Abstract
Background
Since the pandemic began in 2020, Remdesivir has been widely used for the treatment of coronavirus disease-2019 (COVID-19). Here, we describe a case of a patient with COVID-19 who developed transient complete atrioventricular (AV) block and bradycardia after initiating treatment with Remdesivir.
Case summary
A 72-year-old male with a history of atrial fibrillation and lung cancer was hospitalized for COVID-19. Electrocardiogram (ECG) on admission demonstrated atrial fibrillation and right bundle branch block. He was started on a course of Dexamethasone and Remdesivir. Within 24 h of starting Remdesivir, he was noted to be in atrial fibrillation with ventricular rates between 30 and 40 b.p.m. On Day 5 of Remdesivir therapy, ECG demonstrated complete AV block. Having completed the Remdesivir regimen, during the next 48 h, he was closely monitored, and the AV block resolved spontaneously. As he remained asymptomatic and had an adequate chronotropic response with activity, pacemaker implantation was not recommended.
Discussion
Despite the widespread use of Remdesivir, there is little known information about its cardiac toxicity. Daily ECGs and close cardiac surveillance of patients who develop severe bradycardia or AV block are essential. Discontinuation of the medication usually results in the resolution of these cardiac disturbances.
Keywords: Remdesivir, SARS-CoV2, COVID-19, Bradycardia, AV block, Heart block, Case report
Learning points
Cardiac dysrhythmias can occur in patients with coronavirus disease-2019 (COVID-19) and should have close heart rhythm monitoring.
Use of Remdesivir in COVID-19 may be associated with bradycardia and heart block particularly in patient with pre-existing cardiac conduction disease.
Introduction
On 1 May 2020, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the use of antiviral drug Remdesivir in adults and children hospitalized with severe coronavirus disease-2019 (COVID-19).1 The Adaptive COVID-19 Treatment Trial-1 (ACTT-1) showed that the median time to recovery was 10 days in the Remdesivir group compared to 15 days in the placebo group.2 In October 2020, the FDA approved the use of Remdesivir for the treatment of COVID-19 in hospitalized patients.3 Despite the widespread use of Remdesivir, there is little known information about its cardiac toxicity.
Timeline
| Time | Events |
|---|---|
| 8 December 2020 | Onset of fever, cough, shortness of breath |
| 15 December 2020 | Positive SARS-CoV2 result |
| 23 December 2020 | Patient was hospitalized with worsening coronavirus disease-2019 symptoms |
| 24 December 2020 | Patient was started on Remdesivir and Dexamethasone |
| 25 December 2020 | Within 24 h, patient was in atrial fibrillation with slow ventricular rates between 30 and 40 b.p.m |
| 26 December 2020 | Patient was transferred to the intensive care unit as the patient was requiring 40 L/min high flow oxygen |
| 29 December 2020 | Complete atrioventricular (AV) block noted on Day 5 of Remdesivir therapy. Patient was transferred to the cardiac care unit the same day |
| 30 December 2020 | Patient was on 15 L/min high flow oxygen |
| 31 December 2020 | Resolution of AV block following discontinuation of Remdesivir along with improvement in heart rates |
| 15 January 2021 | Patient was discharged home on 2 L/min oxygen |
| 2 February 2021 | Patient died |
Case presentation
A 72-year-old male with a history of lung cancer, with left lower lung lobectomy and right upper lobe wedge resection, chronic obstructive pulmonary disease, and atrial fibrillation presented with 2 weeks duration of fever, cough, shortness of breath, and poor appetite. Nine days before admission, he tested positive for COVID-19 by a real-time polymerase chain reaction. He had a 60-pack-year smoking history. At home, he was taking 81 mg of aspirin once daily, 20 mg of rivaroxaban once daily, 40 mg of atorvastatin once daily, 40 mg of furosemide once daily, and albuterol 90 μg/actuation inhaler, which were continued while he was hospitalized.
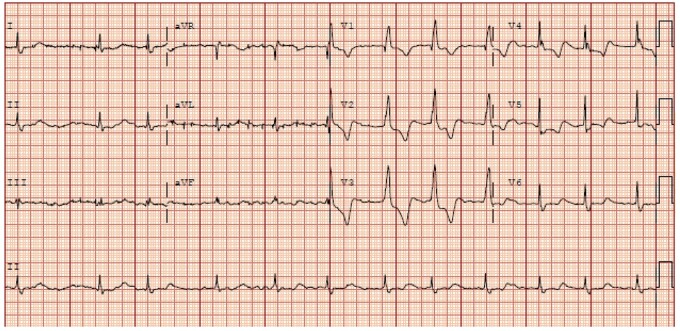
During his first hour of admission, he required 2 L/min of supplemental oxygen to maintain adequate saturation, although the rest of his vitals were stable. Laboratory test results were significant for the following: C-reactive protein 304.87 mg/L (0–10 mg/L); lactate 3.2 mEq/L (0.2–1.9 mEq/L); lactate dehydrogenase 659 U/L (100–220 IU/L); d dimer 2374 ng/mL (0–300 ng/mL); aspartate transaminase 45 U/L; and alanine transaminase 36 U/L (6–45 IU/L). Venous blood gas revealed pH 7.46 (7.32–7.42), pCO2 39 mmHg (42–50 mmHg), and pO2 34 mmHg (30–50 mmHg). The chest radiograph demonstrated bilateral patchy airspace disease. Electrocardiogram (ECG) demonstrated atrial fibrillation and right bundle branch block (RBBB). QRS interval was 132 ms (Figure 1). Within 3 h of presentation, the QRS complexes reverted to narrow complexes. Hence, he was in intermittent RBBB.
Figure 1.
Electrocardiogram done on initial presentation showing right bundle branch block and atrial fibrillation with ventricular rates between 68 and 86 b.p.m.
Remdesivir was started on Day 1 of his hospitalization, with a loading dose of 200 mg on Day 1 and 100 mg daily for four additional days. He was started on Dexamethasone 6 mg daily for 10 days. Within 24 h of initiation of Remdesivir, he was noted to be in atrial fibrillation with slow ventricular rates between 30 and 40 b.p.m. He was also requiring 40 L/min of oxygen through a high flow nasal cannula and subsequently transferred to the intensive care unit. Two sets of troponins I were 0.028 and 0.022 ng/mL (0.03–0.04 ng/mL).
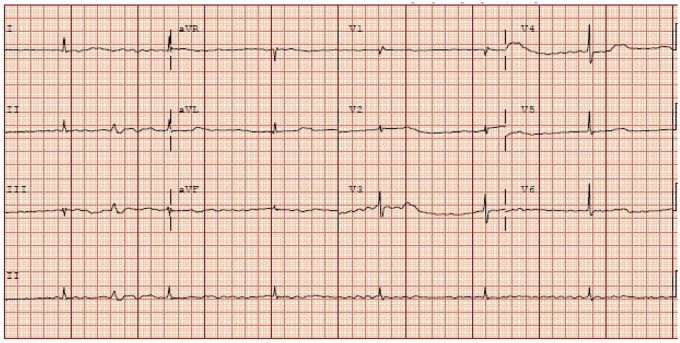
On Day 5 of Remdesivir therapy, he was noted to be persistently bradycardic. ECG demonstrated atrial fibrillation with slow, fixed ventricular response, consistent with complete atrioventricular (AV) block (Figure 2). Echocardiography done on that day showed an ejection fraction of 60% with moderate pulmonary hypertension, dilated right ventricle, mild aortic insufficiency, moderate tricuspid insufficiency, and mildly reduced right ventricular function.
Figure 2.
Electrocardiogram done on Day 5 of Remdesivir treatment showing atrial fibrillation with slow, fixed ventricular response, consistent with complete atrioventricular block. Right bundle branch block has resolved.
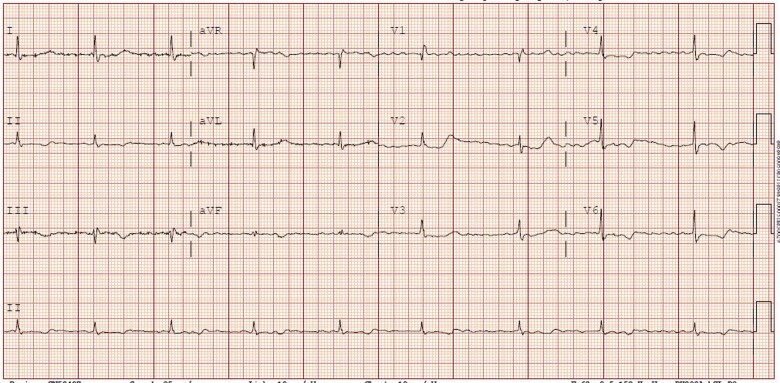
Having completed the Remdesivir regimen, during the next 48 h, he was closely monitored, and the AV block resolved spontaneously. ECG done on Day 10 demonstrated atrial fibrillation with ventricular rates between 52 and 58 b.p.m. (Figure 3). He was noted to be clinically improving, with his supplemental oxygen requirements decreasing from 40 to 15 L/min. As he remained asymptomatic and had an adequate chronotropic response with activity, pacemaker implantation was not recommended. Over the next 2 weeks, his oxygen requirements improved, and his heart rate remained between 48 and 60 b.p.m. He was discharged home after a 3-week stay in the hospital on 2 L/min of supplemental oxygen.
Figure 3.
Electrocardiogram done on Day 10 showing atrial fibrillation with ventricular rates between 52 and 58 b.p.m.
He died ∼2 weeks after being discharged from the hospital. The cause of his death was not known.
Discussion
Remdesivir is a broad-spectrum antiviral drug and an inhibitor of the viral RNA-dependent RNA polymerase. Once it enters the cell, it is rapidly converted by esterases into different nucleoside metabolites. The pharmacologically active triphosphate form has a half-life of 11 h, and there is another dephosphorylated nucleoside metabolite (GS-441524) that has a half-life of 20–25 h. The nucleoside metabolites compete with Adenosine triphosphate (ATP) for incorporation into viral RNA, causing premature chain termination and inhibition of viral replication. GS-441524 has potent in vitro inhibitory activity against middle east respiratory syndrome coronavirus, SARS-CoV1, and SARS-CoV2.4,5 The most common reported side effects of Remdesivir are elevation of hepatic enzymes, rash, anaemia, renal impairment, and diarrhoea.6
The ACTT-1 trial reported a 1.1% incidence of cardiac arrest and 4% atrial fibrillation in the Remdesivir group (compared to 1% and 2%, respectively, in the placebo group).2 There have been two prior case reports of patients who developed severe bradycardia following the use of Remdesivir.7,8 These patients’ heart rates improved following discontinuation of Remdesivir. Patients with COVID-19 have also been noted to have bradyarrhythmias, likely due to viral myocarditis, hypoxia, or damage to the pacemaker cells from inflammatory cytokines,9 however, the timing of bradycardia in our case indicates contribution from Remdesivir. He also scored 3 points on the Naranjo Adverse Drug Reaction Probability Scale, indicating possible probability of adverse drug reaction (total score 1–4).10
Our patient had a history of atrial fibrillation. He was not on any AV nodal blocking agents or negative chronotropic agents during or prior to his hospitalization. The QRS complexes in Figure 2 were narrow compared to the baseline ECG (Figure 1). This could be due to slow junctional escape rhythm with normalization of RBBB conduction or ventricular escape rhythm resulting in matched conduction delay and narrow QRS complexes. The fact sheet for EUA for Remdesivir recommends against coadministration of chloroquine phosphate or hydroxychloroquine sulphate with Remdesivir due to risk of reduced antiviral activity.11 There are no known interactions of Remdesivir with any of the other medications the patient was on.
The mechanism of cardiac disturbances in these patients is unclear. One postulated mechanism is possibly related to Remdesivir’s affinity towards the RNA polymerases and subsequent mitochondrial dysfunction, resulting in cardiotoxicity.12 Another possible explanation is related to Remdesivir’s resemblance to adenosine triphosphate. Adenosine causes coronary vasodilation through its action on A2A receptors on cardiac cells. Additionally, it has a short-lived effect on the A1 receptors on cardiac cells, causing blockage of AV nodal conduction.13 Remdesivir may influence the AV node through this mechanism, given its status as a nucleoside analogue. In our case, the patient developed severe bradycardia within 24 h of administering Remdesivir, implying a temporal relationship. Moreover, the AV block resolved spontaneously after 48 h of discontinuation of Remdesivir, consistent with the pharmacokinetics of its active metabolites.
Conclusion
Little information exists regarding the potential cardiac toxicity of Remdesivir. Baseline ECG should be obtained on all patients before initiating Remdesivir. Daily ECGs and close cardiac surveillance of patients who develop severe bradycardia or AV block are essential. Extra caution should be exercised in patients on dipyridamole due to the enhanced effect of adenosine on cardiac cells. Discontinuation of the medication usually results in the resolution of these cardiac disturbances. The decision to pursue electrophysiological studies or to implant a pacemaker should be made on an individual basis. Further investigation and research are needed to assess the mechanisms surrounding cardiac toxicity and Remdesivir in COVID-19 patients and in patients with pre-existing cardiac comorbidities or AV block.
Lead author biography

Dr Vijairam Selvaraj, MD, MPH, FACP is an Assistant Professor of Medicine and Clinician Educator at Warren Alpert Medical School of Brown University. He is the site director at Miriam Hospital for the sub-internship program for fourth-year medical students at Warren Alpert Medical School of Brown University. He is also Adjunct Clinical Assistant Professor at Johnson and Wales University for the Physician Assistant program. He presently works in the Hospital Medicine Department at Miriam Hospital in Providence, Rhode Island. He is passionate about medical education, clinical research, bedside ultrasound, and ECG teaching.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Center for Drug Evaluation and Research. Emergency use authorization (EUA) for remdesivir. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/EUA%20Review%20Remdesivir_050120.pdf (1 May 2020).
- 2. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC. et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Center for Drug Evaluation and Research. Combined cross-discipline team leader, division director, and ODE director summary review for NDA 214787. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000Sumr.pdf (21 October 2020).
- 4. Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ. et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020;11:222–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gubitosa JC, Kakar P, Gerula C, Nossa H, Finkel D, Wong K. et al. Marked sinus bradycardia associated with Remdesivir in COVID-19: a case and literature review. JACC Case Rep 2020;2:2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta AK, Parker BM, Priyadarshi V, Parker J.. Cardiac adverse events with Remdesivir in COVID-19 infection. Cureus 2020;12:e11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amaratunga EA, Corwin DS, Moran L, Snyder R.. Bradycardia in patients with COVID-19: a calm before the storm? Cureus 2020;12:e8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA. et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–245. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration (FDA). Fact sheet for healthcare providers. Emergency Use Authorization (EUA) of Veklury (Remdesivir). https://www.samc.com/assets/documents/covid19/nursing/remdesivir_eua-hcp-fact-sheet-8-2020.pdf (7 March 2021).
- 12. Varga ZV, Ferdinandy P, Liaudet L, Pacher P.. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol 2015;309:H1453–H1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zipes DP, Libby P, Bonow RO, Mann DL, Tomaselli GF, Braunwald E, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. Philadelphia, PA: Elsevier/Saunders; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.