Abstract
Background and aims:
South Asians are at increased risk for cardiovascular disease (CVD). Aortic valve calcium (AVC) is associated with CVD risk and aortic stenosis. Elevated Lp(a) is a heritable risk factor for CVD and AVC. AVC prevalence and its association with Lp(a) have not been studied in South Asians.
Methods:
Among participants in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study (n=695), AVC prevalence and extent were compared to four race/ethnic groups in the Multi-Ethnic Study of Atherosclerosis (MESA) (n=4671). Multivariable regression was performed to evaluate associations between Lp(a) and AVC stratified by race/ethnic groups, adjusting for cardiovascular risk factors.
Results:
After age and sex adjustment, South Asians had higher median Lp(a) (17.0 mg/dL) compared to Whites (12.9 mg/dL), Hispanics (13.1 mg/dL) and Chinese Americans (12.9 mg/dL), and Blacks had highest Lp(a) levels (35.1 mg/dL). There were no differences in the odds of AVC in South Asians compared with Whites or Hispanics, after age and sex adjustment (p= 0.64 and 0.63, respectively). Odds of AVC was lower in Chinese (OR 0.35;95%CI 0.23–0.54) and somewhat lower in Blacks compared with South Asians (OR 0.76;0.56–1.04). There were no associations between Lp(a) and AVC presence or extent in South Asians. Lp(a) was associated with AVC only among Blacks and Whites.
Conclusions:
Although present in Whites and Blacks, there were no associations between Lp(a) and AVC in South Asians. These differences may be due to statistic power or race specific modifying factors that influences the effect of Lp(a) particles on AVC pathogenesis.
Keywords: Lipoprotein (a), Aortic valve calcium, South Asians, MASALA
Graphical Abstract

Introduction
South Asians are at increased risk for atherosclerotic cardiovascular disease (ASCVD) compared with many other groups.[1, 2] Although the etiology of higher risk of ASCVD in South Asians is multifactorial, dyslipidemia plays an important role. Dyslipidemia in South Asians is characterized by high levels of triglycerides, low levels of HDL cholesterol, and elevated levels of lipoprotein (a) [Lp(a)].[3] Lp(a) is a subclass of low-density lipoproteins distinguished by its apolipoprotein(a) component and is highly heritable.[4] An elevated Lp(a) level is an independent risk factor for ASCVD. [5–7]
Aortic valve calcium (AVC), measured from cardiac CT scans, is a subclinical measure associated with ASCVD events [8] and a potential precursor of clinical calcific aortic valve disease. The prevalence of AVC in South Asians has not been described in comparison with other racial/ethnic groups. The development of AVC is related mechanistically to atherosclerotic risk factors, including elevated LDL cholesterol. [9] Genetic variation in the LPA gene, mediated by Lp(a) levels, is strongly associated with AVC and clinical aortic stenosis.[10] We previously demonstrated that elevated Lp(a) is associated with AVC in White and Black individuals, but this association was not seen in Hispanic or Chinese participants;[11] however, the association between Lp(a) and AVC in South Asians is not known.
Materials and methods
Study populations
The Mediators of Atherosclerosis in South Asians Living in America (MASALA) study was designed to identify factors that lead to heart disease in South Asians. MASALA is a community based prospective cohort of 906 participants of South Asian descent from two clinical sites (San Francisco Bay area at the University of California, San Francisco and the greater Chicago area at Northwestern University). MASALA participants are 98% immigrant from a variety of countries from South Asia with 84% from India, 5% from Pakistan and <5% from Sri Lanka, Bangladesh, Nepal or Myanmar. [12] The baseline examination occurred from 2010 to 2013 and included participants 40–84 years of age without known clinical cardiovascular disease. [12] The second clinical examination occurred from 2015 to 2018 and included 749 (83%) returning participants.
The MASALA study uses methods similar to the Multi-Ethnic study of Atherosclerosis (MESA) in order to compare South Asians to other ethnic groups. MESA is a longitudinal cohort study of 6,814 participants recruited from 6 different clinical centers (Johns Hopkins University, Baltimore, MD; Columbia University, New York, NY; Northwestern University, Chicago, IL; UCLA, Los Angeles, CA; University of Minnesota, Twin cities, MN; Wake Forest University, Winston Salem, NC) and includes White, Black, Hispanic and Chinese American participants. The participants were between the ages of 45–84 years at baseline (2000–2002), with no clinical evidence of cardiovascular disease. The MESA study design has been described in detail previously. [13]
For both MASALA and MESA, the self-reported data collected included demographic information, medication use and tobacco use.[12] Other measures included systolic blood pressure, use of blood pressure medicines, weight, height, waist circumference, fasting plasma glucose and the standard lipid profile, which included fasting total and HDL cholesterol, and triglycerides. Type 2 diabetes was defined as fasting plasma glucose ≥ 126 mg/dL or use of anti-diabetes medications. [12, 14].
Lipid measurements
Lp(a) mass concentration was measured in baseline specimens in MESA by the Health Diagnostics Laboratory (Richmond, Virginia) and in MASALA by the University of Minnesota using the same instrument and assay in both labs. The assay consists of a latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan), which includes five independent calibrators of varying apo(a) sizes to control for apo(a) size heterogeneity. [12, 15, 16] MESA participants who were taking lipid-lowering medication at baseline (n=1090) and a subcohort of 1000 MESA participants with limited specimen volume did not have Lp(a) measurements performed. Fasting total cholesterol, triglycerides and HDL-C were measured. LDL-C was calculated with the Friedewald equation. [11]
Aortic valve calcium
AVC was measured using non-contrast gated cardiac computed tomography imaging acquired similarly in both studies at the second visit for MASALA and the first visit for MESA. MESA participants were each scanned twice at the first visit, and the first scan results were used for this analysis. MASALA participants were scanned once at the second visit. The MESA scanning protocol has been previously published.[17, 18]. The scans were interpreted at the Los Angeles Biomedical Research institute at Harbor University of California Los Angeles (UCLA) by readers blinded to the clinical information. [11, 19, 20] Aortic valve calcium (AVC) was measured using the Agatston method to determine the presence and extent of calcium and excluded the aortic annulus. [21]
Institutional review board approval was obtained at all MASALA and MESA sites, and all participants gave informed consent.
Data analyses
In MESA, there were 2 participants with missing AVC measurements and 2141 without Lp(a), leading to a sample size of 4671 for this analysis. In MASALA, 171 participants from the recruited 906 participants were excluded from these analyses based on age <45 at exam 2, to ensure similar age distribution to MESA, and 40 were missing AVC measurements leading to a sample size of 695 from MASALA. The cross-sectional analysis from the MESA baseline exam was compared to a cross-sectional analysis from MASALA exam 1 where Lp(a) was measured, and exam 2, which includes AVC. The prevalence of AVC was determined in South Asian, White, Black, Chinese and Hispanic participants. Multivariable logistic and linear regressions were used to compare the age and sex adjusted odds and odds ratios of AVC and the distribution of Lp(a) among South Asians and each of the other racial/ethnic groups. Multivariable linear and logistic regressions were performed to evaluate the associations between Lp(a) and AVC stratified by racial/ethnic group. Lp(a) was evaluated as a continuous variable with log transformation and also at cut points >30 or >50 mg/dL, as used in prior MESA analyses.[11] AVC was evaluated as a dichotomous variable (present versus absent) and as a continuous variable [log (AVC+1)]. The presence of AVC was defined as Agatston score > 0. Multivariable models were adjusted for age, sex, and CVD risk factors including smoking (current versus former/never), antihypertensive medications, diabetes, systolic blood pressure, total and HDL cholesterol and body mass index (BMI). The covariates in MASALA were taken from exam 2, when the CT scans were performed. The interaction between continuous Lp(a) and extent of AVC by South Asian compared to Whites and to Blacks was examined by including an interaction term in the regression models.
Results
Sample characteristics
Table 1 summarizes the characteristic of MESA and MASALA participants. Mean age was lower in MASALA compared to the other ethnicities in MESA. MASALA also included a higher proportion of female participants compared to MESA. South Asians in MASALA had the lowest prevalence of current smokers (3%) compared to other ethnicities in MESA. Chinese Americans also had a lower percentage of smokers (6%) compared to Blacks (18%), Whites (11%) and Hispanics (14%). South Asian participants had a mean BMI comparable to Whites (South Asians 26.5 kg/m2 and Whites 27 kg/m2), whereas Blacks had the highest mean BMI (30 kg/m2) and Chinese the lowest mean BMI at (23.8 kg/m2). South Asians had the highest prevalence of diabetes (20%) compared to other ethnicities, whereas Whites had the lowest (5%). South Asians had the lowest mean total cholesterol (187 mg/dL) while Whites had the highest mean HDL-C (53 mg/dL). Blacks had the highest mean systolic blood pressure (132 mmHg), with 45.6% participants on antihypertensive medications.
Table 1:
Participant characteristics
| MASALA | MESA | ||||
|---|---|---|---|---|---|
| N | S. Asians N= 695 | Black N=1345 | White N=1705 | Hispanic N=1063 | Chinese N=558 |
| Age (years) | 59.3 ±9.2 | 61.7 ±10.3 | 62.4 ±10.3 | 61.2 ±10.4 | 62.3 ±10.4 |
| Female (%) | 57.0 | 54.0 | 52.3 | 51.5 | 51.4 |
| Current smoking (%) | 3.0 | 18.0 | 11.0 | 13.6 | 5.6 |
| On BP medications (%) | 36.7 | 45.6 | 28.9 | 28.6 | 24.7 |
| SBP (mmHg) | 127.7 ±17.3 | 131.5 ±21.9 | 122.8 ±19.9 | 126.3 ±21.6 | 124 ±21.8 |
| HDL cholesterol (mg/dL) | 51.0 ±14.8 | 52.4 ±15.4 | 53.0 ±16.2 | 47.2 ±12.8 | 50.1 ±13.3 |
| Total cholesterol (mg/dL) | 187.1 ±40.8 | 191.1 ±36.3 | 199 ±34.6 | 199.3 ±37.3 | 195.3 ±31.2 |
| Body mass index (kg/m2) | 26.5 ±4.0 | 30.0 ±5.8 | 27.5 ±5.1 | 29.4 ±5.1 | 23.8 ±3.3 |
| Diabetes (%) | 20.0 | 12.6 | 4.6 | 14.9 | 7.5 |
| Aortic valve calcium prevalence (%) | 10.7 | 11.7 | 14.6 | 13.2 | 6.6 |
| Aortic valve extent (AU median IQR)a | 47 (12–158) | 64 (17–127) | 67 (18–163) | 68 (29–228) | 32 (11–62) |
| Lp(a) mg/dL (median IQR) | 17.0(9.0–32.0) | 35.1 (20.4–61.6) | 12.9 (5.8–29.5) | 13.1 (6.3–28.8) | 12.9 (7.7–23.4) |
| Lp(a) > 30 mg/dL (%) | 26.6 | 57.3 | 24.7 | 24.3 | 19.3 |
| Lp(a) > 50 mg/dL (%) | 13.2 | 33.0 | 14.9 | 13.1 | 9.7 |
Mean ± standard deviation or percent or median and interquartile range.
MASALA exam 2. MESA exam 1.
Agatston units among participants with AVC present.
Median Lp(a) and prevalence of AVC in different ethnicities
The unadjusted median Lp(a) and interquartile ranges and the prevalence of Lp(a) > 30 mg/dl, Lp(a) > 50 mg/dl and AVC, stratified by race/ethnicity, are listed in Table 1. South Asians had higher median Lp(a) (17 mg/dL) compared to Whites (12.9 mg/d), Hispanics (13.1 mg/dL) or Chinese (12.9 mg/dL) Americans. Blacks had the highest median Lp(a) levels at 35.1 mg/dL (all differences compared to South Asians, age and sex adjusted p<0.001). Similar patterns were seen for Lp(a) > 30 mg/dl. There were no significant differences in the odds of AVC in South Asians compared with Whites or Hispanics, after adjusting for differences in age and sex (p= 0.64 and 0.63, respectively). The odds of AVC was lower in Chinese than in South Asians (OR 0.35; 95% CI 0.23–0.54, p<0.001), with a trend towards lower AVC in Blacks compared with South Asians (OR 0.76; 0.56–1.04, p= 0.08).
Lp(a) associations with AVC
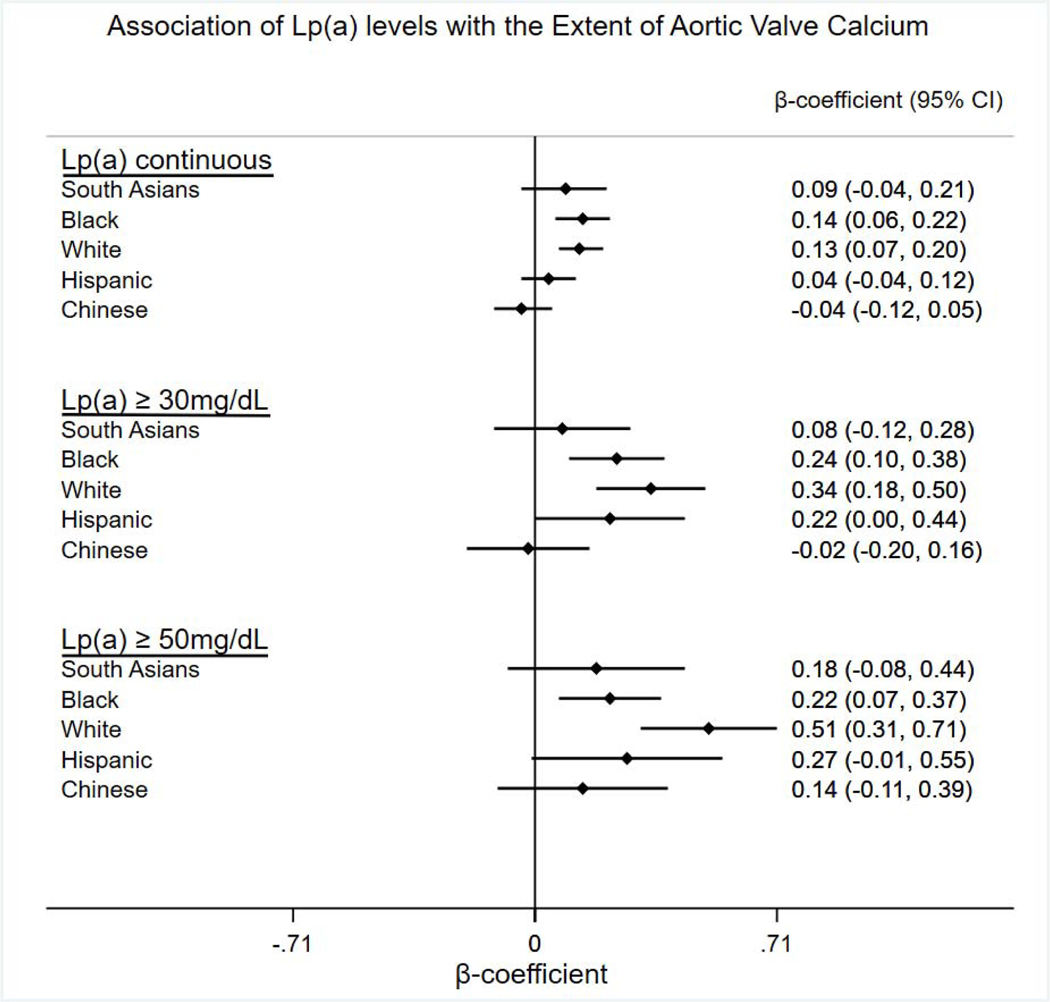
Table 2 shows the associations between Lp(a) levels and AVC stratified by the four race/ethnic groups in MESA and South Asians in MASALA. No statistically significant associations were observed between Lp(a) levels and AVC in South Asians or Chinese; however, Lp(a) was significantly associated with AVC in White and Black participants. There were trends towards significant associations between Lp(a) levels >30 mg/dl and >50 mg/dl and AVC in Hispanics, but not with Lp(a) as a continuous measure. An interaction test demonstrated no statistically significant differences in the associations between log Lp(a) and log (AVC+1) between South Asians and either Whites (p=0.37) or Blacks (p=0.35). Participants on lipid lowering medications were excluded from Lp(a) measurements in MESA. In MASALA, 33.7% of participants were taking statin medication; however, there was no association between statin use and AVC in MASALA (p<0.33). Additionally, there was no association between Lp(a) and AVC in non-statin users in MASALA as well (p<0.40).
Table 2:
Association of Lp(a) levels with the extent of aortic valve calcium (AVC)
| MASALA | MESA | ||||
|---|---|---|---|---|---|
| S. Asians N= 695 | Black N=1345 | White N=1705 | Hispanic N=1063 | Chinese N=558 | |
| log Lp(a) continuous | |||||
| ß coefficient (95% CI) | 0.09 (−0.04,0.21) | 0.14 (0.06, 0.22) | 0.13 (0.07, 0.20) | 0.04 (−0.04,0.12) | −0.04 (−0.12,0.05) |
| p value | 0.18 | 0.0009 | <0.0001 | 0.35 | 0.41 |
| Lp(a) ≥ 30 mg/dL | |||||
| ß coefficient (95% CI) | 0.08 (−0.12, 0.28) | 0.24 (0.10, 0.38) | 0.34 (0.18, 0.50) | 0.22 (0.00, 0.44) | −0.02 (−0.20, 0.16) |
| p value | 0.45 | 0.001 | <0.0001 | 0.05 | 0.84 |
| Lp(a) ≥ 50 mg/dL | |||||
| ß coefficient (95% CI) | 0.18 (−0.08, 0.44) | 0.22 (0.07, 0.37) | 0.51 (0.31, 0.71) | 0.27 (−0.01, 0.55) | 0.14 (−0.11, 0.39) |
| p value | 0.18 | 0.005 | <0.0001 | 0.06 | 0.26 |
Linear regression models adjusted for age, sex, systolic BP, use of anti-hypertensive medications, current smoking, diabetes, total and HDL cholesterol.
In addition to continuous AVC as the outcome in the analyses, we also tested associations between Lp(a) and the presence of AVC (Agatston score > 0) adjusted for the covariates mentioned above. Similar results were seen as in the prior analyses as shown in Table 3. There were associations between Lp(a) and the presence of AVC in Whites and Blacks, with trends in Hispanics and no associations in South Asians. There was an associations between Lp(a) >50 mg/dl and the presence of AVC in Chinese, with no associations with Lp(a)> 30 mg/dl or Lp(a) as a continuous measure.
Table 3:
Association of Lp(a) levels with the presence of aortic valve calcium (AVC)
| MASALA | MESA | ||||
|---|---|---|---|---|---|
| S. Asians N= 695 | Black N=1345 | White N=1705 | Hispanic N=1063 | Chinese N=558 | |
| log Lp(a) continuous | |||||
| Odds Ratio (95% CI) | 1.39 (0.93, 2.07) | 1.49 (1.18, 1.88) | 1.38 (1.19, 1.59) | 1.05 (0.88, 1.27) | 1.05 (0.66, 1.67) |
| p value | 0.11 | 0.001 | <0.0001 | 0.57 | 0.83 |
| Lp(a) ≥ 30mg/dL | |||||
| Odds Ratio (95% CI) | 1.45 (0.77, 2.74) | 1.85 (1.24, 2.74) | 2.12 (1.52, 2.96) | 1.48 (0.94, 2.33) | 1.35 (0.52, 3.51) |
| p value | 0.26 | 0.002 | <0.0001 | 0.09 | 0.54 |
| Lp(a) ≥ 50mg/dL | |||||
| Odds Ratio (95% CI) | 1.55 (0.71, 3.37) | 1.73 (1.20, 2.51) | 2.71 (1.85, 3.97) | 1.72 (0.98, 3.03) | 3.73 (1.10, 12.7) |
| p value | 0.27 | 0.003 | <0.0001 | 0.06 | 0.03 |
Logistic regression models adjusted for age, sex, systolic BP, use of anti-hypertensive medications, current smoking, diabetes, total and HDL cholesterol; AVC presence, defined as Agatston score>0
Discussion
South Asians in MASALA had higher median Lp(a) compared to Whites, Hispanics and Chinese Americans in MESA, with Blacks having the highest median Lp(a). After age and sex adjustment, South Asians had similar AVC prevalence to Whites and Hispanics, higher than in Chinese Americans, and somewhat higher when compared to Blacks. We found no statistically significant associations between Lp(a) levels and AVC in South Asians, after adjusting for cardiovascular risk factors. As previously demonstrated in MESA [11], we also found significant associations between Lp(a) and AVC in Whites and Blacks, but there were no consistent associations between Lp(a) and AVC in Hispanics and Chinese Americans.
A potential reason for the lack of association between Lp(a) and AVC in South Asians is the smaller sample size than in Whites and Blacks. This limits statistical power in detecting the relationship between Lp(a) and AVC in South Asians. The point estimates for South Asians are all in the same direction as those for White and Blacks. As previously shown, there is a strong genetic link between Lp(a) and AVC and incident aortic valve stenosis, so we expected to find a robust association in South Asians.[10].
It is interesting to note that Blacks have higher Lp(a) levels than South Asians, yet Blacks have a somewhat lower prevalence of AVC than South Asians. Lp(a) is associated with AVC in Blacks but not in South Asians. In contrast, South Asians have higher Lp(a) levels than Whites, but similar AVC prevalence, but there is no association of Lp(a) with AVC in South Asians whereas there is an association in Whites. These results suggest that the associations between Lp(a) and AVC could be driven by race specific factors that influence the effect of Lp(a) particles on the pathogenesis of AVC, and perhaps other factors may play a role other than plasma Lp(a) levels alone. The observed lack of association in South Asians is likely multifactorial. There may be racial/ethnic differences in the composition of Lp(a) particles or in Lp(a) associated factors, such as apolipoprotein-C III, autotaxin or oxidized phospholipids. [22–25]. Prior studies in MASALA also demonstrated no association between Lp(a) levels and coronary artery calcium [26], and racial/ethnic differences in associations between Lp(a) and carotid plaque were noted in MESA.[27]
There is a growing interest in novel therapies aimed at lowering Lp(a). [28] This highlights the importance of understanding the association between Lp(a) and AVC in South Asians, since it is hypothesized that reducing Lp(a) levels might reduce AVC and risk for aortic stenosis.
Strengths of this study include the standardization of Lp(a) and AVC measurements. Lp(a) mass concentrations were measured with the same assay in both MESA and MASALA. This is also the only study looking at Lp(a) and AVC in South Asians in comparison to other race/ethnic groups. Limitations of the study include the difference in timing of the AVC and Lp(a) measurements in MASALA. AVC was measured in exam 2 while Lp(a) was measured during exam 1; however, Lp(a) is genetically determined making it stable over time. [29] There were smaller sample sizes in South Asians and Chinese, compared to those for Whites and Blacks, and the prevalence of AVC was lower in Chinese. There was 91% power to find a significant association in Whites, with an alpha=0.05. To see the same association in South Asians (n=695), the power was only 56%. However, this is the largest known sample of South Asians who have had both Lp(a) and AVC measured, to our knowledge. Another potential limitation is that 33.7% of MASALA participants were taking statins while MESA participants on lipid lowering agents were excluded from this analysis; however, there was no association between statins and AVC in MASALA (p<0.33), suggesting that statin use is not an important confounder. In addition, randomized trials have not demonstrated an effect of statin therapy on the progression of aortic valve disease. [30] Furthermore, MASALA participants were recruited from the San Francisco Bay and Chicago metropolitan region and are predominantly of Indian descent, which limits the generalizability of the results to all South Asians in the United States. Additional larger studies, with greater statistical power, are required to truly understand the potential association between Lp(a) and its relationship to AVC and aortic stenosis in South Asians.
Conclusions
In summary, there was no statistically significant association between Lp(a) and AVC in South Asians. This lack of association despite the strong genetic link between Lp(a) and AVC is likely multifactorial, and additional larger studies need to be conducted to truly understand race specific differences and other variables that affect this association.
Figure 1:
Associations between Lp(a) levels and extent of aortic valve calcium stratified by racial/ethnic groups.
aBeta coefficients and 95% confidence intervals.
bLinear regression analysis adjusted for age, sex, and CVD risk factors including smoking (current versus former/never), antihypertensive medications, diabetes, systolic blood pressure, total and HDL cholesterol and body mass index (BMI). cLp(a) continuous= Log (AVC + 1).
Highlights.
South Asians are at increased risk of atherosclerotic cardiovascular disease
Lipoprotein (a) levels are associated with aortic valve calcium
South Asians have higher median Lipoprotein (a) than Whites, Hispanics and Chinese
Lipoprotein (a) is associated with aortic valve calcium in Whites and Blacks
No association between Lipoprotein (a) and Aortic valve calcium in South Asians
Financial support
The MASALA study was supported by the NIH grant no. 1R01HL093009, 2R01HL093009, and R01 HL120725. Data collection at UCSF was also supported by NIH/NCRR UCSF-CTSI grant number UL1 RR024131. Lp(a) measurements were supported by the Cliff Lede Family Charitable Foundation.
The MESA research was supported by R01 HL071739 and contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
The sponsors did not play a significant role in the analysis, interpretation, and presentation of these results.
The graphical abstract was designed by Oluwaseun Fashanu MBBS, MPH using STATA.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest
Parag Joshi: Grant support: AHA, NovoNordisk, NASA; consulting: Regeneron, Bayer; Equity—G3 Therapeutics; Site investigator (funds to institution): Novartis, AstraZeneca. Michael Blaha: Grants: NIH, FDA, AHA, Aetna Foundation, Amgen Foundation; advisory boards: Amgen, Sanofi, Regeneron, Novartis, Novo Nordisk, Bayer, Akcea; consulting: Zogenix, Tricida, Gilead; executive committee: HORIZONSLp(a) – Novartis. Matthew Budoff: Grants: General electric.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed ST, et al. , Premature Coronary Heart Disease in South Asians: Burden and Determinants. Curr Atheroscler Rep, 2018. 20(1): p. 6. [DOI] [PubMed] [Google Scholar]
- 2.Volgman AS, et al. , Atherosclerotic Cardiovascular Disease in South Asians in the United States: Epidemiology, Risk Factors, and Treatments: A Scientific Statement From the American Heart Association. Circulation, 2018. 138(1): p. e1–e34. [DOI] [PubMed] [Google Scholar]
- 3.Makshood M, Post WS, and Kanaya AM, Lipids in South Asians: Epidemiology and Management. Current Cardiovascular Risk Reports, 2019. 13(8): p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglund L. and Ramakrishnan R, Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler Thromb Vasc Biol, 2004. 24(12): p. 2219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R, et al. , Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med, 2009. 361(26): p. 2518–28. [DOI] [PubMed] [Google Scholar]
- 6.Erqou S, et al. , Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. Jama, 2009. 302(4): p. 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamstrup PR, et al. , Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. Jama, 2009. 301(22): p. 2331–9. [DOI] [PubMed] [Google Scholar]
- 8.Owens DS, et al. , Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC. Cardiovascular imaging, 2012. 5(6): p. 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JG, et al. , Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. Jama, 2014. 312(17): p. 1764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanassoulis G, et al. , Genetic associations with valvular calcification and aortic stenosis. N Engl J Med, 2013. 368(6): p. 503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao J, et al. , Lipoprotein(a) Levels Are Associated With Subclinical Calcific Aortic Valve Disease in White and Black Individuals: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol, 2016. 36(5): p. 1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanaya AM, et al. , Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol, 2013. 36(12): p. 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bild DE, et al. , Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol, 2002. 156(9): p. 871–81. [DOI] [PubMed] [Google Scholar]
- 14.Flowers E, et al. , Body Composition and Diabetes Risk in South Asians: Findings From the MASALA and MESA Studies. Diabetes Care, 2019. 42(5): p. 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcovina SM, et al. , Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin Chem, 2000. 46(12): p. 1956–67. [PubMed] [Google Scholar]
- 16.Huffman MD, et al. , Evaluating the Potential Association Between Lipoprotein(a) and Atherosclerosis (from the Mediators of Atherosclerosis Among South Asians Living in America Cohort). Am J Cardiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr JJ, et al. , Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology, 2005. 234(1): p. 35–43. [DOI] [PubMed] [Google Scholar]
- 18.Detrano RC, et al. , Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology, 2005. 236(2): p. 477–84. [DOI] [PubMed] [Google Scholar]
- 19.Katz R, et al. , Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation, 2006. 113(17): p. 2113–9. [DOI] [PubMed] [Google Scholar]
- 20.Kanaya AM, et al. , Comparing coronary artery calcium among U.S. South Asians with four racial/ethnic groups: the MASALA and MESA studies. Atherosclerosis, 2014. 234(1): p. 102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budoff MJ, et al. , Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol, 2006. 13(2): p. 166–72. [DOI] [PubMed] [Google Scholar]
- 22.Capoulade R, et al. , ApoCIII-Lp(a) complexes in conjunction with Lp(a)-OxPL predict rapid progression of aortic stenosis. Heart, 2020. 106(10): p. 738–745. [DOI] [PubMed] [Google Scholar]
- 23.Tsimikas S, et al. , Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation, 2009. 119(13): p. 1711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nsaibia MJ, et al. , Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J Intern Med, 2016. 280(5): p. 509–517. [DOI] [PubMed] [Google Scholar]
- 25.Torzewski M, et al. , Lipoprotein(a) Associated Molecules are Prominent Components in Plasma and Valve Leaflets in Calcific Aortic Valve Stenosis. JACC Basic Transl Sci, 2017. 2(3): p. 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huffman MD, et al. , Evaluating the Potential Association Between Lipoprotein(a) and Atherosclerosis (from the Mediators of Atherosclerosis Among South Asians Living in America Cohort). Am J Cardiol, 2019. 123(6): p. 919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steffen BT, et al. , Race-Based Differences in Lipoprotein(a)-Associated Risk of Carotid Atherosclerosis. Arterioscler Thromb Vasc Biol, 2019. 39(3): p. 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsimikas S, et al. , Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med, 2020. 382(3): p. 244–255. [DOI] [PubMed] [Google Scholar]
- 29.Kronenberg F, Human Genetics and the Causal Role of Lipoprotein(a) for Various Diseases. Cardiovascular drugs and therapy, 2016. 30(1): p. 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, et al. , The effect of statins on valve function and calcification in aortic stenosis: A meta-analysis. Atherosclerosis, 2016. 246: p. 318–24. [DOI] [PubMed] [Google Scholar]