Abstract
Background
Checkpoint inhibitors (CPIs) are used to treat solid organ metastatic malignancies. They act by triggering a vigorous immune response against tumoural cells, preventing their proliferation and metastasis. However, this is not a selective response and can cause immune-related adverse events (irAEs). The kidney can potentially be damaged, with an incidence of irAEs of 1–4%. The most frequent type of toxicity described is acute interstitial nephritis (AIN).
Methods
We conducted a study of patients with solid organ metastatic malignancies treated with immunotherapy who developed acute renal injury and underwent kidney biopsy in the last 14 months at the Vall d’Hebron University Hospital.
Results
In all, 826 solid organ malignancies were treated with immunotherapy in our centre, 125 of them (15.1%) developed acute kidney injury (AKI), 23 (18.4% of AKI) visited the nephrology department and 8 underwent kidney biopsy. The most frequent malignancy was lung cancer, in five patients (62%), followed by two patients (25%) with melanoma and one patient (12%) with pancreatic cancer. Four patients (50%) had already received previous oncological therapy, and for the remaining four patients (50%), CPI was the first-line therapy. Five patients (62%) were treated with anti-programmed cell death protein 1, three patients (37%) received anti-programmed death ligand 1 and two (25%) patients were treated in combination with anti-cytotoxic T-lymphocyte antigen 4. The time between the start of CPI and the onset of the AKI ranged from 2 to 11 months. The most frequent urine findings were subnephrotic-range proteinuria, with a mean protein:creatinine ratio of 544 mg/g (standard deviation 147) and eosinophiluria. All patients were biopsied after being diagnosed with AIN. Three patients (37%) received treatment with pulses of methylprednisolone 250–500 mg/day and five patients (62%) received prednisone 1 mg/kg/day. Seven patients (87%) experienced recovery of kidney function and one patient (12%) progressed to chronic kidney disease.
Conclusions
We report on eight patients with CPI-related AIN diagnosed in the last 14 months at our centre. The novel immunotherapy treatment of metastatic solid organ malignancies carries a higher risk of irAEs. The kidney is one of the most commonly affected organs, frequently presenting as an AIN and exhibiting a favourable response to steroid treatment.
Keywords: AKI, acute interstitial nephritis, checkpoint inhibitors, immunotherapy, kidney biopsy
INTRODUCTION
According to the Global Cancer Observatory, the incidence of cancer worldwide in 2018 was estimated at 18 078 957, resulting in nearly 10 million deaths [1]. In 2015, the World Health Organization reported cancer as the first and second leading causes of death of individuals <70 years of age in most countries. Moreover, with life expectancy progressively increasing, the high prevalence of comorbidities and the globalization of a Western lifestyle (diet, smoking, sedentary lifestyle, etc.), cancer is expected to become the leading cause of death in the coming years [2]. Given the excessive economic and social burdens this represents, the scientific community has been rushing to develop new and more effective therapies. As a result, and under the premise of stimulating the immune system to attack cancer cells, extensive research has been done, resulting in the discovery of cancer immunotherapy about two decades ago. To date, several agents have emerged, including vaccine-based therapies, oncolytic viruses, T cell–directed therapies, bi-specific antibodies and checkpoint inhibitors (CPIs) [3]. Based on the results of many clinical trials, significant improvements in the survival rates of patients treated with these drugs have been observed [4, 5]. Further studies are needed to determine the duration of this effect, possible resistance mechanisms and side effects.
The tumoural microenvironment has many ways of escaping the recognition of the immune system, which allows for its growth and dissemination, resulting in metastasis. An important mechanism is the expression of ligands for inhibitory receptors like cytotoxic T–lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on T cells and other immune cells causing a downregulation of its activation. Here lies the rationale for CPIs, which bind to CTLA-4 and PD-1 to activate immune cells from a quiescent state, triggering a vigorous response against tumoural cells [3]. However, this mechanism is not selective and has the potential to suppress tolerance to self-antigens, thus increasing the risk of immune-related adverse events (irAEs). Although any organ can be involved, the most commonly affected organs are the skin, gastrointestinal tract, endocrine glands and the liver, comprising an incidence of 15–90% [6, 7]. With regards to the kidney, a meta-analysis of 48 clinical trials demonstrated an incidence of 2–4%, and higher if combination therapy is used [6, 8]. However, recent series have reported an incidence as high as 29% [9]. The most common kidney lesion associated with CPIs is acute interstitial nephritis (AIN). Nonetheless, immune complex–mediated glomerulonephritis, podocytopathies, thrombotic microangiopathy and electrolyte disturbances have also been described [10]. Our objective was to study biopsy-proven renal diseases secondary to CPIs in our centre in the last 14 months.
MATERIALS AND METHODS
This is a retrospective study of patients with metastatic solid organ malignancies treated with CPIs in a period of 14 months at Vall d’Hebron University Hospital. We found 125 patients who developed AKI based on the Acute Kidney Injury Network (AKIN) criteria. A total of 826 solid organ malignancies were treated with CPIs in our centre; 125 patients (15.1%) developed an AKI, 23 patients (18.4% of AKI) visited the nephrology department and 8 patients underwent kidney biopsy. Subsequently, data that included gender, age, ethnicity, comorbidities, current medication [with special emphasis in non-steroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers], type of malignancy, type of oncological treatment, first and last dose of CPI, class of CPI, number of cycles received, extrarenal irAEs, baseline renal function, stage of AKI at presentation, sediment findings, proteinuria, urine cytology, serological information, renal biopsy diagnosis and treatment were recorded.
Renal biopsy techniques included light microscopy (haematoxylin and eosin and saffron, periodic acid–Schiff and Masson's trichrome), immunofluorescence and immunohistochemistry. Two biopsy cores were obtained under ultrasound guidance using a 16-gauge needle, with the cores containing at least one glomerular and one arterial profile and sufficient tubulointerstitial tissue.
A follow-up time of at least 3 months was used to established renal function recovery or progression to chronic kidney disease (CKD) according to Kidney Disease: Improving Global Outcomes guidelines.
RESULTS
A total of 826 solid organ malignancies were treated with CPIs in our centre; 125 patients (15.1%) developed an AKI and 23 patients (18.4% of AKI) visited the nephrology department. Eight patients who visited the nephrology clinic (34.8%) with metastatic solid organ malignancies under treatment with CPIs underwent renal biopsy over the study period. The male:female ratio was 1:1. The median age was 67 years, all patients were Caucasian, the most prevalent comorbidity was hypertension (50%) and one patient had type 2 diabetes (12%). Five patients (62%) had a history of either NSAIDs or PPI use. Most of the patients (62%) had metastatic lung cancer; the remaining had melanoma (12%) or pancreatic cancer (12%). Four patients (50%) had previously received other types of oncological treatment without remission. For the other 50%, CPIs represented the first line of treatment. The immunotherapies used were the following: five patients (62%) were treated with anti-PD-1, one (12%) in combination with an anti-CTLA-4; three patients (37%) were treated with anti-PD-L1, one (12%) in association with an anti-CTLA-4. Three patients presented with irAEs: one patient (12%) had previously been diagnosed with immune-mediated esophagitis and two patients (24%) presented with arthralgias related to CPI treatment.
Seven patients (87%) had normal baseline kidney function and one patient had CKD Stage 3a. The time between the CPI administration and the presentation of kidney disease ranged from 2 to 11 months [mean 5.8 months (SD 3.5)]. Two patients who were treated with an anti-PD-L1 had the earliest observable onset. Regarding the presentation, six patients (75%) met AKIN Stage 3 criteria and the other two showed AKIN Stages 2 and 1. None of the patients required renal replacement therapy. The prevailing urine findings were subnephrotic proteinuria in seven patients (87%) with a mean urine protein:creatinine ratio of 544 mg/g (SD 147) and eosinophiluria in five patients (62%). One patient (12%) exhibited eosinophilia. Two patients (25%) had microscopic isomorphic haematuria. The serologic tests demonstrated metabolic acidosis in five patients (62%); characteristically, one of these patients had persistent hypokalaemia with urine and blood findings compatible with renal tubular acidosis type I (RTA). No other electrolyte disturbances were observed. The autoimmunity laboratory workup was negative and complement levels and immunoglobulins were normal. Each patient had a diagnosis of AIN when biopsied (Figure 1). No significant changes in the glomerular compartment were seen. No immunoglobulins or complement deposits were observed on immunofluorescence or immunohistochemistry. No granulomas were observed.
FIGURE 1.
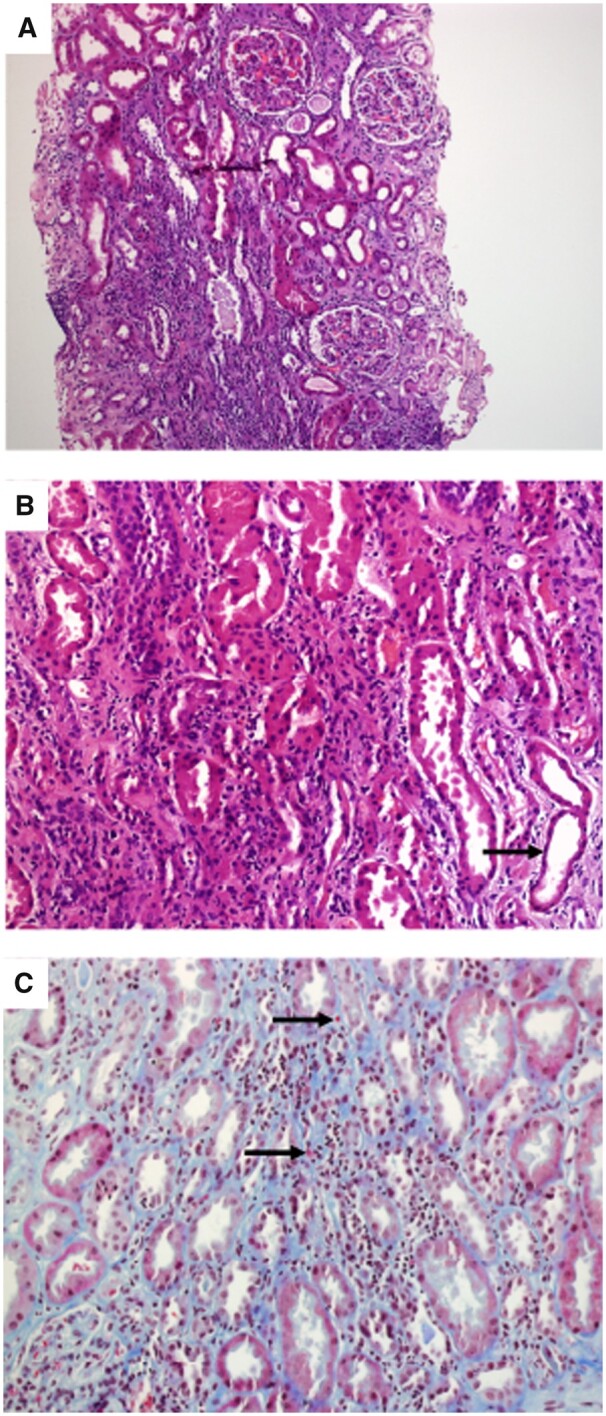
Kidney biopsy findings (Patient 1). (A) Tubulo-interstitial inflammatory infiltrates and no structural abnormalities in the glomeruli are observed (haematoxylin and eosin, ×10). (B) Predominantly monocytic inflammatory infiltrates in the interstitial compartment. Tubular epithelium simplification suggesting acute tubular injury (arrow) (haematoxylin and eosin, ×20). (C) Inflammatory cells in the basolateral aspect of the tubular epithelium (arrow), scattered eosinophils in the interstitium (Masson's trichrome, ×20).
Three patients (37%) received intravenous methylprednisolone pulses for the first 3 days after being diagnosed. Two patients were given 500 mg/day and one patient was given 250 mg/day. The other five patients (62%) started treatment with oral prednisone 1 mg/kg/day. For each of the patients, the management approach included a full oral dose of prednisone for 1.5 weeks in five patients (62%) and a longer period for the remaining three patients (37%), which ranged from 3 to 5 weeks. Subsequently, prednisone was tapered from 5 to 10 mg/week. At the end of the follow-up, two patients (25%) had finished treatment. One patient completed 6 months of steroids and the other 4 months. The remaining patients are still receiving prednisone. Seven patients (87%) stopped their CPI treatment since the AKI diagnosis—none of which have restarted. A patient who had been administered steroids stopped after 14 weeks of treatment and demonstrated kidney function recovery, but exhibited a recurrence 8 weeks later. The patient received three pulses of methylprednisolone 250 mg/day, followed by oral prednisone.
At the 3-month follow-up, seven patients (87%) presented with complete recovery of kidney function, including the patient who exhibited a recurrence during steroid tapering. Only one patient (12%) progressed to CKD. Table 1 shows a summary of the baseline characteristics of the patients and oncological diseases. Table 2 summarizes clinical features at the time of AKI presentation and outcome.
Table 1.
Baseline characteristics of the patients, the oncological disease, the AKI presentation and outcome
| Patient number | Age/gender/ race | Comorbidities | Malignancy | Type of CPI and duration | Baseline serum creatinine (mg/dL) | Cancer outcome | Non-renal irAEs |
|---|---|---|---|---|---|---|---|
| 1 | 59/F/C | GERD | Lung | Anti-CTLA-4 and anti PD-L1 (four cycles) | 0.7 | PD | No |
| 2 | 67/F/C | GERD | Pancreas | Anti-PD-L1 and MEK inhibitor (two cycles) | 0.8 | PD | No (fever) |
| 3 | 83/F/C | HT, dyslipidaemia | Melanoma | Anti-PD-1 and anti-LAG-3 antibody (three cycles) | 0.7 | CR | Arthralgia |
| 4 | 85/M/C | DM, HT, dyslipidaemia, GERD, CKD | Lung | Anti-PD-1 and anti-cMET antibody (seven cycles) | 1.3 | PD | No |
| 5 | 68/F/C | HT, GERD | Lung | Anti-PD-1 (three cycles) | 1.1 | SD | No |
| 6 | 63/M/C | GERD | Lung | Anti-PD-L1 (one cycle) | 0.9 | PR | Arthralgia |
| 7 | 75/M/C | GERD, HT | Melanoma | Anti-PD-1 and EGFR inhibitor (one cycle) | 0.9 | CR | Esophagitis |
| 8 | 61/M/C | – | Lung | Anti-PD-1 and RAS inhibitor (five cycles) | 0.9 | PD | No |
CR, complete response; GERD, Gastroesophageal reflux disease; HT, Hypertension; MEK, mitogen-activated protein; PD, partial disease; PR, partial response; SD, stable disease.
Table 2.
Clinical features and treatment of AKI
| SCr at AKI presentation (mg/dL) | Urine sediment | UPCR (mg/g) | Autoimmunity | Nephrotoxic drugs | Electrolitic disturbances | Kidney biopsy diagnosis | Induction treatment | Steroid tapering | Renal outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.8 |
Eos Leuk |
680 |
|
Ibuprofen, omeprazole | Hypokalaemia and normal anion gap metabolic acidosis (RTA) | AIN | 1 mg/kg/day |
|
Recovery |
| 2 | 4.4 | Eos Leuk Hem | 741 | No available data | Omeprazole | – | AIN | 1 mg/kg/day |
|
Recovery |
| 3 | 3.5 | Eos Leuk | 475 |
|
– | – | AIN | 1 mg/kg/day |
|
Recovery, but relapse at Month 3 after steroid tapering |
| 4 | 2.2 | Eos Leuk | 230 | No available data | Omeprazole | Elevated anion gap metabolic acidosis | AIN | 1 mg/kg/day |
|
Recovery |
| 5 | 3.1 | Leuk | 519 |
|
Omeprazole | Elevated anion gap metabolic acidosis | AIN | 1 mg/kg/day |
|
Recovery |
| 6 | 4.2 | Leuk | 624 |
|
Ibuprofen, omeprazole | – | AIN | 500 mg/day × 3 |
|
CKD |
| 7 | 6.2 | Leuk Hem | 485 |
|
Omeprazole | Elevated anion gap metabolic acidosis | AIN | 250 mg/day × 3 |
|
Recovery |
| 8 | 3.7 |
|
600 |
|
– | – | AIN | 500 mg/day × 3 |
|
Recovery |
SCr: serum creatinine; UPCR: urine protein:creatinine ratio; Eos: eosinophiluria; Leuk: leucocyturia; Hem: haematuria; ANA: anti-nuclear antibody; anti-DNA: anti-deoxyribonucleic acid antibody; ANCA: anti-neutrophilic cytoplasmatic antibody; anti-GBM: anti-glomerular basement membrane antibody; RTA, renal tubular acidosis; AD: accumulated data.
DISCUSSION
Novel immunotherapy has considerably increased the survival rate of patients with solid organ malignancies, at the cost of a variety of irAEs. Virtually any organ can be affected, with the most common irAEs involving the skin, gastrointestinal tract, endocrine system, liver and kidneys, with incidences of 36, 18, 13, 1–9 and 1–4%, respectively [7]. However, recent studies have shown a higher incidence of renal irAEs—up to 29% [9]. In general terms, the exact physiopathological mechanism of damage in the kidney has not yet been established, although it seems that a few mechanisms might be involved. The most plausible hypothesis is the production of autoantibodies against kidney tissue, anti-tumour T-cell-mediated damage due to cross-reactivity with healthy kidney components, previous loss of tolerance of drug-specific effector T-cells by drugs known to cause immune-mediated kidney injury (such as NSAIDs or PPIs) and an inflammatory lesion due to cytokines and chemokines produced by T-cell activation [11]. Wolchok et al. [12] studied whether a genetic predisposition to immune-related AEs was present in patients with melanoma treated with ipilimumab, but they did not find any association with human leucocyte genotypes.
As previously mentioned, the most commonly reported nephrotoxicity presentation was AIN, and less frequently, immune-mediated glomerulonephritis, podocytopathies and thrombotic microangiopathy. Surprisingly, the time of onset can vary depending on the type of CPI used for treatment: CTLA-4 antagonist toxicity can start as early as 6–12 weeks after its initiation; in contrast, PD-1 inhibitors may take 3–6 months to provoke kidney injury. This difference can be explained by the fact that CLTA-4 acts on T lymphocytes at an early stage, triggering an activation and proliferation of T cells while reducing T-regulatory cells. On the other hand, PD/PLD1 activates peripheral T cells inducing a more tumour-specific response [3]. It is worth mentioning that this damage can also be developed once the drug has been stopped [7, 13]. The largest series of biopsy-proven nephropathies associated with the use of an anti-PD-1 (pembrolizumab) reported 12 patients who exhibited a kidney lesion with a median time of onset of 9 months (range 1–24). In contrast to our study, three types of damage were observed on kidney biopsy: AIN, acute tubular damage and minimal change disease [14]. We did not have electron microscopy available for our biopsy samples.
In this article, we reported eight cases of biopsy-proven AIN in patients with solid organ metastatic malignancies treated with CPIs. We did not find gender predominance, in contrast to other larger series, which have found a white male predominance [10]. The median age of patients was 67 years. The most prevalent comorbidities were hypertension and diabetes mellitus. Most patients had ongoing treatment with PPIs and some had taken NSAIDs, which possess the potential to act as nephritogenic drugs, producing drug-specific T cells that, after being left in a quiescent state by regulatory mechanisms like PD-1 receptors, are reactivated by CPIs [9]. In concordance with other series, the main urinary findings were subnephrotic proteinuria and eosinophiluria [10]. A large number of our patients had metabolic acidosis with a high anion gap; however, one patient presented with persistent normal anion gap metabolic acidosis and hypokalaemia. Further workup led to the diagnosis of a tubular acidification deficit concordant with RTA. Drug-related and autoimmune aetiologies were ruled out. An adequate response to discontinuing the CPI, potassium, bicarbonate replacement and steroid was seen. Three similar cases have been described in the literature favouring immune-mediated damage of the distal tubule proton pump secondary to CPI treatment [15–17].
After ruling out other potential aetiologies like contrasts, medications and volume status, and having the biopsy diagnosis of AIN, all of our patients discontinued the CPI treatment and received induction therapy with steroids. The more severe cases of AKI Stage 3 were treated with intravenous methylprednisolone and those with AKI Stage 2 or 1 received oral prednisone. According to the American Society of Clinical Oncology (ASCO) guidelines, steroids should be administered when a Grade 2 toxicity (creatinine 2–3 times above baseline) is established. Likewise, a kidney biopsy should be considered with Grade 3 toxicity (creatinine >3 times above baseline or >4 mg/dL) [18]. On the other hand, Perazella and Sprangers [19] established an algorithm that suggests starting steroids in AKI Stage 2/3 if other aetiologies have been excluded and if the patient has other irEAs, sterile pyuria and/or white blood cell cast; otherwise it is suggested to wait until the kidney biopsy diagnosis. We have maintained oral prednisone in our patients for up to 6 months with a strict, monitored tapering schedule—an analogous approach has been suggested in other studies. Previous series of cases have shown that prolonged steroid treatment is related with better renal outcome than conservative management or shorter therapeutic schedules [13].
The majority of our patients experienced kidney function recovery with steroid treatment, but one patient progressed to CKD. A similar rate of recovery has been described in the literature with AIN induced by CPIs [10]. Moreover, immune-mediated glomerulonephritis, podocytopathies or thrombotic microangiopathy could have the requirement of stronger immunosuppressive therapy. Mycophenolate mofetil, cyclosporine, infliximab and other agents have been used with variable results [10]. In one case we observed a relapse of AIN after completing 14 weeks of steroid therapy—the patient was treated with pulses of methylprednisolone, with kidney function recovery. It has been proposed to reinstate CPIs on an individualized decision, balancing the risks and benefits on a case-by-case basis [19]. More studies are needed to evaluate the efficacy of low-dose prednisone when CPI reintroduction for treating the cancer is needed.
In 2018, the ASCO published guidelines for the management of irAEs [18]. More recently, Perazella and Sprangers [19] developed an algorithm that considers treatment with steroids and kidney biopsy at earlier AKI stages compared with the ASCO guidelines, especially if the patient has evidence of irAEs involving other organs, pyuria and/or white blood cells casts.
The main limitations of the study are the small sample size and the inability to report the specific type of CPI that each patient received because of confidentiality agreements with the respective sponsor of the clinical trials. All of the reported patients were included in clinical trials.
In conclusion, all of our biopsied patients treated with CPIs were diagnosed of AIN. Thus we recommend obtaining a biopsy if irAEs involving the kidney are suspected, not only to confirm the diagnosis, but also to establish a treatment and prognosis given the fact that many of these patients carry an important comorbidity that could have an impact on renal survival. In the same line, basal kidney function should be checked before starting immunotherapy and during treatment. These strategies will allow the clinician to provide an early diagnosis and treatment of AIN and subsequently avoid the development of CKD.
ACKNOWLEDGEMENTS
The authors are current recipients of research grants from the Fondo de Investigación Sanitaria-Feder, ISCIII (PI17/00257) and Redinren (RD16/0009/0030). M.B. performed this work as the basis of her thesis at the Department de Medicina of Universitat Autònoma de Barcelona.
CONFLICT OF INTEREST STATEMENT
M.J.S. reports conflicts of interest with NovoNordisk, Janssen, Boehringer, Eli Lilly, AstraZeneca and Esteve. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.World Health Organization. Global Health Observatory. Geneva: World Health Organization, 2018. http://who.int/gho/database/en/ (30 May 2019, date last accessed) [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2018; 68: 394–424 [DOI] [PubMed] [Google Scholar]
- 3. Osipov A, Murphy A, Zheng L. From immune checkpoint to vaccines. The past, present and future of cancer immunotherapy. Adv Cancer Res 2019; 143: 63–144 [DOI] [PubMed] [Google Scholar]
- 4. Hamid O, Robert C, Daud A. et al. Five-year survival outcomes in patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019; 30: 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodi FS, Chesney J, Pavlick AC. et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicenter, randomized, controlled, phase 2 trial. Lancet Oncol 2016; 17: 1558–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Champiat S, Lambotte O, Barreau E. et al. Management of immune checkpoint blockage dysimmune toxicities: a collaborative position paper. Ann Oncol 2016; 27: 559–574 [DOI] [PubMed] [Google Scholar]
- 7. Postow MA, Sidlow R, Hellmann MD.. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168 [DOI] [PubMed] [Google Scholar]
- 8. Manohar S, Kompotiatis P, Thongprayoon C. et al. Programmed cell death protein inhibition is associated with acute kidney injury and hypocalcemia: a meta-analysis. Nephrol Dial Trasplant 2019; 34: 108–117 [DOI] [PubMed] [Google Scholar]
- 9. Wanchoo R, Karam S, Uppal NN. et al.; on behalf of Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors. Adverse renal effects of immune check point inhibitors: a narrative review. Am J Nephrol 2017; 45: 160–169 [DOI] [PubMed] [Google Scholar]
- 10. Mamlouk O, Selamet U, Machado S. et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointertstitial nephritis: single center experience. J Immunother Cancer 2019; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perazella MA, Shirali AC.. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol 2018; 29: 2039–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolchok JD, Weber JS, Hamid O. et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun 2010; 10: 9. [PMC free article] [PubMed] [Google Scholar]
- 13. Cortazar FB, Marrone KA, Troxell ML. et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016; 90: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izzidine H, Mathian A, Champiat S. et al. Renal toxicities associated with pembrolizumab. Clin Kidney J 2019; 12: 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Bitar S, Weerasinghe C, El-Charabaty E. et al. Renal tubular acidosis an adverse effect of PDI inhibitor immunotherapy. Case Rep Oncol Med 2018; 2018: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balakrishna P, Villegas A.. Hypokalemic paralysis secondary to immune checkpoint inhibitor therapy. Case Rep Oncol Med 2017; 11: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charmetant X, Teuma C, Lake J. et al. A new expression of immune checkpoint inhibitors’ renal toxicity: when distal tubular acidosis precedes creatinine elevation. Clin Kidney J 2020; 13: 42–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brahmer JR, Lancchetti C, Schneider BJ.. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36: 1714–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perazella MA, Sprangers B.. AKI in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 2019; 14: 1077–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]


