Abstract
Background:
Contrast-induced encephalopathy (CIE) is a rare condition that occurs after intravenous or intra-arterial contrast agent administration. Patients generally show different ranges of neurological deficits, which generally resolve themselves spontaneously within 24–48 h or in rare cases within 2 weeks.
Case Description:
We report a case of CIE in a 54-year-old woman during retreatment for recanalization of communicating anterior artery aneurysm and with no history of allergic reaction to contrast agent. After the procedure, the patient developed right hemiplegia and complete aphasia; an MRI performed at 6 days excluded any signs of new ischemia and revealed a hyperintense signal on FLAIR sequences in the left cortical precentral gyrus corresponding to a hyperintense signal on DWI, suggesting a vasogenic edema. After 6 months, she clinically improved even if her previous neurological status was never restored while radiological findings did not change.
Conclusion:
According to the literature, many risk factors may play a role in the pathogenesis of CIE: we hypothesized that, among all of them, chronic hypertension and previous cerebral ischemic lesions were the most important in our case. Further studies are necessary to find the correlation between possible risk factors and neurotoxicity.
Keywords: Brain aneurysm, Cerebral angiography, Contrast agent, Encephalopathy, Neurological deficit

INTRODUCTION
Contrast-induced encephalopathy (CIE) is a rare complication that may occur after intravenous or intra-arterial contrast agent administration. Patients experience a wide range of neurological deficits, often mimicking a postprocedural stroke, which generally resolve themselves spontaneously within 24–48 h or in a few cases 10–15 days. The prognosis is not always favorable: in a limited number of cases, a persistent (>15 days) or permanent neurological impairment and even death have been described. Moreover, only presumable risk factors have been reported.
We report the case of a patient with a previous cerebral ischemia and no history of allergic reaction to contrast agent who developed a permanent neurological impairment after contrast medium administration during a cerebral angiography. Furthermore, we update a literature review regarding adverse clinical outcomes following contrast medium exams.
CASE REPORT
A 54-year-old woman was admitted to our hospital in a comatose state. Radiological examinations revealed a subarachnoid hemorrhage from a ruptured aneurysm of the anterior communicating artery. She underwent urgent external ventricular drainage positioning followed by cerebral angiography, resulting in a successful occlusion of the aneurysm with endovascular coiling. After the procedure, she developed a status epilepticus, which was efficaciously treated. A cerebral angiography showed a moderate distal vasospasm and an MRI demonstrated an ischemic area in the left precentral frontal lobe with no clinical correlation. She was discharged and transferred to a rehabilitation hospital.
Seven months later, a follow-up cerebral angiography showed a recanalization of the aneurysm, which required retreatment. At that point, she had also developed severe hypertension and was able to suspend antiepileptic treatment. During retreatment, intravenous nimodipine was administered because of ongoing vasospasm throughout the procedure. Endovascular treatment was prolonged due to a complex vascular anatomy: after unsuccessfully attempting to perform a stent-assisted coiling procedure, a coiling-only procedure was carried out, thus resulting in a much larger volume of contrast medium administration. Final sequences documented no vessel occlusion or emboli. The patient emerged from general anesthesia with severe right hemiparesis and complete aphasia. A postoperative CT scan showed an edematous area in the left cerebral hemisphere, an abnormal subarachnoid contrast enhancement zone (probably secondary to contrast extravasation) and a focal left parasagittal frontal hyperdense area [Figure 1]. A new CT scan performed at about 20 h after a neurological deflection showed a new left parasagittal frontal hypodense area at the vertex and in the left precentral gyrus (territory of the middle cerebral artery). It also showed a resorption of the subarachnoid hyperdensity, supporting the hypothesis of contrast medium extravasation due to disruption of the blood–brain barrier (BBB) [Figure 1]. An EEG showed a significant epileptic activity, whereupon the patient was administered antiepileptic drugs.
Figure 1:
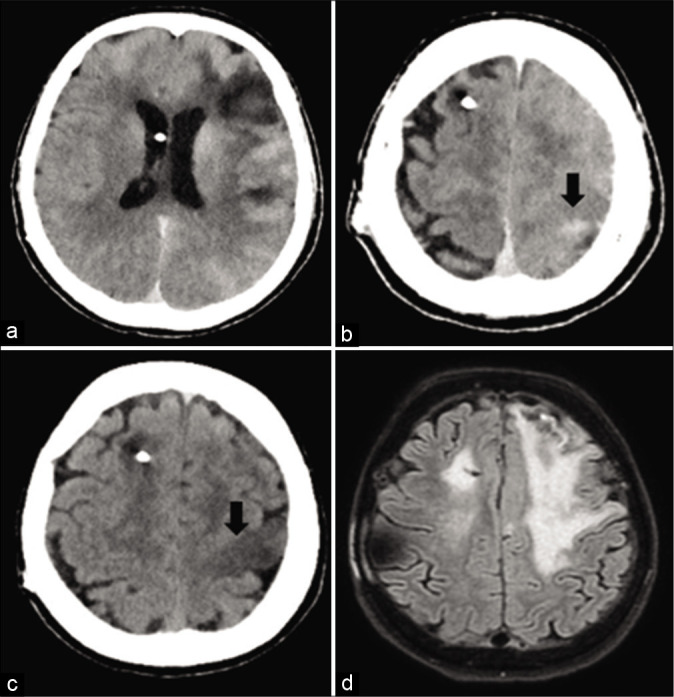
(a and b): a postoperative CT scan shows an abnormal subarachnoid contrast enhancement zone and a focal left parasagittal frontal hyperdense area. (c) A CT scan performed at 20 h shows a resorption of the subarachnoid hyperdensity and a new left parasagittal frontal hypodense area. (d) An MRI performed at 6 days reveals a hyperintense signal on FLAIR sequences in the left cortical precentral gyrus (as in case of vasogenic edema), excluding any signs of new ischemia.
During the following days, her speech and right hemiparesis gradually improved, although she could only compose short sentences and was still not able to walk autonomously. An MRI performed at 6 days revealed a hyperintense signal on FLAIR sequences in the left cortical precentral gyrus (as in case of vasogenic edema), corresponding to a hyperintense signal on DWI sequences with no alteration of the ADC maps, thus excluding any signs of new ischemia [Figure 1]. An MRI performed 6 months later showed the same radiological alterations [Figure 2].
Figure 2:
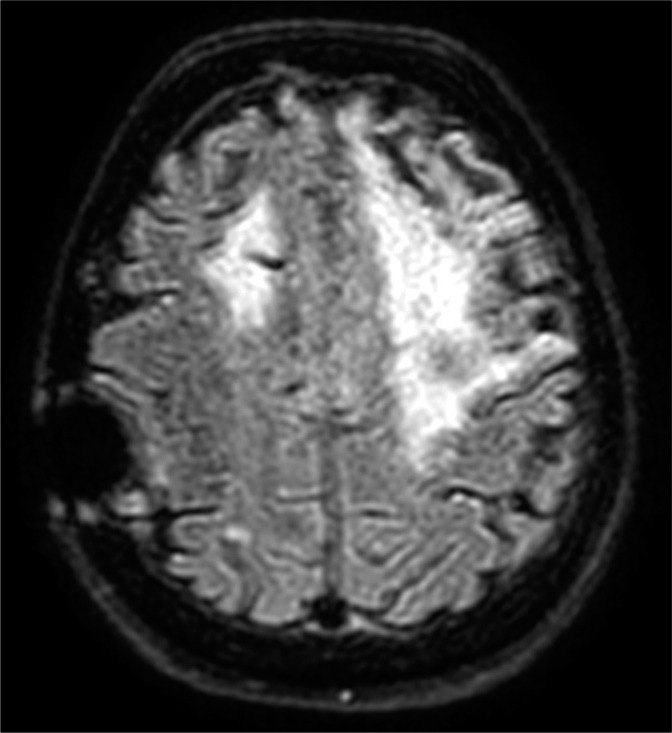
An MRI performed at 6 months shows the same hyperintense signal on FLAIR.
After 6 months of rehabilitation, the patient improved clinically, being left with a mild dysarthria and a mild right monoparesis, but having regained the capacity to walk and live autonomously.
The patient’s clinical course and radiological findings suggested a possible permanent neurotoxic effect of contrast medium during coil embolization.
DISCUSSION
CIE is a form of neurotoxicity arising from contrast medium. It is mostly described as a transient phenomenon with a favorable prognosis.[6] Cortical blindness is the most frequent clinical symptom, but patients may experience confusion, transient global amnesia, seizures, and focal neurological deficits such as hemiplegia or aphasia, or coma. A minority (approximately 15%) may develop permanent neurological deficits or fatal cerebral edema.[1,4,8] No exact time period between contrast injection and appearance of clinical symptom has been established, but they usually appear soon after contrast administration and completely resolve within 24–48 h or in a few cases 10–15 days. Diagnosis is undertaken by demonstrating typical CT findings in a symptomatic patient when ischemic or hemorrhagic stroke has been excluded.[5] Chronic hypertension is the most important risk factor for the development of CIE, as it predisposes the patient to cerebral autoregulation dysfunction and facilitates disruption of the BBB. Others risk factors are renal failure, diabetes mellitus, previous reactions to contrast agents, intracranial pathology, and the use of a large volume of contrast medium.[8,7] The posterior vascular territories seem to be the most susceptible to damage because of their sympathetic innervation, which predisposes one to a decrease in vascular autoregulatory capacity.[3] Neuroimaging plays an important role in differential diagnosis. A CT scan may show alterations mimicking cortical/subcortical contrast enhancement or edematous, ischemic, and hemorrhagic lesions. An MRI may show the presence of a vasogenic edema with alteration on T2, FLAIR, and DWI sequences and no differences in ADC sequences. There is no specific treatment because of the often transient nature of CIE. Some authors have described the use of corticosteroids or mannitol; others have proposed close observation with supportive and symptomatic treatment.[1] We decided to maintain a low-dose corticosteroid since there was evidence of areas of vasogenic edema.
Some possible pathological mechanisms have been proposed. The contrast medium may directly alter the tight junctions between capillary endothelial cells in the brain or may cause a blood displacement and consequently tissue hypoxia. Furthermore, high concentrations of contrast medium may cause clumping of red blood cells and consequently occlusion of arterial branches or even the hyperosmolarity of the contrast medium may cause BBB breakdown.
Selective cortical injury following hypoxic-ischemic insults has been described.[2] In our case, the patient was suffering from chronic hypertension and also had a previous history of cerebral ischemia. We hypothesize that her risk factors played a role in the pathophysiology of CIE. We may also speculate about the persistence of neurological impairment: due to underlying conditions such as hypertension and an inveterate injury of the BBB related to the ischemic lesion, the involved area may have undergone greater damage, which was not only directly related to the presence of contrast medium but persisted also after its washing out. This lack of rapid regression is an even rarer outcome in an already rare condition, which has been described only sporadically in the literature.
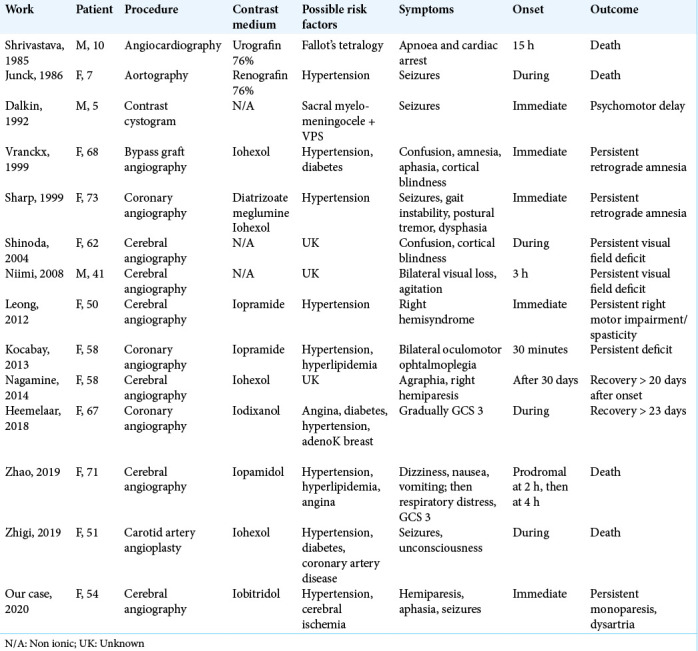
Table 1 includes cases of CIE with adverse clinical outcomes reported to date in the literature, updating and integrating the previous work by Leong and Fanning (2012).[6] The first three cases were observed in children and were likely due to an excess of contrast medium. Other cases have mostly been reported in women aged 41–73. The procedures involved are principally endovascular ones, such as cerebral or coronary artery angiographies; only one case occurred after a nonendovascular procedure (cystogram), performed on a child with a ventricular-peritoneal shunt. Of a total of 14 patients, 7 suffered from chronic hypertension, while none had a previous history of cerebral ischemia. The outcome was always adverse, but in different ways. Of increasing severity, 2/14 had a recovery after >20 days, 2/14 had persistent amnesia, 5/14 had persistent visual or motor deficits, 1/14 developed psychomotor delay, and 4/14 died. No unequivocal cause, risk factor, or pathophysiological cascade are present in all these cases. We hypothesize that patients with a worse outcome may have had another unrecognized factor that, combined with the previous medical history, may have increased the probability of BBB disruption through “hypersensitivity” to contrast medium.
Table 1:
Literature review of CIE regarding adverse clinical outcomes following contrast medium exams.
CONCLUSION
CIE is a form of neurotoxicity arising from contrast media, generally transient but occasionally leading to permanent complications or death. Further studies are necessary to investigate the possible influence of already known risk factors in its pathogenesis and to rule out the presence of new contributing factors.
Footnotes
How to cite this article: Cristaldi PM, Polistena A, Patassini M, de Laurentis C, Giussani C, Remida P. Contrast-induced encephalopathy and permanent neurological deficit: A case report and literature review. Surg Neurol Int 2021;12:273.
Contributor Information
Paola Maria Francesca Cristaldi, Email: paolamfcristaldi@gmail.com.
Alessandra Polistena, Email: alepolistena@gmail.com.
Mirko Patassini, Email: mirko.patassini@gmail.com.
Camilla de Laurentis, Email: camilla.delaur@gmail.com.
Carlo Giussani, Email: carlo.giussani@unimib.it.
Paolo Remida, Email: paolo.remida@libero.it.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Donepudi B, Trottier S. A seizure and hemiplegia following contrast exposure: Understanding contrast-induced encephalopathy. Case Rep Med. 2018;2018:9278526. doi: 10.1155/2018/9278526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frontera JA, Pile-Spellman J, Mohr JP. Contrast-induced neurotoxicity and selective cortical injury. Cerebrovasc Dis. 2007;24:148–51. doi: 10.1159/000103621. [DOI] [PubMed] [Google Scholar]
- 3.Guimaraens L, Vivas E, Fonnegra A, Sola T, Soler L, Balaguer E, et al. Transient encephalopathy from angiographic contrast: A rare complication in neurointerventional procedures. Cardiovasc Intervent Radiol. 2010;33:383–8. doi: 10.1007/s00270-009-9609-4. [DOI] [PubMed] [Google Scholar]
- 4.Hamra M, Bakhit Y, Khan M, Moore R. Case report and literature review on contrast-induced encephalopathy. Future Cardiol. 2017;13:331–5. doi: 10.2217/fca-2016-0075. [DOI] [PubMed] [Google Scholar]
- 5.Kocabay G, Karabay CY. The diagnosis of contrast-induced neurotoxicity. Vascular. 2014;22:391–2. doi: 10.1177/1708538113487334. [DOI] [PubMed] [Google Scholar]
- 6.Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol. 2012;18:33–41. doi: 10.1177/159101991201800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Dangas G. Commentary: New insights into the risk factors of contrast-induced encephalopathy. J Endovasc Ther. 2011;18:545–6. doi: 10.1583/11-3476C.1. [DOI] [PubMed] [Google Scholar]
- 8.Zhao W, Zhang J, Song Y, Sun L, Zheng M, Yin H, et al. Irreversible fatal contrast-induced encephalopathy: A case report. BMC Neurol. 2019;19:46. doi: 10.1186/s12883-019-1279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


