Abstract
SARS-CoV-2 has been reported as a possible triggering factor for the development of several autoimmune diseases and inflammatory dysregulation. Here, we present a case report of a woman with a history of systemic lupus erythematosus and antiphospholipid syndrome, presenting with concurrent COVID-19 infection and immune thrombotic thrombocytopenic purpura (TTP). The patient was treated with plasma exchange, steroids, and caplacizumab with initial good response to therapy. The course of both TTP and COVID-19 disease was mild. However, after ADAMTS-13 activity was normalized, the patient experienced an early unexpected TTP relapse manifested by intravascular hemolysis with stable platelet counts requiring further treatment. Only 3 cases of COVID-19 associated TTP were reported in the literature thus far. We summarize the literature and suggest that COVID-19 could act as a trigger for TTP, with good outcomes if recognized and treated early.
Keywords: SARS-CoV-2, COVID-19, Immune thrombotic thrombocytopenic purpura, Caplacizumab
Introduction
Coronavirus 2 (SARS-CoV-2) can cause autoimmune and/or auto-inflammatory dysregulation. Autoimmune thrombotic thrombocytopenic purpura (TTP) as a complication of SARS-CoV-2 infection has been reported in 3 cases thus far [1, 2, 3]. We report a woman with COVID-19 infection and acquired TTP who relapsed early during the acute infection.
Case Presentation
A 62-year-old woman was transferred to our hospital for plasma exchange. She presented to another hospital due to acute epigastric abdominal pain 7 days earlier. She had a history of systemic lupus erythematosus (SLE), antiphospholipid syndrome (APLS), CVA, hyperlipidemia trigeminal neuralgia, and hypothyroidism. She was treated with low-dose prednisone, hydroxychloroquine, eltroxin, pregabalin, rosuvastatin, carbamazepine, ramipril, and clopidogrel. On admission, she had normal vital signs and mild epigastric tenderness. Hemoglobin (HgB) was 11.7 g/dL, the platelet count was 84 × 109/L, and the total leukocyte count was 12 × 109/L. The Cr level was 0.43 mg/dL, and there was a slight elevation in liver enzymes. Lactate dehydrogenase (LDH) was not measured. Abdominal X-ray and ultrasonography was normal. Past platelet counts were in the range of 100–140 × 109/L. The presumed diagnosis was peptic ulcer disease, and she was discharged.
Two days prior to the patient's referral to our hospital, she presented again with worsening right lower quadrant and flank abdominal pain. Vital signs were normal. The abdomen was soft and slightly tender, with 2 ecchymoses measuring 4 cm in the RLQ. Neurological examination was normal. Abdominal computed tomography showed a mild enlargement of the right kidney with perinephric fat infiltration and a mild dilatation of the right ureter and collecting ducts. HgB was 9.5 g/dL, platelets 41 × 109/L, and leukocyte count 5.7 × 109/L. Cr was 0.99 mg/dL, LDH was 937 U/L (normal range up to 450 U/L), haptoglobin was <10 mg/dL, PT and aPTT were normal, and fibrinogen was high 667 mg/dL. D-dimers were elevated to 4,536 ng/mL (normal up to 500 ng/mL). Peripheral blood smear showed schistocytes (online suppl. Fig. 1; see www.karger.com/doi/10.1159/0005514283 for all online suppl. material). Immune TTP (iTTP) was suspected. There was no evidence for DIC as coagulation tests were normal and there were no thrombotic complications, making catastrophic aPLS unlikely. A nasopharyngeal swab for SARS-CoV-2 RNA in the nasopharynx was negative. ADAMTS-13 activity was measured and was nondetectable (0%) by a chromogenic ELISA (Technoclone, Vienna Austria) with borderline anti-ADAMTS-13 antibody levels of 17I U/mL (normal up to 15I U/mL). She received 2 units of fresh frozen plasma and was transferred to initiate emergent plasma exchange treatments.
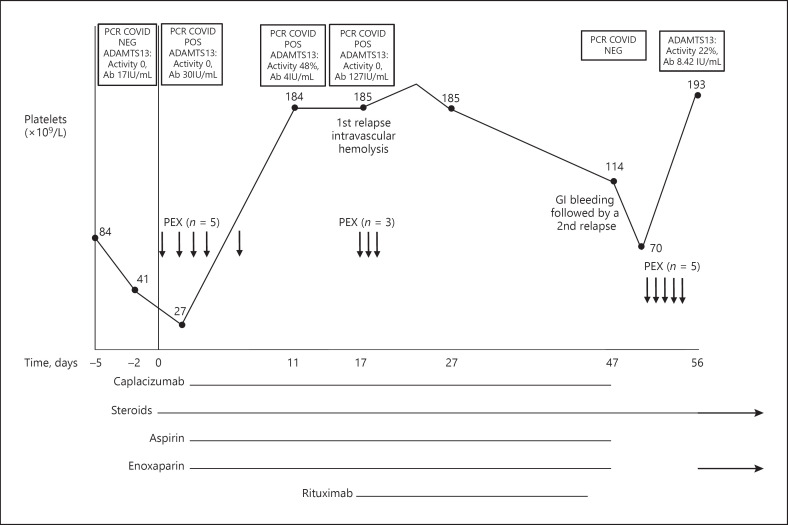
Upon admission to our hospital, the patient was in a good general condition. LDH was 1329 U/L, and platelet count was 32 × 109/L. Daily single volume plasma exchange was commenced along with intravenous hydrocortisone (100 mg × 3 per day) and folic acid supplementation. ADAMTS-13 activity and inhibitor were retested and were reported to be 0.07 (normal range: 0.40–1.3 IU/mL) and 30 IU/mL (normal range 0–15 IU/mL), respectively, which reconfirmed the diagnosis of acquired autoimmune TTP. Indirect and direct Coombs tests were negative. A second nasopharyngeal swab test for the detection of SARS-CoV-2 RNA was positive, and she was transferred to the COVID-19 ward. She had a mild cough and low-grade fever, with moderate interstitial infiltrates compatible with COVID-19 pneumonia (online suppl. Fig. 2). After the first plasma exchange, the abdominal pain resolved. By the third day of plasma exchange therapy, the patient's platelet count increased to 91 × 109/L, while the HgB level was 7.2 g/dL and LDH was 666 U/L. She was transfused with 1 leukoreduced RBC unit. Aspirin and enoxaparin dosed at 1 mg/kg once daily were administrated daily. Treatment with caplacizumab was started before the fourth plasma exchange treatment. After 5 plasma exchanges, platelets increased to 184 × 109/L, and plasma exchange was discontinued. Repeat ADAMTS-13 test was normal, with an activity of 0.48 IU/mL and inhibitor of 4.1 IU/mL. The patient was discharged home 11 days after admission to our hospital. During her hospital stay, she was clinically stable with no worsening respiratory symptoms. Upon discharge, she was still positive for SARS-CoV-2. At home, she continued treatment with prednisone 1 mg/kg once daily, aspirin, enoxaparin, folic acid, and daily caplacizumab. Six days later, she was rehospitalized with an HgB drop to 7.5 g/dL and weakness. The platelet count was 185 × 109/L, LDH increased to 865 U/L, Cr was 0.46 mg/dL, D-dimers 1,225 ng/mL, the direct Coombs test was positive, the indirect Coombs was positive, and anti-Jka antibody was detected in serum and RBC eluate. We first assumed the patient had developed hemolysis of the single blood unit she had received; however, ADAMTS-13 activity was again undetected with a higher inhibitor of 127 IU/mL, suggesting relapsed TTP. She was treated with 3 additional plasma exchange courses, steroids, and caplacizumab. Rituximab 375 mg/m2 was added once weekly for 4 cycles. HgB and LDH remained stable for 21 days after the first dose of rituximab, when the first negative test for COVID-19 received. Three weeks after discharge, she was hospitalized again with severe anemia and melena. HgB was 3.8 g/dL, while the platelet count was 114 × 109/L. LDH was within normal limits. Caplacizumab, LMWH, and aspirin were withheld. Endoscopic evaluation could not find the source of bleeding. After stopping caplacizumab, the platelet count decreased to 70 × 109/L, ADAMTS-13 activity was undetected, and she was diagnosed with a second TTP relapse. Plasma exchange was resumed for 5 more cycles, until the platelet count was above 150 × 109/L for 2 days. Due to gastrointestinal bleeding, she only received LMWH (with no antiplatelet or caplacizumab). After plasma exchange, platelet count, HgB levels, and ADAMTS-13 were normalized. Fig. 1 summarizes the clinical course, treatment, and PCR positivity.
Fig. 1.
Association between coronavirus 2 (SARS-CoV-2) PCR positivity, platelet count, ADAMTS13 activity, ADAMTS13 antibody titer, treatment institution, and relapse is shown above. COVID = SARS-CoV-2. ADAMTS13 activity reference range: 40–130%; ADAMTS13 inhibitor reference range: 0–15.00 IU/mL. Day 0 = admission to our hospital and initiation of plasma exchange.
Discussion
To the best of our knowledge, only 2 cases of TTP in an acutely infected COVID-19 patient have been reported thus far, although nasopharyngeal swab for the detection of SARS-CoV-2 RNA was positive in only one of them (1,2). Another case of relapsed TTP was reported in a woman after following a recovery from COVID-19 infection. She was tested to be seropositive for SARS-CoV-2 IgG with a negative nasopharyngeal swab [3] (Table 1).
Table 1.
iTTP associated with COVID-19 infection: comparison of cases described in the literature and our case
| Reference | Gender/age, years | TTP diagnosis time relative to SARS-CoV-2 infection | SARS-CoV-2 swab | COVID 19 disease severity | TTP treatment | TTP course |
|---|---|---|---|---|---|---|
| Our case | Female/62 | Concomitant | Positive | Mild | PEX (n = 5, 1st relapse n = 3, 2nd relapse n = 5), steroids, caplacizumab, and rituximab | Two relapses |
| Hindilerden et al. [1] | Female/74 | Concomitant | Positive | Mild | PEX (n = 11) Steroids | Lethargy − resolved |
| Albiol et al. [2] | Female/57 | Concomitant | Negative (referred to as false negative, followed by positive serology) | Mild | Steroids, intravenous immunoglobulin PEX (n = 7) |
Uneventful |
| Capecchi et al. [3] Female/55 | Relapsed TTP following recovery from SARS CoV-2 | Negative (positive serology suggesting a recent infection) | Mild Severe post-COVID pulmonary complications | Plasma infusion PEX (n = 14) Steroids caplacizumab | Severe neurological symptoms, multi-organ failure, DIC Intracranial hemorrhage | |
iTTP, immune thrombotic thrombocytopenic purpura.
We present a woman with a history of SLE and APLS, admitted with concurrent COVID-19 infection and acquired TTP. TTP has been described in association with SLE and APLS [4, 5]. Considering the rarity and complexity of this case, we have yet to decipher whether COVID-19 was the triggering factor for TTP or whether these were 2 diseases occurring simultaneously. Our patient suffered from abdominal pain 1 week before diagnosis of TTP, while the platelet count was 84 × 109/L. Since the patient had a mild chronic thrombocytopenia, TTP was not suspected upon her ER visit. Five days later, when diagnosis of TTP was confirmed, the first nasopharyngeal swab for SARS-CoV-2 was negative. Since the SARS-CoV-2 test performed approximately 24 h later in our hospital was positive, it is plausible that the first swab test was falsely negative, a common pitfall in the diagnosis of an early-stage COVID-19 infection. Therefore, the possibility of a COVID-19-triggered TTP is plausible, and iTTP could be a presenting manifestation for SARS-CoV-2 infection even before PCR positivity. Furthermore, it was demonstrated that normal ADAMTS13 activity in clinical remission are associated with a low risk of TTP relapse [6]. The fact that TTP recurred very early in our patient after ADAMTS-13 inhibitor normalized was surprising and supports the hypothesis that there was a continued trigger for the disease, such as COVID-19 infection.
COVID-19-associated coagulopathy leading to a prothrombotic state was shown to be associated with elevated von Willebrand factor (VWF) antigen and activity [7]. In a previous study, ADAMTS-13 levels were significantly reduced in all COVID-19 patients [8]. ADAMTS-13 plasma levels less than 30% were significantly associated with a higher mortality [9]. In our case, no clinical thrombotic complications have occurred, despite unmeasurable ADAMS13 activity and the presence of antiphospholipid antibodies. However, the patient was treated early with enoxaparin aspirin and caplacizumab.
Clopidogrel-associated TTP was first identified in 1999 and is one of the most common drugs associated with TTP according to the FDA safety databases [10, 11]. Clopidogrel-associated TTP onset usually occurs within 2 weeks of drug initiation, and only in a minority of cases, ADAMTS-13 activity is reduced [10]. Therefore, clopidogrel as the triggering factor for TTP in this patient is unlikely. Still, the patient was advised to discontinue clopidogrel and switch to aspirin.
Caplacizumab is a humanized anti-VWF antibody which inhibits interaction between VWF large multimers and platelets [12]. Capecchi M et al. reported a case of a woman with relapsed TTP associated with a recent COVID-19 infection [3]. This woman had a life-threatening presentation of TTP with altered consciousness and multi-organ failure. Treatment with caplacizumab started early, with a platelet count rising within 6 h after the first injection. Our patient did not develop neurological symptoms and eventually had a mild course of TTP, although she relapsed early. Since caplacizumab is not immunosuppressive, it is not predicted to have an impact on COVID-19 disease. We decided to prescribe caplacizumab in order to try and avoid rituximab administration during acute SARS-CoV-2 infection as advised by guidelines [13] and also in order to reduce the number of plasma exchange treatments in view of the complexity of treatment at the COVID-19 department. Presuming that SARS-CoV-2 infection was a triggering factor to TTP, we also assumed it was safer to discharge the patient home while still positive for SARS-CoV-2 with the combination of steroids and caplacizumab. Indeed when she relapsed, thrombocytopenia did not develop probably because of caplacizumab treatment.
The fact that our patient had O+ blood type is consistent with the literature stating that for these patients, it take a shorter time to recover as measured by a fewer number of plasma exchanges needed for platelet recovery, in comparison to non-O blood group patients [14]. A delayed hemolytic transfusion reaction results in RBC hemolysis 1–28 days following blood transfusion. Delayed hemolytic transfusion reaction incidence is ∼1:20,000 [15]. In our patient, anti-Jka was seen. Multiple factors affect RBC alloimmunization including discrepancies of RBC antigens between the donor and recipient, HLA haplotypes, autoimmune diseases, and the presence of inflammation [16]. It is plausible that the both the inflammatory state caused by COVID-19 and autoimmunity contributed to the development of anti-Jka following a single RBC transfusion, despite the fact that the Jka antigen is not highly immunogenic.
In conclusion, SARS-CoV-2 infection may trigger the development of autoimmune TTP. Patients may have a favorable course if diagnosed and treated early. Early relapse may occur, despite initial response to therapy.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors declare no conflict of interest.
Author Contributions
All authors were involved in patient treatment. G.S. and M.K.C. wrote the manuscript. L.S., Y.S. V.Y., and A.G.G. have critically reviewed the manuscript.
References
- 1.Hindilerden F, Yonal-Hindilerden I, Akar E, Kart-Yasar K. Covid-19 associated autoimmune thrombotic thrombocytopenic purpura: report of a case. Thromb Res. 2020 Jul 5;195:136–8. doi: 10.1016/j.thromres.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albiol N, Awol R, Martino R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID-19. Ann Hematol. 2020;99((7)):1673–4. doi: 10.1007/s00277-020-04097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capecchi M, Mocellin C, Abbruzzese C, Mancini I, Prati D, Peyvandi F. Dramatic presentation of acquired TTP associated with COVID-19. Haematologica. 2020 Jul 2;105:e540–1. doi: 10.3324/haematol.2020.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porta C, Caporali R, Montecucco C. Autoimmunity in thrombotic thrombocytopenic purpura. Semin Thromb Hemost. 2005;31((6)):633–40. doi: 10.1055/s-2005-925469. [DOI] [PubMed] [Google Scholar]
- 5.Fayyaz A, Igoe A, Kurien BT, Danda D, James JA, Stafford HA, et al. Haematological manifestations of lupus. Lupus Sci Med. 2015 Mar 3;2((1)):e000078. doi: 10.1136/lupus-2014-000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin M, Casper TC, Cataland SR, Kennedy MS, Lin S, Li YJ, et al. Relationship between ADAMTS13 activity in clinical remission and the risk of TTP relapse. Br J Haematol. 2008;141((5)):651–8. doi: 10.1111/j.1365-2141.2008.07107.x. [DOI] [PubMed] [Google Scholar]
- 7.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7((8)):e575–82. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med. 2020;15((5)):861–3. doi: 10.1007/s11739-020-02394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escher R, Breakey N, Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res. 2020;192:174–5. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakarija A, Kwaan HC, Moake JL, Bandarenko N, Pandey DK, McKoy JM, et al. Ticlopidine- and clopidogrel-associated thrombotic thrombocytopenic purpura (TTP): review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989–2008) Kidney Int Suppl. 2009;((112)):S20–4. doi: 10.1038/ki.2008.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakarija A, Bandarenko N, Pandey DK, Auerbach A, Raisch DW, Kim B, et al. Clopidogrel-associated TTP: an update of pharmacovigilance efforts conducted by independent researchers, pharmaceutical suppliers, and the Food and Drug Administration. Stroke. 2004;35((2)):533–7. doi: 10.1161/01.STR.0000109253.66918.5E. [DOI] [PubMed] [Google Scholar]
- 12.Scully M, Cataland SR, Peyvandi F, Coppo P, Knöbl P, Kremer Hovinga JA, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380((4)):335–46. doi: 10.1056/NEJMoa1806311. [DOI] [PubMed] [Google Scholar]
- 13. https://www.hematology.org/covid-19/covid-19-and-ttp.
- 14.Behtaj M, Zhu ML, Bittencourt CE, Ha JP, Maitta RW. Non-O blood group thrombotic thrombocytopenic purpura patients take longer to recover as measured by number of therapeutic plasma exchanges needed for platelet recovery. Thromb Res. 2020 Jan;185:78–84. doi: 10.1016/j.thromres.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood. 2019;133((17)):1831–9. doi: 10.1182/blood-2018-10-833988. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickson JE, Tormey CA. Understanding red blood cell alloimmunization triggers. Hematology Am Soc Hematol Educ Program. 2016;2016((1)):446–51. doi: 10.1182/asheducation-2016.1.446. [DOI] [PMC free article] [PubMed] [Google Scholar]