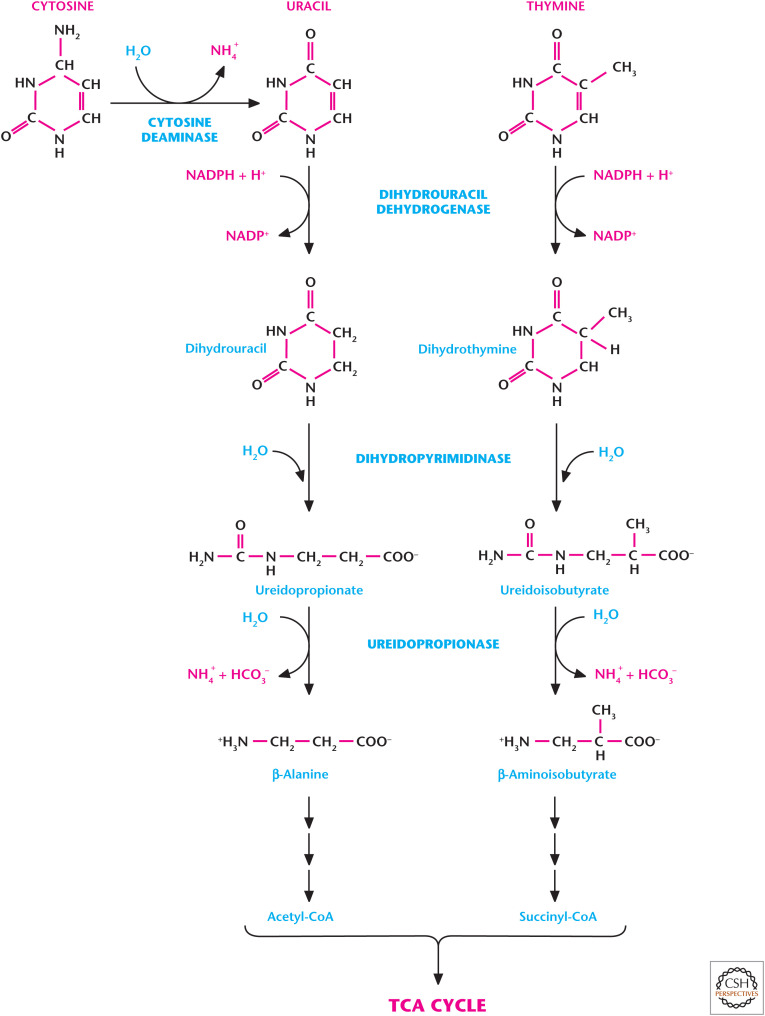
Unlike the purine ring, the pyrimidine ring can be completely degraded. Pyrimidine bases are water soluble, and the pyrimidine ring can undergo degradation to metabolites that can feed into metabolic pathways, such as the TCA cycle. Thus, pyrimidine salvage pathways are not as significant as purine salvage pathways. Catabolism of the pyrimidine nucleotides leads ultimately to β-alanine or β-aminoisobutyrate production, as well as NH3 and CO2. UMP and CMP are degraded to uracil and cytosine, which generate β-alanine, which is subsequently converted to malonyl-CoA and used for fatty acid synthesis (Fig. 13). dTMP is degraded to thymine, which is converted to β-aminoisobutyrate, and next converted to methylmalonyl-CoA, and then to succinyl-CoA used in the TCA cycle. β-Aminoisobutyrate and β-alanine can also be excreted.
Figure 13.
Catabolism of pyrimidine rings. Pyrimidine rings of cytosine and thymine can be completely degraded. Catabolism of the cytosine and thymine leads ultimately to β-alanine or β-aminoisobutyrate production, as well as NH3 and CO2. (Adapted from Moran et al. 2012, p. 570, by permission of Pearson Education Inc., Upper Saddle River, New Jersey.)
BOX 1.
GOUT
Clinical problems associated with nucleotide metabolism in humans are predominantly the result of abnormal catabolism of the purines and not pyrimidine. Remember, pyrimidine ring structure can be fully degraded, whereas the purine ring degradation results in the relatively insoluble compound uric acid. Elevated levels of uric acid can result in the uric acid crystal formation in the joints that characterizes gout or can form kidney stones. Gout is initiated by high levels of uric acid in the blood (hyperuricemia), as a result of either the overproduction or underexcretion of uric acid. The uric acid crystals engage the caspase-1-activating inflammasome, resulting in the production of interleukin-1β and IL-18. This produces severe inflammation and arthritis in the joints. In the majority of people, hyperuricemia is caused by underexcretion of uric acid, whereas a less common cause of hyperuricemia is from the overproduction of uric acid. Several identified mutations in the gene for PRPP synthetase result in increased availability of PRPP, causing overproduction of purines. Rare inherited disorders, such as Lesch–Nyhan syndrome, caused by a deficiency in HGPRT also cause hyperuricemia by decreasing salvage of hypoxanthine and guanine, therefore, increasing availability of PRPP. Administering allopurinol, a structural analog of hypoxanthine that inhibits xanthine oxidase, can treat gout. Allopurinol increases the levels of the more soluble metabolites xanthine, hypoxanthine, and guanine compared with uric acid.
NUCLEOTIDES AND SIGNALING
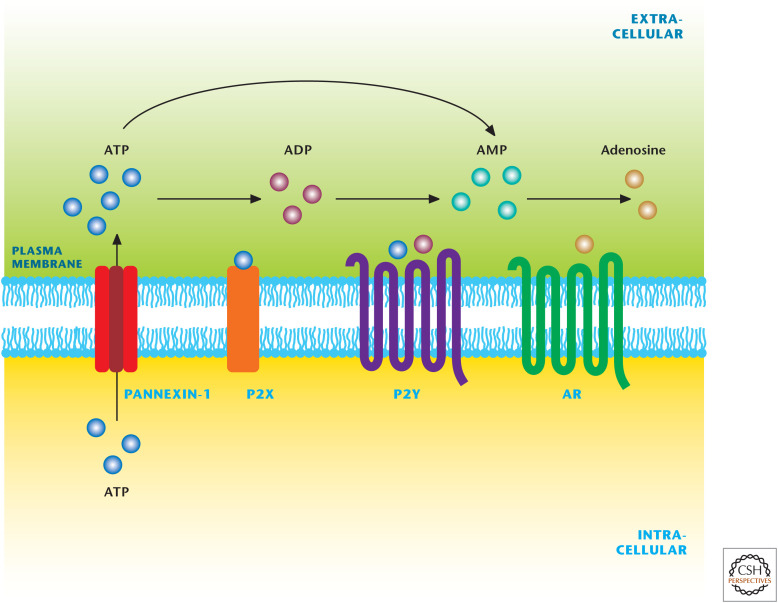
The cyclic nucleotides cAMP and cGMP are well-recognized signaling molecules. However, nucleotides themselves, as well as their breakdown products, have also emerged as important signaling molecules interacting with specific receptors (Fig. 14). Experiments dating back more than 50 years suggested that some nerves release ATP, and this later led to the proposal of “purinergic nerves.” Today, it is well recognized that extracellular ATP can regulate multiple physiological processes, including neurotransmission and inflammation, by activating nucleotide receptors called P2 purinoreceptors. ATP activates a family of P2X and P2Y receptors, whereas ADP activates only P2Y receptors. These receptors contain intrinsic pores and allow flow of ions that are coupled to intracellular second-messenger systems through heteromeric G proteins. ATP and ADP activate P2Y.
Figure 14.
Nucleotide signaling. Nucleotides are signaling molecules that regulate multiple physiological processes, including neurotransmission and inflammation. Extracellular ATP activates a family of ionotropic receptors (P2X) and metabotropic receptors (P2Y). Extracelluar ADP activate P2Y receptors. Extracellular adenosine activates G-protein-coupled cell-surface receptors, which are divided into four subtypes: A1, A2A, A2B, and A3 receptors. (Adapted from http://en.wikipedia.org/wiki/Purinergic_receptor#mediaviewer/File:Purinergic_signalling.jpg.)
Extracellular ATP can also be degraded to AMP and adenosine by the ectonucleotideases CD39 and CD73, respectively. Extracellular adenosine activates G-protein-coupled cell-surface receptors that are divided into four subtypes: A1, A2A, A2B, and A3. A1 and A3 receptors are coupled with G proteins, but can also elicit the release of calcium ions from intracellular stores. A2A and A2B receptors stimulate adenylyl cyclase by Gs proteins (Box 2). A2B receptors can also activate phospholipase C through Gq proteins. All the adenosine receptors can activate mitogen-activated protein kinase (MAPK) pathways, including extracellular signal-regulated kinase 1, -2, Jun amino-terminal kinase, and p38 MAPK. Ultimately, adenosine receptor signaling depends on the level of extracellular adenosine levels. Adenosine deaminase degrades extracellular adenosine, whereas cell-membrane-embedded nucleoside transporters shuttle extracellular adenosine into the intracellular space, thereby decreasing adenosine receptor activity.
BOX 2.
CAFFEINE
Caffeine is a purine that was first synthesized from raw materials by the German chemist Hermann Emil Fischer in 1895. Fischer was well known for discovering purines and awarded the Nobel Prize in Chemistry in 1902 “in recognition of the extraordinary services he has rendered by his work on sugar and purine syntheses.” Today, caffeine is unquestionably the most widely consumed substance that influences daily behavior. There is general perception that caffeine is “bad.” However, like alcohol, there are data to indicate that moderate consumption of caffeinated drinks can be beneficial. Recent case-control and prospective studies have linked caffeine consumption with a reduced risk of type 2 diabetes, chronic liver cirrhosis, and neurodegenerative diseases, such as Parkinson and Alzheimer diseases, dementia, and type 2 diabetes. Furthermore, long-term caffeine use is not associated with increased morbidity. The main pharmacological target of caffeine is to antagonize adenosine receptors, particularly A2A receptors. These observations, along with laboratory-based experiments linking adenosine to inflammation, cancer, and neurodegeneration, have promoted the development of specific adenosine receptor antagonists, such as Istradefylline, that have undergone clinical trials for Parkinson disease.
Footnotes
From the recent volume Navigating Metabolism by Navdeep S. Chandel
Additional Perspectives on Metabolism available at www.cshperspectives.org
SUGGESTED READING
*Reference is also in this collection.
- Antonioli L, Blandizzi C, Pacher P, Haskó G. 2013. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13: 842–857. 10.1038/nrc3613 [DOI] [PubMed] [Google Scholar]
- *.Chandel NS. 2020. NADPH—the forgotten reducing equivalent. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. 2012. Association of coffee drinking with total and cause-specific mortality. N Engl J Med 366: 1891–1904. 10.1056/NEJMoa1112010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett RH, Grisham CM. 1999. Biochemistry, 2nd ed. Thomson Brooks/Cole, Belmont, CA. [Google Scholar]
- Idzko M, Ferrari D, Eltzschig HK. 2014. Nucleotide signalling during inflammation. Nature 509: 310–317. 10.1038/nature13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LA, Horton RA, Scrimgeour G, Perry M. 2012. Principles of biochemistry, 5th ed. Pearson, Glenville, IL. [Google Scholar]
- Nelson DL, Cox MM. 2013. Lehninger principles of biochemistry, 6th ed. WH Freeman, New York. [Google Scholar]
- Neogi T. 2011. Clinical practice. Gout. N Engl J Med 364: 443–452. 10.1056/NEJMcp1001124 [DOI] [PubMed] [Google Scholar]
- Nordlund P, Reichard P. 2006. Ribonucleotide reductases. Annu Rev Biochem 75: 81–70. 10.1146/annurev.biochem.75.103004.142443 [DOI] [PubMed] [Google Scholar]