Abstract
Background
The sodium-glucose cotransporter 2 inhibitor canagliflozin has been shown to reduce the risk of cardiovascular and renal events in patients with Type 2 diabetes mellitus and high risk. Pooled analyses of data from early studies and interim data from the CANagliflozin cardioVascular Assessment Study (CANVAS) suggested that canagliflozin might lead to increases in serum potassium, particularly the 300 mg dose in patients with renal impairment, which is important because high serum potassium is associated with increased cardiovascular and renal risk. We examined the effect of canagliflozin on serum potassium levels and hyperkalemia rates in the completed CANVAS Program.
Methods
The CANVAS Program (n = 10,142) was comprised of two comparable double-blind, randomized, placebo-controlled trials (CANVAS and CANVAS-Renal). Participants received canagliflozin 100 or 300 mg or placebo. Serum potassium measurements were performed in a central laboratory0 and assessed at ∼6-month intervals.
Results
In the CANVAS Program, mean potassium levels were generally consistent with canagliflozin and placebo, overall and by baseline estimated glomerular filtration rate (eGFR; ≥60, 45 to<60 and <45 mL/min/1.73 m2). The risk of increased or decreased potassium was similar with canagliflozin and placebo overall and by baseline eGFR (all P-heterogeneity ≥0.56) or use of renin–angiotensin–aldosterone system inhibitors (all P-heterogeneity ≥0.71); levels did not appear different by canagliflozin dose. Hyperkalemia {hazard ratio (HR) [95% confidence interval (CI)] 1.60 (0.92–2.81)} and serious hyperkalemia [HR (95% CI) 0.75 (0.27–2.11)] adverse events were not different across groups.
Conclusions
In the CANVAS Program, there were no meaningful effects of canagliflozin on serum potassium in the overall population or key subgroups. Hyperkalemia adverse events were uncommon and occurred at comparable rates with canagliflozin and placebo.
Keywords: canagliflozin, hyperkalemia, potassium, SGLT2 inhibitor, Type 2 diabetes
INTRODUCTION
The sodium-glucose cotransporter 2 (SGLT2) inhibitors canagliflozin, empagliflozin and dapagliflozin are glucose-lowering agents that have been approved for reduction of the risk of major adverse cardiovascular (CV) events, CV death and hospitalization for heart failure, respectively, in patients with Type 2 diabetes mellitus (T2DM) and high CV risk [1–6]. Recently, canagliflozin was also approved to reduce renal risk and the risk of hospitalization for heart failure in patients with T2DM and high renal risk [1, 7]. The cardiorenal protective effects of canagliflozin may be partially mediated by glucose lowering. However, effects on other biomarkers such as blood pressure, body weight and albuminuria may also play a role [8–10]. No negative effects of canagliflozin on CV biomarkers have been demonstrated, except for a possible transient effect on serum potassium reported in small studies available at the time of original regulatory approval [1, 11, 12]. This may be of importance since changes in serum potassium have been associated with increased CV and renal risk, including in those with reduced renal function [13–15]. It is also important to understand whether there is a justifiable concern about coadministration of canagliflozin with angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) therapy, which is the standard of care for albuminuric chronic kidney disease (CKD) in the setting of T2DM but necessitates monitoring for elevated serum potassium [16–20].
The effects of the SGLT2 inhibitor canagliflozin on serum potassium in patients with T2DM have been analyzed across the Phase 3 development program of canagliflozin. Generally, only small, nonsignificant mean percent changes from baseline in serum potassium have been observed with canagliflozin and placebo regardless of baseline renal function, with no notable between-group differences [11, 12, 21, 22]. Several analyses examined the effects of canagliflozin on serum potassium in patients with various levels of baseline renal function based on the expectation that serum electrolytes may be affected by renal function and in a pooled analysis of four studies that evaluated subgroups of patients by baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 and 45 to <60 mL/min/1.73 m2 [including interim data from the CANagliflozin cardioVascular Assessment Study (CANVAS) trial], there was a numerically higher number of episodes of elevated serum potassium [potassium > upper limit of normal (ULN) and >15% increase from baseline] with canagliflozin 300 mg (but not 100 mg) versus placebo in both eGFR subgroups, which was hypothesized to be related to the mild diuretic effects of canagliflozin; the incidence of hyperkalemia adverse events was generally similar with the canagliflozin 100 and 300 mg doses and placebo [11]. In a separate pooled analysis of Phase 3 studies of patients with T2DM and Stage 3 CKD (eGFR 30 to <60 mL/min/1.73 m2; including interim data from the CANVAS trial), the incidence of elevated serum potassium was numerically higher with canagliflozin 300 mg (but not 100 mg) compared with placebo, with events more frequently observed in patients with Stage 3b CKD who either received canagliflozin 300 mg, had elevated potassium before treatment and/or were on multiple medications that reduce potassium excretion; however, there was no difference in the incidence of hyperkalemia with canagliflozin and placebo [12].
Based on early data, including interim data from the CANVAS trial, a warning of increased risk of hyperkalemia in patients with renal impairment, especially those taking medications that interfere with potassium excretion or the renin–angiotensin–aldosterone system (RAAS), was included in the Warnings and Precautions section of the initial canagliflozin package insert [23, 24]. Here, we report analyses of several potassium measurements and hyperkalemia rates from the completed CANVAS Program that provide evidence supporting the recent removal of the hyperkalemia warning from the canagliflozin package insert.
MATERIALS AND METHODS
Study design and participants
The CANVAS Program (n = 10,142) consisted of two comparable, double-blind, randomized, placebo-controlled trials [CANVAS (n = 4330) and CANVAS-Renal (CANVAS-R; n = 5812)] that have been described previously [4]. Eligible participants had T2DM with hemoglobin A1C ≥7% to ≤10.5% and were either ≥30 years of age with symptomatic atherosclerotic CV disease or ≥50 years of age with two or more CV risk factors. Patients with screening eGFR <30 mL/min/1.73 m2 were excluded; there were no other kidney-related eligibility criteria. Participants in the CANVAS study were randomized 1:1:1 to canagliflozin 100 or 300 mg or placebo. In CANVAS-R, participants were randomized 1:1 to canagliflozin 100 mg or placebo, with an optional increase to canagliflozin 300 mg or matching placebo starting from Week 13.
Assessments
Serum potassium measurements were obtained at baseline and then at Weeks 6 and 18 for CANVAS, Weeks 13 and 26 for CANVAS-R, Week 52 for both studies and every 6 months after that throughout the course of the studies. All measurements were performed at a central laboratory. The values meeting a predefined limit of change criteria were prespecified for analysis and assessed using results obtained at any time up to 2 days after the last dose of the study drug.
Outcomes
In the CANVAS Program, mean serum potassium over time was assessed overall and by baseline eGFR (≥60, 45 to <60 and <45 mL/min/1.73 m2). In the CANVAS Program, analyses of the incidence of participants with serum potassium meeting the predefined limit of change criteria were prespecified and evaluated overall, by baseline eGFR and by baseline RAAS inhibitor use. In the CANVAS study only, analyses of the incidence of participants with serum potassium meeting predefined limits of change criteria were prespecified and evaluated by canagliflozin dose (100 and 300 mg); dose response could not be evaluated in CANVAS-R due to the option to up-titrate canagliflozin dose during the study. The prespecified limit of change criteria for decreased serum potassium was serum potassium <3.4 mEq/L (lower limit of normal) and >15% decrease from baseline. The prespecified limit of change criteria for increased serum potassium was serum potassium >5.4 mEq/L (ULN) and >15% increase from baseline. Analysis of serum potassium measurements ≥6.5 mEq/L was also predefined. Post hoc assessments of serum potassium ≥5.5 and ≥6.0 mEq/L were also evaluated overall and by baseline eGFR (≥60, 45 to <60, and <45 mL/min/1.73 m2). Safety analyses included all investigator-reported adverse events of hyperkalemia that included the terms hyperkalemia or blood potassium increased collected from the initiation of the CANVAS study in December 2009 through 7 January 2014. In the CANVAS-R study, which started in 2014, only serious adverse events or adverse events leading to discontinuation were collected; thus, data for serious adverse events of hyperkalemia are reported for the entire CANVAS Program.
Statistical analysis
Treatment effects of combined canagliflozin doses versus placebo on serum potassium levels were evaluated using the CANVAS Program integrated dataset and were based on the intention to treat analysis. The visual representation of the pattern of adjusted mean serum potassium was provided using a restricted maximum likelihood repeated measures approach. This analysis included the fixed, categorical effects of treatment, visit, study, category of baseline eGFR (≥60, 45 to <60, and <45 mL/min/1.73 m2) and treatment-by-visit interaction, as well as the continuous, fixed covariates of baseline serum potassium value and baseline-by-visit interaction, assuming an unstructured within-subject covariance. The least squares (LS) mean change in serum potassium from baseline to the end of study was estimated using an analysis of covariance including the effects of study, treatment, category of baseline eGFR and baseline serum potassium value. To evaluate the risk of increased or decreased potassium, hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox regression model with treatment and category of baseline eGFR as the explanatory variables, stratified by history of CV disease and study as appropriate. Annualized incidence rates were calculated per 1000 participant-years of follow-up. A similar Cox model was used in the assessment of hyperkalemia risk based on an on-treatment approach that includes post-treatment events up to the last study medication plus 30 days.
RESULTS
Participants
The CANVAS Program enrolled a total of 10 142 participants: 4330 in CANVAS and 5812 in CANVAS-R. A total of 9734 participants (96%) completed the trials with a mean and median follow-up of 188.2 and 126.1 weeks, respectively. Baseline characteristics of these participants have been previously reported [4]. Briefly, the mean age of participants was 63.3 years, 35.8% were women, the mean eGFR was 76.5 mL/min/1.73 m2 and the mean serum potassium level was 4.4 mEq/L. Approximately 80% of patients were taking RAAS inhibitors, and 5% had a baseline eGFR <45 mL/min/1.73 m2. Of participants in the CANVAS study, 1445 and 1443 were on canagliflozin 100 and 300 mg, respectively.
Serum potassium levels
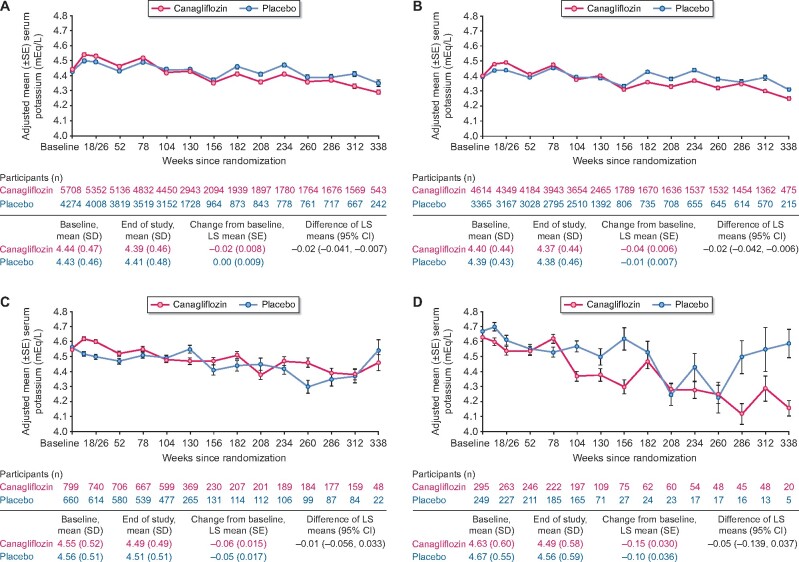
Mean serum potassium levels were generally consistent over time in the canagliflozin and placebo groups, overall and by baseline eGFR (≥60, 45 to <60, and <45 mL/min/1.73 m2; Figure 1). Over the course of the study, the difference of LS mean change (95% CI) from baseline in serum potassium between canagliflozin and placebo was −0.02 (−0.04 to −0.01) mEq/L overall, and −0.02 (−0.04 to −0.01), −0.01 (−0.06 to 0.03) and −0.05 (−0.14 to 0.04) mEq/L in participants with baseline eGFR ≥60, 45 to <60, and <45 mL/min/1.73 m2, respectively.
FIGURE 1:
Mean serum potassium in the CANVAS Program (A) overall, and by baseline eGFR (B) ≥60 mL/min/1.73 m2, (C) 45 to <60 mL/min/1.73 m2 and (D) <45 mL/min/1.73 m2. SE, standard error.
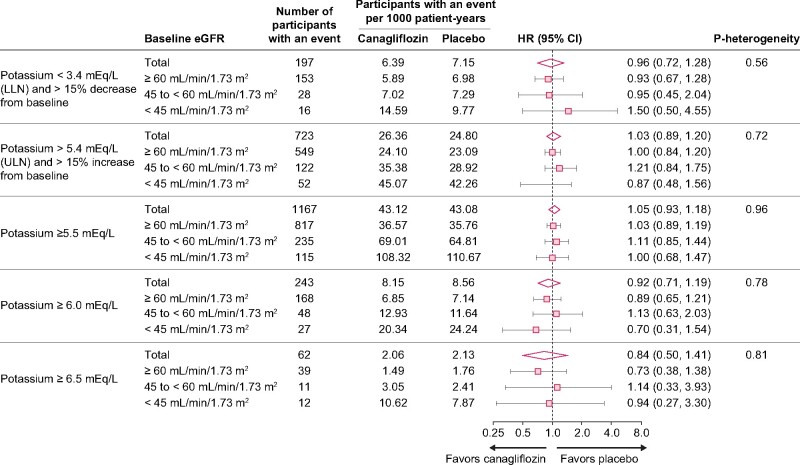
The risk of increased serum potassium during the study was similar with canagliflozin and placebo, using a number of thresholds for increased serum potassium, some of which included a requirement for a minimum change from baseline in serum potassium (Figure 2). The risk of decreased serum potassium was also similar with canagliflozin and placebo. These results were generally consistent across categories of renal function, as illustrated by the similar results across eGFR subgroups (≥60, 45 to <60, and <45 mL/min/1.73 m2; all P-heterogeneity ≥0.56).
FIGURE 2:
Participants with serum potassium values outside of specified ranges in the CANVAS Program overall and by baseline eGFR (intent-to-treat analysis, n = 10 140). LLN, lower limit of normal.
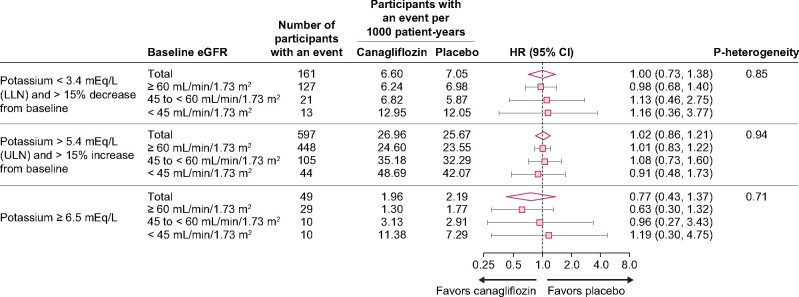
In participants who received RAAS blockers at baseline in the CANVAS Program, the risk of increased or decreased serum potassium was not different between canagliflozin and placebo in the overall population or across baseline eGFR levels (Figure 3; all P-heterogeneity ≥0.71).
FIGURE 3:
Participants with baseline RAAS inhibitor use with serum potassium values outside of predefined limit of change criteria in the CANVAS Program overall and by baseline eGFR (n = 8114 randomized participants with baseline RAAS inhibitor use and baseline serum potassium measurements). LLN, lower limit of normal.
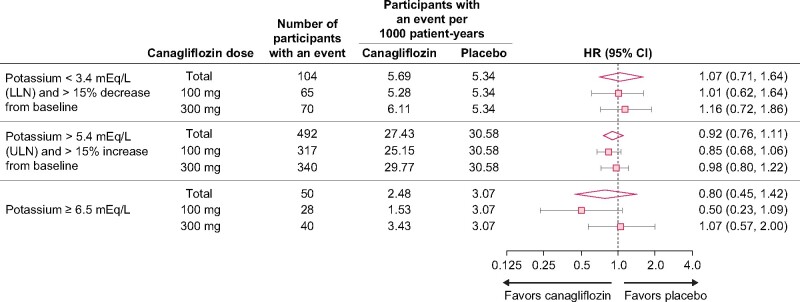
In analyses of CANVAS study participants who were randomized to treatment with canagliflozin 100 or 300 mg or placebo, the risk of increased or decreased serum potassium values outside the predefined limits of change was consistent by dose of canagliflozin (Figure 4).
FIGURE 4:
Participants with serum potassium values outside of predefined limit of change criteria by canagliflozin dose in the CANVAS study (n = 4328 randomized participants with baseline serum potassium measurements in the CANVAS study). LLN, lower limit of normal.
Safety
Adverse events of hyperkalemia were uncommon and not statistically different between treatment arms. In the CANVAS study through 7 January 2014, adverse events of hyperkalemia occurred in 6.9 and 4.4 participants per 1000 patient-years with canagliflozin and placebo, respectively [HR (95% CI) 1.60 (0.92–2.81)]. Across the CANVAS Program, serious adverse events of hyperkalemia occurred in 0.4 and 0.6 participants per 1000 patient-years with canagliflozin and placebo, respectively [HR (95% CI) 0.75 (0.27–2.11)]. During the entire study period, fatal hyperkalemia occurred in one study participant in the placebo arm; no participants in the canagliflozin arm experienced fatal hyperkalemia.
DISCUSSION
In the CANVAS Program, no meaningful effects on average serum potassium were seen with canagliflozin versus placebo overall or when evaluated by baseline eGFR. Moreover, no significant differences in the risk of increased or decreased serum potassium were observed with canagliflozin versus placebo regardless of a variety of variables, including canagliflozin dose, RAAS inhibitor use and baseline eGFR. In addition, the incidence of hyperkalemia adverse events was not different between canagliflozin and placebo. These results provide additional evidence that canagliflozin has no effect on serum potassium and does not increase the risk of hyperkalemia.
Based on data from the completed CANVAS Program, the warning for hyperkalemia was removed from the Warnings and Precautions section of the INVOKANA US package insert in October 2018, and monitoring of serum potassium is no longer required [25]. Removal of this warning reverses the initial concern that concomitant use of canagliflozin may increase the risk of hyperkalemia known to be associated with ACE inhibitor or ARB therapy.
Hyperkalemia is a frequent occurrence in clinical practice in people with diabetes, especially in those with reduced kidney function [26, 27], and patients at high risk of hyperkalemia should be monitored for changes in potassium as clinically appropriate. Treatment with RAAS inhibitors, which are recommended to reduce CV and renal disease progression in patients with T2DM and CKD, further increases the risk of hyperkalemia [28–30], and monitoring of serum potassium is required for some ACE inhibitors and ARBs [18–20]. As physicians are concerned about mitigating the risk of hyperkalemia-induced cardiac arrhythmia, they may reduce the dose or stop the use of RAAS inhibitors in their patients based on concerns related to hyperkalemia [28, 29]. Thus, patients often may not derive adequate cardiorenal protective benefits from these drugs [14].
SGLT2 inhibitors are a novel therapeutic opportunity for patients with T2DM and CKD. The lack of new treatments for and increased prevalence of T2DM and CKD over the past several decades has presented a challenge for clinicians. The evidence that this therapeutic class reduces blood pressure, may reduce albuminuria and may also slow the progression of kidney disease, as suggested by the CV outcomes trials of canagliflozin, dapagliflozin and empagliflozin, highlights the therapeutic potential of this class to optimize clinical outcomes in patients with T2DM and CKD. Interestingly, these consistent observations of reduced blood pressure, as well as the slowing of loss of kidney function over time, occurred in these CV outcome trials despite the use of drugs that block the RAAS in ∼80% of patients [4, 6, 31]. In the first dedicated study of an SGLT2 inhibitor in patients with T2DM and CKD, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), canagliflozin demonstrated a 30% reduction in the relative risk of the primary outcome, end-stage kidney disease, doubling of serum creatinine or death from renal or CV causes, as well as benefits in other CV and renal outcomes in a population where 99.9% of patients were on RAAS inhibitors at baseline [7].
In contrast to previous analyses that included interim data from the CANVAS trial, the results of our analyses using data from the completed CANVAS Program provide encouragement that, from a safety standpoint, there are no apparent changes in serum potassium that could interfere with the use of canagliflozin in patients with T2DM and moderately impaired renal function whether used with or without RAAS inhibitors, though only 5% of participants had a baseline eGFR <45 mL/min/1.73 m2. The removal of the warning for hyperkalemia and requirement for monitoring of serum potassium from the US label of canagliflozin makes it consistent with the labeling of other SGLT2 inhibitors, which do not require potassium monitoring [2, 3, 25, 32]. The neutrality of SGLT2 inhibitors with respect to potassium may allow more opportunity for the expansion of the use of SGLT2 inhibitors in patients with T2DM and CKD. The results of CREDENCE, which showed no increase in the risk of hyperkalemia with canagliflozin versus placebo in patients with T2DM and CKD, further support this expansion [7]. Additional dedicated studies in patients with T2DM and CKD [Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease(DAPA-CKD) and EMPA-KIDNEY trials] are currently underway and will provide more clinical insight into the concomitant use of SGLT2 inhibitors with ACE inhibitor or ARB therapy in patients with reduced kidney function, both with and without diabetes [33, 34].
Strategies to control chronic hyperkalemia are often difficult to achieve. Reduction in dietary potassium is difficult to follow over prolonged periods of time. Increasing the use of diuretics may facilitate kaliuresis but also may be associated with volume depletion and prerenal azotemia [30, 35]. The chronic use of potassium-binding therapies may also be complicated by side effects and costs [36, 37]. Thus, the opportunity to identify a novel therapeutic strategy that may help control serum glucose, reduce blood pressure and delay progression of CKD without affecting serum potassium is an important advantage for managing patients with CKD. These results from the clinical trial experience of >10 000 participants in the CANVAS Program suggest that the SGLT2 inhibitor canagliflozin has a negligible effect on serum potassium when compared to placebo, whether or not patients are on ACE inhibitor or ARB therapy. This lack of adverse effect on serum potassium may prove to be important for future efforts to enhance renal protection in patients with T2DM and CKD.
Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
FUNDING
This study was funded by Janssen Research & Development, LLC. The study sponsor was involved in the collection and analysis of the data.
CONFLICT OF INTEREST STATEMENT
M.R.W. is a scientific consultant for Janssen. A.S. is a full-time employee of Axio Research. T.S., D.B. and R.O. are full-time employees of Janssen Research & Development, LLC. D.d.Z. reports serving on advisory boards and/or as a speaker for Bayer, Boehringer Ingelheim, Fresenius, Mundipharma and Mitsubishi Tanabe; serving on steering committees and/or as a speaker for AbbVie and Janssen; and serving on data safety and monitoring committees for Bayer. V.P. reports receiving research support from the Australian National Health and Medical Research Council (Senior Research Fellowship and Program Grant); serving on steering committees for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Novartis and Pfizer; and serving on advisory boards and/or speaking at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PharmaLink, Relypsa, Retrophin, Roche, Sanofi, Servier and Vitae.
ACKNOWLEDGEMENTS
Medical writing support was provided by Dana Tabor, PhD, of MedErgy, and was funded by Janssen Global Services, LLC.
REFERENCES
- 1.INVOKANA® (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ: Janssen Pharmaceuticals; September 2019
- 2.JARDIANCE® (empagliflozin) tablets, for oral use [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; October 2018
- 3.FARXIGA® (dapagliflozin) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; October 2019
- 4. Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 7. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 8. Cherney DZI, Odutayo A, Verma S.. A big win for diabetic kidney disease: CREDENCE. Cell Metab 2019; 29: 1024–1027 [DOI] [PubMed] [Google Scholar]
- 9. Verma S, McMurray J.. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018; 61: 2108–2117 [DOI] [PubMed] [Google Scholar]
- 10. Cherney DZ, Odutayo A, Aronson R. et al. Sodium glucose cotransporter-2 inhibition and cardiorenal protection: JACC review topic of the week. J Am Coll Cardiol 2019; 74: 2511–2524 [DOI] [PubMed] [Google Scholar]
- 11. Weir MR, Kline I, Xie J. et al. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr Med Res Opin 2014; 30: 1759–1768 [DOI] [PubMed] [Google Scholar]
- 12. Yamout HM, Perkovic V, Davies M. et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol 2014; 40: 64–74 [DOI] [PubMed] [Google Scholar]
- 13. Hughes-Austin JM, Rifkin DE, Beben T. et al. The relation of serum potassium concentration with cardiovascular events and mortality in community-living individuals. Clin J Am Soc Nephrol 2017; 12: 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo J, Brunelli SM, Jensen DE. et al. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 2016; 11: 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner S, Metzger M, Flamant M. et al. ; for the NephroTest Study group. Association of plasma potassium with mortality and end-stage kidney disease in patients with chronic kidney disease under nephrologist care - the NephroTest study. BMC Nephrol 2017; 18: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes-2019. Diabetes Care 2019; 42: S7–S12 [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 18.AVAPRO® (irbesartan) [package insert]. New York, NY: Bristol-Myers Squibb; April 2011
- 19.COZAAR® (losartan potassium) [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; October 2018
- 20.Zestril® (lisinopril) tablets [packet insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2014
- 21. Yale JF, Bakris G, Cariou B. et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014; 16: 1016–1027 [DOI] [PubMed] [Google Scholar]
- 22. Usiskin K, Kline I, Fung A. et al. Safety and tolerability of canagliflozin in patients with type 2 diabetes: pooled analysis of phase 3 study results. Postgrad Med 2014; 126: 16–34 [DOI] [PubMed] [Google Scholar]
- 23.INVOKANA® (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2015
- 24.INVOKANA™ (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2013
- 25.INVOKANA® (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2018
- 26. Liamis G, Liberopoulos E, Barkas F. et al. Diabetes mellitus and electrolyte disorders. World J Clin Cases 2014; 2: 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montford JR, Linas S.. How dangerous is hyperkalemia? J Am Soc Nephrol 2017; 28: 3155–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delanaye P, Scheen AJ.. Preventing and treating kidney disease in patients with type 2 diabetes. Expert Opin Pharmacother 2019; 20: 277–294 [DOI] [PubMed] [Google Scholar]
- 29. Rastogi A, Arman F, Alipourfetrati S.. New agents in treatment of hyperkalemia: an opportunity to optimize use of RAAS inhibitors for blood pressure control and organ protection in patients with chronic kidney disease. Curr Hypertens Rep 2016; 18: 55. [DOI] [PubMed] [Google Scholar]
- 30. Rafique Z, Weir MR, Onuigbo M. et al. Expert panel recommendations for the identification and management of hyperkalemia and role of patiromer in patients with chronic kidney disease and heart failure. J Manag Care Spec Pharm 2017; 23: S10–S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 32.STEGLATRO™ (ertugliflozin) [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; December 2017
- 33.ClinicalTrials.gov. A study to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease (Dapa-CKD). https://clinicaltrials.gov/ct2/show/NCT03036150 (1 February 2018, date last accessed)
- 34.Boehringer Ingelheim, Medical Research Council Population Health Research Unit, CTSU, University of Oxford (academic lead), Eli Lilly and Company. EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin). ClinicalTrials.gov Identifier: NCT03594110. https://clinicaltrials.gov/ct2/show/NCT03594110 (12 February 2019, date last accessed)
- 35. Greenberg A. Diuretic complications. Am J Med Sci 2000; 319: 10–24 [PubMed] [Google Scholar]
- 36. Bounthavong M, Butler J, Dolan CM. et al. Cost-effectiveness analysis of patiromer and spironolactone therapy in heart failure patients with hyperkalemia. Pharmacoeconomics 2018; 36: 1463–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Das S, Dey JK, Sen S. et al. Efficacy and safety of patiromer in hyperkalemia: a systematic review and meta-analysis. J Pharm Pract 2018; 31: 6–17 [DOI] [PubMed] [Google Scholar]