Despite a slew of press releases from academic research groups (and naive media) over the past 40 years (starting with the Aguayo studies in the early 1980s) announcing the imminent arrival of a cure for spinal paralysis (paraplegia and tetraplegia), the failure of contemporary spinal cord injury (SCI) research stands out egregiously to the eyes of the many patients confined to their wheelchairs. Pace stem cells and a smorgasbord of other “successful” (in rodents) treatments reported in “prestigious” academic journals (e.g., Nature, Science, and many others).[1]
It is not for us to answer the question of what went so awfully wrong (certainly not a lack of funds or support), but it behooves us to bring to everyone’s attention the truly promising avenue that must be trodden to achieve the long-sought cure.
Over the past 70 years, clinical transplantation has made it clear that whenever a body part cannot be fixed by available medical treatments, this body part can be replaced, be it an organ or an appendage. A rather simple, but powerful, assumption.
SCI is generally due to localized tissue disruption. Lost function might thus be recovered by replacing the injured segment of the spinal cord [Figure 1]. To achieve this goal, four obstacles must be cleared:
Figure 1:
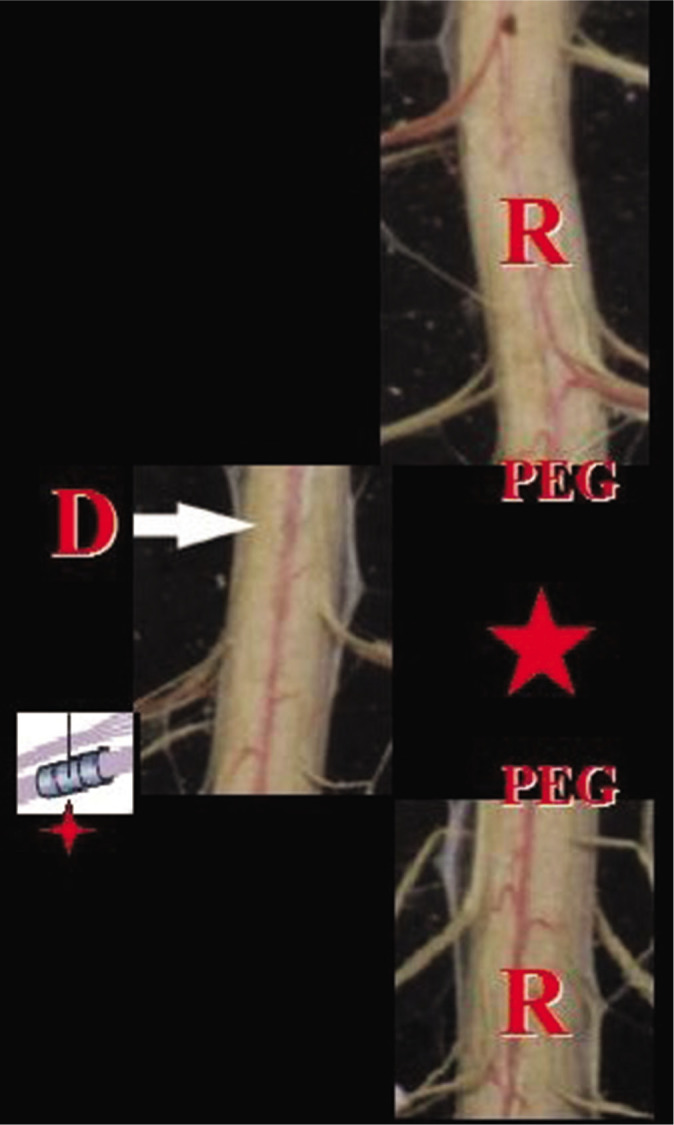
Recipient (r)’s damaged cord segment is removed (star) and a healthy segment is inserted (arrow). Roots are anastomosed with sutureless techniques (four-point star), polyethylene glycol is applied (a spinal cord stimulation apparatus is added after dural closure) and revascularization follows.
Sourcing a homotopic segment of healthy spinal cord
Development of a technology that allows anatomophysiologic integration of the cord transplant (both the cord proper and its roots) with the undamaged cord of the patient
Availability of a microrevascularization technology
Immunologic rejection.
SOURCE OF TRANSPLANTABLE CORD
The idea of replacing a damaged portion of the spinal cord with a healthy one is not new. In 1905, Shirres reported his attempt to graft a segment of healthy canine cord in a human paraplegic patient: initial sensory recuperation was observed at 3 months, but the patient succumbed to infection; of relevance, autopsy showed clear signs of neuroregeneration.[9] This idea bred no further attempts until 40 years later. Woolsey et al.[13] in the USA operated on a 16-year-old male with complete loss of sensorimotor function after he was shot in his right shoulder with the bullet reaching the superior border of T4. Following laminectomy, the injured spinal cord was completely transected and replaced with a cadaveric spinal cord (approximate length: 3 inches) that had been fixed in 10% formalin for 12 days, and cleaned and sterilized with running and distilled water and 70% alcohol. No improvement in the patient’s condition was noted, and the patient died almost 4 months after the surgery. Autopsy showed exceptional preservation of the transplanted graft, although with restricted regeneration and limited tissue reaction. The preservation was attributed to the preoperative use of formalin, and no explanations or related conclusion on the microscopic findings could be made.
In the XXI century, cord segments dovetailing the injured patient’s cord can be harvested from brain-dead organ donors at the same level, thereby respecting the intrinsic anatomy of the damaged cord. A special instrument (GEMIN-o-tome) would allow quick dissection.[2] As can be seen, the cord would simply join a long list of other transplantable body parts. Alternatively, many converging lines of evidence show that cadaveric neural tissue can be salvaged up to 6 h (and perhaps more) postmortem in human bodies.[3] This approach is a mere extension of current efforts aimed at harvesting organs (but also bone marrow) from circulatory-determined death donors (DCD), namely, hyperfresh cadavers, and keeping them viable.[3] Cadaveric spinal cord qualifies as a further extension of this contemporary paradigm.
INTEGRATION OF THE CORD TRANSPLANT
The GEMINI spinal cord fusion protocol enables recovery of sensorimotor function after full cord transection in rodents, canines, and primates (fully reviewed in Canavero et al.[2]; videos available at: http://neuronovosti.ru/serdzhio-kanaverok-peresadke-golovy-gotov/). A Chinese clinical trial testing autologous vascularized hemicord repositioning in paraplegics recently confirmed that GEMINI is effective in fusing a “spinal bridge” at two interfaces simultaneously ([10]; video available at: https://www.youtube.com/watch?v=BxZYUm0rgHY).
Fusion of the cord proper would be followed by reconnection of the dorsal and ventral roots. Spinal nerve root anastomosis is not a new concept. Pioneered by Kilvington in 1907, in the 1960s, Carlsson and Sundin were the first to report on homotopic intradural reconstruction of severed sacral ventral roots using direct repair combined with a tubulization technique: functional reinnervation was demonstrated, including – by later work – in man.[6] In a typical clinical context, a two-level transplant would entail reconnecting eight roots. To expedite this maneuver, a sutureless approach is indicated.[4] The number of axons contained in human ventral roots ranges from ≈ 800 to ≈ 10,000 and that in dorsal roots from ≈2000 to ≈48,000.[7] For comparison, the human sural nerve, a nerve commonly used for grafting procedures, contains ≈1000 axons,[5] and most cranial nerves lie in a similar range.[4] As far as the conus is concerned, the number of roots to be reconnected is high, but still in the realm of feasibility.
MICROREVASCULARIZATION
The transplanted segment must be revascularized. Part and parcel of the GEMINI protocol is polyethylene glycol, a polymer that has been shown to promote angiogenesis in an SCI model.[9] However, vascularization of the donated segment is indicated. By way of illustration, the arterial thoracolumbar territory consists mainly of a ventral (A. spinalis anterior) and two dorsolateral longitudinal trunks (Aa. spinalis posterolateral) linked by two sacral anastomoses. These longitudinal trunks, covering the T8-conus segment, are comparably thick and are fed by radiculomedullary arteries whose diameter ranges from 0.7 mm (Artery of Adamkiewicz) to 0.4 mm.[11] For vessels <0.8 mm, supermicrosurgery has been proposed, consisting of injection of hyaluronic acid in the vessel lumen followed by 12-0 nylon sutures.[8] Sutureless approaches are also possible.[4] A similar argument applies to the venous system. Studies of successful surgical revascularization of the spinal cord in animals are on record.[12]
IMMUNOREJECTION
As per other transplant procedures, immunosuppression is indicated, at least initially. Given the minimal amount of foreign tissue that is inserted into the patient, it is likely that immunosuppression would require a lighter dosing of drugs than, say, face transplants, minimizing its associated toxicity. It must be noted that there are differences between the brain and the cord in neuroimmunological terms, with greater neuroinflammatory responses in the cord.[14,15] This must be allowed for in future trials. Interestingly, cadaveric cord transplants might be reconditioned before transplantation, a process called tolerogenesis.[2]
From this short survey, it is clear that a cure for spinal paralysis is possible. It is only hoped that the international neurosurgical community does not take another 40 years to bring it to fruition.
Footnotes
How to cite this article: Canavero S, Ren X, Kim C. Heterologous spinal cord transplantation in man. Surg Neurol Int 2021;12:295.
Contributor Information
Sergio Canavero, Email: sercan@inwind.it.
Xiaoping Ren, Email: chinarenxg@126.com.
C-Yoon Kim, Email: vivavets@gmail.com.
REFERENCES
- 1.Canavero S, Bonicalzi V. Fall of the titans. The demise of basic neuroscience research. Engineering. 2015;1:409. [Google Scholar]
- 2.Canavero S, Ren XP. New York: Nova Science Publishers; 2020. The Technology of Head Transplantation. [Google Scholar]
- 3.Canavero S. The Frankenstein Effect. United States: Amazon Digital Services LLC; 2019. Extreme Brain Reanimation. Available from: https://wwwamazon.com/extreme-brainreanimation-frankenstein-effect-ebook/dp/b08bdhht54ref=sr_1_9?dchild=1&keywords=sergio+canavero&qid=1600767047&s=books&sr=1-9. [Google Scholar]
- 4.Canavero S. United States: Amazon Digital Services LLC; 2020. The Technology of Brain Transplantation. Available from: https://www.amazon.com/technology-brain-transplantation-sergio-canavero/dp/b08bdwy9q2/ref=sr_1_6?keywords=sergio+canavero&qid=1599227917&sr=8-6. [Google Scholar]
- 5.Frey M, Happak W, Girsch W, Bittner R, Gruber H. Histomorphometric studies in patients with facial palsy treated by functional muscle transplantation: New aspects for the surgical concept. Ann Plast Surg. 1991;26:370–9. doi: 10.1097/00000637-199104000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Amaya SM, Barbe MF, de Groat WC, Brown JM, Tuite GF, Corcos J, et al. Neural reconstruction methods of restoring bladder function. Nat Rev Urol. 2015;12:100–18. doi: 10.1038/nrurol.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhou X, Ma J, Ge Y, Cao X. The diameters and number of nerve fibers in spinal nerve roots. J Spinal Cord Med. 2015;38:532–7. doi: 10.1179/1079026814Z.000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qassemyar Q, Gianfermi M. Supermicrosurgery and hyaluronic acid: Experimental feasability study of a new method. Ann Chir Plast Esthet. 2015;60:e59–65. doi: 10.1016/j.anplas.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Ren X, Kim CY, Canavero S. Bridging the gap: Spinal cord fusion as a treatment of chronic spinal cord injury. Surg Neurol Int. 2019;10:51. doi: 10.25259/SNI-19-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren XP, Henderson P, Kim CY, Canavero S. GEMINI-supported spinal cord transplantation for the treatment of chronic spinal paralysis. Overview and initial clinical translation. In: Preedy VR, Rajendram R, Martin S, editors. Diagnosis and Treatment of Spinal Cord Injury. Amsterdam: Elsevier, Academic Press; 2021. in press. [Google Scholar]
- 11.Schalow G. Feeder arteries, longitudinal arterial trunks and arterial anastomoses of the lower human spinal cord. Zentralbl Neurochir. 1990;51:181–4. [PubMed] [Google Scholar]
- 12.Sindou M, Chignier E, Mazoyer JF, Pialat J, Fischer G, Descotes J, et al. Revascularization of the spinal cord by micro-anastomoses in dogs. Surg Neurol. 1979;12:492–5. [PubMed] [Google Scholar]
- 13.Woolsey D, Minckler J, Rezende N, Klemme R. Human spinal cord transplantation. Exp Med Surg. 1944;2:93–102. [Google Scholar]
- 14.Xuan FL, Chithanathan K, Lilleväli K, Yuan X, Tian L. Differences of microglia in the brain and the spinal cord. Front Cell Neurosci. 2019;13:504. doi: 10.3389/fncel.2019.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Gensel JC. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Exp Neurol. 2014;258:112–20. doi: 10.1016/j.expneurol.2014.04.007. [DOI] [PubMed] [Google Scholar]


