Abstract
Objective
This study was undertaken to estimate the cumulative incidence rate of rheumatoid arthritis (RA) in the Taiwanese population ages 16–84 years, and life expectancy, loss of life expectancy, and lifetime health care expenditures for incident RA in Taiwan after 2003, when biologics began to be prescribed.
Methods
We obtained all claims data for the period 1999 to 2016 from the National Health Insurance program of Taiwan, and validated the data against the Catastrophic Illness Registry to establish the study cohort. We estimated the survival function for RA and extrapolated to lifetime using a rolling‐over algorithm. For every RA case, we simulated sex‐, age‐, and calendar year–matched referents from vital statistics and estimated their life expectancy. The difference between the life expectancy of the referent and the life expectancy of the RA patient was the loss of life expectancy for the RA patient. Average monthly health care expenditures were multiplied by the corresponding survival rates and summed up throughout the lifetime to calculate the lifetime health care expenditures.
Results
A total of 29,352 new RA cases were identified during 2003–2016. There was a decreasing trend in cumulative incidence rate in those ages 16–84 for both sexes. Mean life expectancy after diagnosis of RA was 26.3 years, and mean lifetime cost was $72,953. RA patients had a mean loss of life expectancy of 4.97 years. Women with RA survived 1–2 years longer than men with RA of the same age, which resulted in higher lifetime expenditures for the former. Since the life expectancy for women in Taiwan was 6–7 years higher than that for men, the loss of life expectancy for women with RA was higher than that for men with RA. Annual health care expenditures were similar for both sexes.
Conclusion
Our findings indicate that since biologics became available, RA patients have lived longer and had higher lifetime expenditures, which should be monitored and evaluated for cost‐effectiveness.
INTRODUCTION
Rheumatoid arthritis (RA) causes huge burdens of premature mortality and high medical expenditures (1). With advancements in treatment in recent decades, mortality has decreased, while medical expenditures have increased (2, 3). Although investigators have used the standardized mortality rate to estimate the disease impact on patients conventionally, the impact of RA would be more easily understood by lay people if we could inform them of the life years lost due to the disease (4). In fact, several studies have reported the life years lost attributable to RA, but those studies generally showed large variations, ranging between 1 and 10 years (5, 6, 7, 8, 9, 10). Possible reasons for this variation include cohorts collected before the launch of biologic agents (6, 7, 8), follow‐up periods that covered the time both before and after important drugs (methotrexate or biologics) were launched (5, 9, 10), RA samples from a single hospital (5, 6, 8, 9), and short follow‐up periods (5, 10). In addition, most studies were based on prevalent cases of RA (6, 7, 9, 10). Alternatively, one may directly estimate the loss of life expectancy, which is the difference between the life expectancy of newly diagnosed RA patients (i.e., incident cases) and the life expectancy of their corresponding sex‐ and age‐matched referents in the same calendar year, which could be estimated through life tables and real world data. Such a measure would provide stakeholders with more direct information about disease impact to assist health policy decisions.
Moreover, previous studies of RA have usually used cross‐sectional or short‐term data to calculate health care expenditures, and most of them presented the expenditure as annual costs (11). Estimating long‐term or lifetime costs could provide an overall estimate of the future impact on the health care system after disease occurrence, and would be useful for cost‐effectiveness analysis. However, most previous studies that analyzed lifetime costs used Markov models (12, 13). Markov models are useful for long‐term predictions and policy decisions, but they are generally based on transitional probabilities and related costs extracted from short‐term studies, which should be validated by real‐world data after a long‐term follow‐up.
Hwang et al developed a novel semiparametric method to extrapolate the survival function at the end of follow‐up, thereby improving the accuracy of estimates of life expectancy and loss of life expectancy from the date of diagnosis (14, 15). The main principle of this method is to use data from age‐ and sex‐matched referents simulated from vital statistics of the same calendar year for extrapolation. Using this novel method in a nationwide longitudinal cohort of incident cases of RA with up to 14 years of follow‐up, we estimated the life expectancy, loss of life expectancy, and associated lifetime health care costs attributable to RA, with a particular focus on the period after 2003, when biologics began to be prescribed in Taiwan.
PATIENTS AND METHODS
Study approval
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (B‐ER‐105‐386).
Data source
Taiwan launched the National Health Insurance (NHI) program in 1995. Since then, the public has received comprehensive medical care. The coverage rate of the NHI has been >99% since 2004. In this study, we abstracted claims data from the NHI program after creating interlinkages with the National Mortality Registry and Catastrophic Illness Registry. Data were obtained from the Health and Welfare Data Science Center of the Ministry of Health and Welfare of Taiwan.
Study cohort
Nationwide population‐based data for the period from 1999 to 2016 were retrieved from the claims database of the NHI program. RA cases were defined according to the International Classification of Diseases, Ninth Revision (ICD‐9) CM codes 714.0, 714.1, 714.2, and 714.81, and ICD‐10 CM codes M05, M06.0, and M06.2–M06.9 (16, 17). RA is included in the list of catastrophic illnesses and eligible for exemption from co‐payments in Taiwan. According to NHI regulations, the approval of catastrophic illness status for RA requires clinical and laboratory findings fulfilling the American College of Rheumatology criteria (18, 19). To prevent abuse, all registered cases must first be validated by 2 physicians for quality assurance. All RA cases detected in the NHI program were verified with the Catastrophic Illness Registry in this study. Since the cost of biologic agents has been reimbursed by the Taiwan NHI program since 2003, we included only RA cases newly diagnosed after 2003 in our study cohort. Patients <16 years old at the time of diagnosis were excluded (20). All patients were followed up to the end of 2016 or death. The patients’ survival status was verified by linkage with the National Mortality Registry. The estimate of health care expenditures included both outpatient and inpatient costs related to RA. The analyses of life expectancy and lifetime costs were further stratified by sex and age at diagnosis of RA.
Estimation of the cumulative incidence rate of RA
The cumulative incidence rate can be used to estimate the lifetime risk, or probability that an individual will develop a disease at some point during the life span (21). Although some investigators have proposed defining the lifetime risk as the risk up to age 100 years (22, 23), here we considered the risk from age 16 up to the life expectancy of women in Taiwan, or 84 years, to be the lifetime risk of RA in a general adult population for each calendar year from 2003 to 2016. Using the age‐ and sex‐specific number of new cases abstracted from the NHI database for every 5 consecutive calendar years as the numerator, and the corresponding mid‐year population at risk from vital statistics as the denominator, we calculated the age‐ and sex‐specific incidence rates and cumulative incidence rate for RA. The cumulative incidence rate for the population ages 16–84 years was calculated to estimate the lifetime risk of RA, as follows:
where CIR16–84 is the cumulative incidence rate for the population ages 16–84 years, i = 16–19,20–24,…,80–84, IRi is the incidence rate of the i‐th age group, and Δti is the 5‐year age range.
To compare the characteristics of incident RA populations diagnosed in different calendar years, prevalence rates of major comorbidities in the RA population were collected.
Estimation of life expectancy after diagnosis of RA
Estimates of life expectancy after the diagnosis of RA were obtained using the semiparametric survival extrapolation method proposed by Hwang and Wang (14, 15) and verified mathematically by Fang et al (24). First, we created a reference population of subjects matched to the RA cohort with regard to age, sex, and calendar year according to the hazard function of the life table from Taiwan National Vital Statistics using Monte Carlo methods. Second, we took the logit transformation of the survival ratio of the RA cohort to the matched referents at each time point t and fitted a restricted cubic spline model, which was used to extrapolate the survival curve of the RA cohort for 1 month. Third, assuming the extrapolated 1 month to be the true observation, and omitting the data from the first month used for the previous extrapolation, we refitted the model again and extrapolated it to the next month. By performing the above procedure repeatedly, i.e., using a rolling‐over algorithm (15), and extending the survival curve for the index cohort month‐by‐month, we reached a point where the survival rate was close to 0. For example, if the survival rate were extrapolated for 50 years, or 600 months, the software would repeatedly construct the restricted cubic spline model 600 times until all cases in the index cohort were deceased. Detailed methods and empirical examples have been published previously (4, 15, 25, 26, 27, 28, 29, 30).
The area under the estimated lifetime survival curve is the life expectancy after the diagnosis of RA. The loss of life expectancy was thus the difference between the area of the mean survival curve for the RA cohort and the area of the mean survival curve for the matched referents. This parameter provided us with a measure of the loss of a patient’s lifespan, which is also the burden of RA on the society.
Estimation of lifetime health care expenditures for patients with RA
Methods of estimating lifetime medical costs have been described previously (15, 27). The total lifetime health care cost for RA patients refers to all direct health care expenditures paid by the NHI program from diagnosis of RA to death. We collected reimbursement data (inpatient and outpatient files) from the NHI database to estimate the average health care expenditure for RA patients. We calculated total health care costs, including RA‐related treatments, tests, and procedures, added up the monthly cost for all RA cases, and divided this by the total number of surviving cases in each month to estimate the average monthly health care cost. By multiplying the average monthly health care cost with the corresponding monthly mean probability of survival, we then obtained the lifetime health care cost. To accommodate for the possible increase in health care costs near the end of life, we estimated the monthly mean costs beyond the maximum follow‐up using the mean expenditures of the patients in a specific number of months prior to their death with a weighted average. The number was determined according to the observed costs in the last several months of life of deceased patients with RA, where the weights were dependent on the extrapolated hazard rate of death. Monetary value was expressed in 2019 dollars (US) ($1 [US] = $32.37 [New Taiwan]). The estimates of lifetime costs were calculated using the iSQoL2 package.
Validation of the extrapolation method
We validated the accuracy of the extrapolation method by including only patients enrolled between 2003 and 2009 in the cohort to be extrapolated for 7 years, and compared the results with the 14 years of actual follow‐up data estimated by the Kaplan‐Meier method. Namely, we tested the validity of the extrapolation method by comparing the predicted estimation of 7 years of data with the actual observed data. Using the Kaplan‐Meier estimates as the gold standard, we calculated the relative bias as follows: (estimate from extrapolation – Kaplan‐Meier estimate at the end of 14 years)/Kaplan‐Meier estimate.
RESULTS
Patient characteristics
A total of 29,352 RA cases newly diagnosed since 2003 were included. Three‐fourths of the patients were women.
Cumulative incidence rate
The lifetime risk of RA, expressed as the cumulative incidence ratio in the general Taiwanese population ages 16–84 years, is shown in Table 1. There was a consistent declining trend from 2003 to 2016, with the incidence rate declining from 1.90% to 0.68% in women and from 0.59% to 0.31% in men. Prevalence rates of major comorbidities in RA patients are summarized in Table 2. There was no difference in the prevalence of comorbidities between different calendar periods of diagnosis, except for a mild increase in the prevalence of hyperlipidemia in more recent periods.
Table 1.
Cumulative incidence rate of rheumatoid arthritis in the Taiwanese population ages 16–84 years, stratified by sex and calendar year of diagnosis*
| Year | All | Women | Men |
|---|---|---|---|
| 2003 | 1.24 | 1.90 | 0.59 |
| 2004 | 1.18 | 1.79 | 0.57 |
| 2005 | 1.12 | 1.68 | 0.55 |
| 2006 | 1.06 | 1.60 | 0.51 |
| 2007 | 1.00 | 1.44 | 0.55 |
| 2008 | 0.96 | 1.37 | 0.53 |
| 2009 | 0.94 | 1.34 | 0.52 |
| 2010 | 0.87 | 1.27 | 0.46 |
| 2011 | 0.83 | 1.16 | 0.49 |
| 2012 | 0.84 | 1.17 | 0.49 |
| 2013 | 0.73 | 1.04 | 0.41 |
| 2014 | 0.68 | 0.91 | 0.45 |
| 2015 | 0.64 | 0.89 | 0.38 |
| 2016 | 0.50 | 0.68 | 0.31 |
Values are the percentage of subjects.
Table 2.
Prevalence of major comorbidities in patients with rheumatoid arthritis, stratified by calendar period of disease diagnosis*
|
2003–2007 (n = 12,190) |
2008–2012 (n = 10,565) |
2013–2016 (n = 6,597) |
|
|---|---|---|---|
| Age, mean ± SD years | 52.48 ± 13.94 | 53.60 ± 14.16 | 54.37 ± 14.22 |
| Sex | |||
| No. (%) male | 2,751 (22.57) | 2,635 (24.94) | 1,805 (27.36) |
| No. (%) female | 9,439 (77.43) | 7,930 (75.06) | 4,792 (72.64) |
| Comorbidity, no. (%) | |||
| Cancer | 412 (3.4) | 401 (3.8) | 256 (3.9) |
| Cirrhosis of the liver | 1,411 (11.6) | 975 (9.2) | 516 (7.8) |
| COPD | 1,910 (15.7) | 1,362 (12.9) | 819 (12.4) |
| Diabetes | 984 (8.1) | 973 (9.2) | 707 (10.7) |
| Cardiovascular disease | 1,906 (15.6) | 1,585 (15.0) | 885 (13.4) |
| Hyperlipidemia | 1,610 (13.2) | 1,902 (18.0) | 1,317 (20.0) |
| Stroke | 506 (4.2) | 431 (4.1) | 253 (3.8) |
COPD = chronic obstructive pulmonary disease.
Estimation of life expectancy and loss of life expectancy
The estimated overall mean life expectancy after diagnosis of RA was 26.3 years, and the estimated mean loss of life expectancy was 4.97 years. The mean life expectancy and loss of life expectancy were 23.4 years and 9.68 years, respectively, for women, and 21.6 years and 4.11 years, respectively, for men. Table 3 summarizes the life expectancy and loss of life expectancy for RA patients stratified by sex and age at diagnosis, and Figure 1 shows the association between life expectancy and loss of life expectancy in these groups. Figure 2 shows changes in the estimated loss of life expectancy according to sex, age at diagnosis, and calendar year.
Table 3.
Life expectancy, loss of life expectancy, lifetime health care expenditures, and cost per life year of RA, stratified by sex and age at diagnosis*
| Sex and age at RA diagnosis | No. of deaths | Life expectancy, years | Loss of life expectancy, years | Loss of life expectancy/life expectancy of matched referents, %† | Lifetime health care expenditures, dollars‡ | Mean cost per life year, dollars‡ |
|---|---|---|---|---|---|---|
| Both sexes | ||||||
| Ages 16–49 years (n = 11,766) | 297 | 29.37 ± 4.81 | 14.38 ± 4.81 | 33 | 77,917 ± 7,294 | 2,653 |
| Ages 50–64 years (n = 11,477) | 907 | 21.59 ± 1.50 | 6.01 ± 1.50 | 22 | 70,037 ± 3,429 | 3,244 |
| Ages >65 years (n = 6,109) | 2,011 | 11.82 ± 0.30 | 2.42 ± 0.31 | 17 | 43,649 ± 1,303 | 3,693 |
| Men | ||||||
| Ages 16–49 years (n = 2,324) | 115 | 29.98 ± 6.28 | 8.50 ± 6.30 | 22 | 74,047 ± 7,969 | 2,470 |
| Ages 50–64 years (n = 2,956) | 364 | 22.26 ± 1.32 | 1.93 ± 1.33 | 8 | 68,068 ± 3,895 | 3,058 |
| Ages >65 years (n = 1,911) | 734 | 10.41 ± 0.43 | 2.16 ± 0.44 | 17 | 39,052 ± 1,895 | 3,751 |
| Women | ||||||
| Ages 16–49 years (n = 9,442) | 182 | 31.92 ± 6.38 | 13.11 ± 6.38 | 29 | 81,832 ± 9,179 | 2,564 |
| Ages 50–64 years (n = 8,521) | 543 | 19.44 ± 1.82 | 9.36 ± 1.82 | 33 | 67,314 ± 4,144 | 3,463 |
| Ages >65 years (n = 4,198) | 1,277 | 12.35 ± 0.34 | 2.61 ± 0.34 | 17 | 45,269 ± 1,510 | 3,666 |
Except where indicated otherwise, values are the mean ± SEM.
The life expectancy of age‐, sex‐, and calendar year–matched referents was defined as the life expectancy after diagnosis of rheumatoid arthritis (RA) plus the loss of life expectancy for RA.
$1 dollar (US) = $32.37 dollars (New Taiwan).
Figure 1.
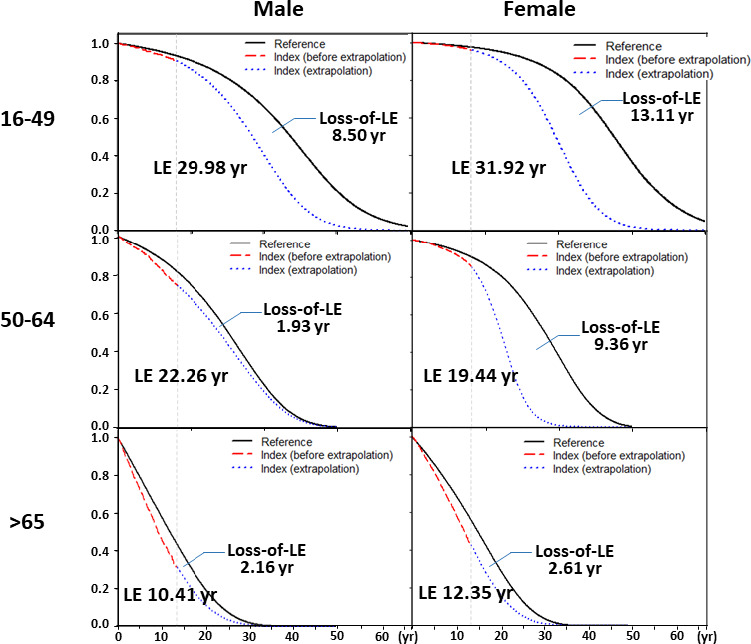
Life expectancy (LE) and loss of life expectancy after diagnosis of rheumatoid arthritis (RA), stratified by sex and age (in years) at diagnosis. Black lines represent sex‐ and age‐matched referents simulated from Taiwanese national life tables; red lines represent data from the RA cohort; blue lines represent extrapolation from the RA cohort. The area between the curve for matched referents and that for the RA cohort is the loss of life expectancy.
Figure 2.
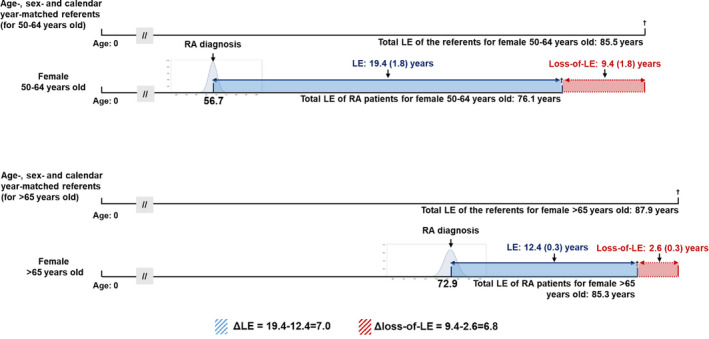
Comparison of loss of life expectancy (LE) estimates, adjusted for age, sex, and calendar year, in multiple cohorts. If a female patient with rheumatoid arthritis (RA) was diagnosed between 50 and 64 years of age, her life expectancy after diagnosis would be 19.4 years, or 7.0 years longer than that of a patient diagnosed at age >65 years (who had a life expectancy after diagnosis of 12.4 years). The loss of life expectancy was the difference between the life expectancy in the index cohort and that in the age‐, sex‐, and calendar year–matched referents simulated from life tables. Thus, the comparison of 2 loss of life expectancies, or Δloss of life expectancy (9.4−2.6 = 6.8), would be a difference‐in‐differences adjusted for the potential confounders. The apparent advantage of diagnosis at a younger age would be much less apparent after such an adjustment. Values are the mean ± SEM.
Lifetime health care expenditures and cost per life year
After biologic agents became available, the mean expected lifetime cost for RA patients was $72,953, which was higher for women than for men ($73,112 versus $63,557), and the mean cost per year was similar for women and men ($3,123 versus $2,942). Regardless of sex, the older the age at diagnosis of RA, the higher the average annual cost of treating RA (Table 3).
Validation of the extrapolation method
Table 4 indicates the differences between the month‐by‐month rolling‐over extrapolation of 7 years based on the first 7 years of data and the observed 14‐year real world estimates calculated by the Kaplan‐Meier method. The relative biases were generally <5%.
Table 4.
Validation of the extrapolated estimates of life expectancy after diagnosis of RA based on 7 years of follow‐up of the RA cohort compared with 14 years of actual follow‐up, calculated by the Kaplan‐Meier method*
| Sex and age at diagnosis | Age at diagnosis, mean ± SD years | Censoring rate at end of 7th year, % |
Estimate using the extrapolation based on the first 7 years to the 14th year, months |
Actual follow‐up of 14 years by Kaplan‐Meier estimate, months | Relative bias, %† |
|---|---|---|---|---|---|
| All | |||||
| Men (n = 3,824) | 55.33 ± 14.14 | 92.5 | 143.40 ± 2.96 | 140.32 ± 0.80 | 2.2 |
| Women (n = 12,718) | 51.97 ± 13.86 | 96.8 | 155.97 ± 7.79 | 154.06 ± 0.37 | 1.2 |
| Ages 16–49 years | |||||
| Men (n = 1,350) | 40.27 ± 7.62 | 98.3 | 155.19 ± 14.29 | 159.83 ± 0.65 | –2.9 |
| Women (n = 5,599) | 39.59 ± 7.94 | 99.2 | 164.02 ± 5.54 | 164.20 ± 0.24 | –0.1 |
| Ages 50–65 years | |||||
| Men (n = 1,459) | 57.02 ± 4.27 | 95.1 | 150.76 ± 5.86 | 146.32 ± 1.19 | 3.0 |
| Women (n = 4,800) | 56.53 ± 4.24 | 98.1 | 152.33 ± 9.41 | 157.69 ± 0.46 | –3.4 |
| Ages >65 years | |||||
| Men (n = 1,015) | 72.94 ± 5.75 | 81.2 | 104.42 ± 6.95 | 104.94 ± 1.89 | –0.5 |
| Women (n = 2,319) | 72.43 ± 5.67 | 88.3 | 127.33 ± 8.13 | 121.62 ± 1.30 | 4.7 |
Only data for rheumatoid arthritis (RA) cases diagnosed between 2003 and 2009 were included in the extrapolation. Except where indicated otherwise, values are the mean ± SEM.
Relative bias = (extrapolated estimate – Kaplan‐Meier estimate)/Kaplan‐Meier estimate.
DISCUSSION
This study estimated the lifetime risk, life expectancy, loss of life expectancy, and lifetime health care costs for RA. Before making more inferences, however, we must first demonstrate that these estimates are accurate. Our study had the following strengths. First, after excluding prevalent RA cases diagnosed before or during 1999–2002, we included only incident cases to establish a nationwide cohort and followed up the patients for 14 years. Therefore, we can be assured of the representativeness of our cases, and that the actual survival and health care costs were not confounded by different disease stages. Second, all RA cases included were validated through the Catastrophic Illness Registry. Thus, the cohort did not include patients with other types of inflammatory arthritis, and inpatient and outpatient costs were generally comprehensive and accurate. Moreover, since all RA patients can be waived from co‐payment under our NHI system, they usually adhere to Taiwan NHI to minimize financial impact. Third, we validated our extrapolation method, using a cohort collected for the first 7 years, through comparison with an actual Kaplan‐Meier estimate, by extrapolating to the 14th year. Given that censoring rates for all of the different age groups were >90% (except for those >65 years old), all relative biases were generally <5% (Table 4). We anticipate that our estimate of lifetime survival based on 14 years of follow‐up is relatively accurate. Fourth, since our estimates of loss of life expectancy were based on referents matched to the RA patients for age, sex, and calendar year of diagnosis, the results were not confounded by these factors, including different medical technologies in use in different calendar years. Therefore, we tentatively conclude that our findings are generally accurate, and the possible pathophysiologic mechanisms should be explored further.
Because the cumulative incidence rate can be directly compared across different calendar years in populations in the same age range (31), we calculated the cumulative incidence rate instead of prevalence to explore the time trend of disease occurrence and its possible pathophysiology. Since the life expectancy in Taiwan has been approaching 80–84 years among women, we calculated the cumulative incidence rate in the population ages 16–84 years as an estimate of lifetime risk. Our results were much lower than those reported in the US, which were 3.6% and 1.7% for women and men, respectively (23). The major reason for such a difference is still unknown, but there are several possible explanations. First, there is temporal and geographic variability in the occurrence of RA (32). A previous study found that the incidence in Asia is lower than that in North America (33). Second, the RA incidence rates in high‐income Asia have been on the decline, and our estimates were for the period from 2003 to 2016, which would be expected to be lower than the lifetime risk estimated in 2000 for the US population (23). Third, since our estimate of the cumulative incidence rate in the population ages 16–84 simply uses the mid‐year population as the denominator for the calculation of the age‐specific incidence rate, the age‐specific incidence rates and lifetime risk would be slightly underestimated because of old age and competing risks of mortality due to other diseases. Thus, we only performed estimation up to the age of 84, or life expectancy in Taiwan, to minimize the aforementioned confounding. Since the estimate of the cumulative incidence rate in the population ages 16–84 years assumes that subjects do not die of other diseases before reaching the age of 85 years (21), caution must be used in counseling people, and the estimate is not useful for people >85 years old.
Our study also found that the occurrence of RA in Taiwan is declining, which deserves some exploration. The occurrence of RA may be affected by genetic and environmental factors (33), including smoking, periodontitis, hormone replacement therapy, and dietary patterns. From 2008 to 2017, the population of smokers in Taiwan decreased by 40% (34), which might partially explain the decline in RA incidence. However, since RA predominantly affects women, and cigarette consumption among women in Taiwan has remained at ~5% during the last 2 decades, we believe that changes in smoking habits cannot explain the declining trend in RA incidence in women. A study of periodontitis in Taiwan found that the disease prevalence increased from 1997 to 2013 (35). Because there are consistent decreasing trends in RA incidence rates among women >50 years of age (Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41597/abstract), increased rates of prescription for hormone replacement therapy cannot explain this tendency. In addition, healthy dietary patterns may reduce the incidence of RA, but the prevalence of metabolic syndrome in Taiwan has increased in the last 2 decades (36), implying that the decreasing occurrence of RA in Taiwan is unrelated to healthy dietary patterns.
Table 2 also shows that there were no major changes in the prevalence rates of comorbidities, including diabetes, in the newly diagnosed RA cases, except for a mild increase in the rate of hyperlipidemia in recent periods. A plausible reason may be the recently enhanced accessibility of rheumatology care. In fact, the number of board‐certified rheumatologists in practice in Taiwan increased from 151 in 2003 to 254 in 2016. The high accessibility of Taiwan’s NHI, as well as the waived co‐payments for patients with RA, make it easier for people with rheumatic symptoms to seek medical attention from a rheumatologist at a medical center without a referral (37). Thus, patients with early undifferentiated arthritis are usually more likely to receive disease‐modifying antirheumatic drug treatment, which may stop the progression from preclinical RA to true RA. However, further studies are warranted to corroborate this hypothesis.
Previous studies used different methods of estimation of loss of life expectancy and showed that the life expectancy of RA patients decreased by 4–11 years for women and 1–10 years for men compared with the general population (5, 6, 7, 8, 10). However, many of those studies were conducted with small sample sizes. In addition, as follow‐up periods increase, age‐ and sex‐related factors, associated complications, and comorbidities may influence survival rates. So direct comparison may not be appropriate between different cohorts comprising patients of different ages and sex and between studies examining different calendar periods. Alternatively, we used national life tables to simulate referents matched for age, sex, and calendar year of diagnosis to estimate loss of life expectancy (Table 3), which would adjust for potential confounding by these factors (Figure 2) and could be used for the evaluation of outcomes of different medical technologies, including biosimilars. Our nationwide cohort followed up for 14 years showed loss of life expectancy ranging from 2 to 13 years for women with RA and from ~2 to 8 years for men with RA after adjustment for age, sex, and calendar year of diagnosis, which seems to corroborate previous results (10).
Although severe disease activity in RA can be well controlled by biologics, thus reducing mortality from severe RA (38), we still found some loss of life expectancy for patients with RA compared with the general population. Because of the limited financial resources in different insurance systems, there is still no consensus about how early or what amount of biologics can be prescribed and how long should they be continually prescribed after remission of RA. There may be adverse effects associated with the use of biologics. Thus, future studies are warranted to evaluate the cost‐effectiveness of different reimbursement policies. Moreover, our study showed that women and those who had disease onset at a younger age had a higher loss of life expectancy when compared with matched referents (Table 3). Since cardiovascular disease probably accounts for premature mortality in these groups of RA patients (39, 40), health care professionals should pay attention to patients’ risk factors from the beginning of treatment.
Previous studies of the lifetime costs of RA have generally been based on the Markov model (41) and used short‐term data, which might be confounded by different RA stages when making long‐term predictions (11). Moreover, most of those studies were performed before biologic agents were launched, so their findings do not reflect the current real‐world situation. Therefore, our method provides a viable alternative to estimate disease burden over the lifetime. We found that the average annual costs in different age groups of both sexes were similar, and the older the age at diagnosis, the higher the average annual costs (Table 3) (42). Thus, the longer life expectancy for women may be the major reason why their lifetime costs were higher than those for men.
The following limitations of this study must be acknowledged. First, the disease burden of RA, especially in the biologic era, should include effects on quality of life and functional disability. These data were not included in the present study and warrant further investigation. Second, although our cases came from the Catastrophic Illness Registry and may be accurate in diagnosis, there might be some delay from RA onset to diagnosis because of the caution taken to prevent abuse of co‐payment waivers. Thus, the life expectancy may be a conservative estimate. However, such an effect would be cancelled out by comparison of loss of life expectancy between RA patients and the general population. Third, because the censoring rates of the youngest age group were all >98%, our estimation with 14 years of follow‐up has limited accuracy, with high SEMs. Since biologic agents may further improve survival, longer periods of follow‐up are warranted in the future to obtain a more accurate estimate. Fourth, this study only included direct medical costs and did not cover out‐of‐pocket expenditures and costs from loss of productivity and social services related to functional disability. Thus, the lifetime costs would be underestimated from a societal perspective, and future studies are warranted to quantify these issues. Fifth, since the mortality rate was low in men ages 16–49 years, the estimate of the confidence limits of loss of life expectancy may be highly inaccurate, and inferences regarding this group must be made with care. Finally, because the occurrence of RA has great geographic and ethnic variation, any generalization of our results for lifetime risk should be made with caution.
In conclusion, the mean life expectancy after diagnosis of RA was 26.3 years, and the lifetime cost was estimated to be $72,953 after biologics became available. However, there was still a mean loss of life expectancy of 4.97 years, indicating a health disparity that must be resolved. Future studies are needed to evaluate the effects of biologic agents on functional disability, quality of life, and cost‐effectiveness from a societal perspective that includes productivity loss and/or social services (long‐term care, etc.) and to provide evidence for reaching a consensus in the policy decision for RA treatment.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Wang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Chiu, Lan, Chen, Wang.
Acquisition of data
Lu, Wang.
Analysis and interpretation of data
Chiu, Lu, Lan, Chen, Wang.
Supporting information
Fig S1
Supported by the Ministry of Science and Technology of Taiwan (grants MOST 107‐2627‐M‐006‐007 and MOST 108‐2627‐M‐006‐001) and by China Medical University Hospital (grant DMR‐109‐172).
No potential conflicts of interest relevant to this article were reported.
References
- 1. Jonsson B, Kobelt G, Smolen J. The burden of rheumatoid arthritis and access to treatment: uptake of new therapies. Eur J Health Econ 2008;8:S61–86. [DOI] [PubMed] [Google Scholar]
- 2. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 2002;359:1173–7. [DOI] [PubMed] [Google Scholar]
- 3. Jacobsson LT, Turesson C, Nilsson JA, Petersson IF, Lindqvist E, Saxne T, et al. Treatment with TNF blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis 2007;66:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu PH, Wang JD, Keating NL. Expected years of life lost for six potentially preventable cancers in the United States. Prev Med 2013;56:309–13. [DOI] [PubMed] [Google Scholar]
- 5. Lassere MN, Rappo J, Portek IJ, Sturgess A, Edmonds JP. How many life years are lost in patients with rheumatoid arthritis? Secular cause‐specific and all‐cause mortality in rheumatoid arthritis, and their predictors in a long‐term Australian cohort study. Intern Med J 2013;43:66–72. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell DM, Spitz PW, Young DY, Bloch DA, McShane DJ, Fries JF. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum 1986;29:706–14. [DOI] [PubMed] [Google Scholar]
- 7. Gabriel SE, Crowson CS, O’Fallon WM. Mortality in rheumatoid arthritis: have we made an impact in 4 decades? J Rheumatol 1999;26:2529–33. [PubMed] [Google Scholar]
- 8. Minaur NJ, Jacoby RK, Cosh JA, Taylor G, Rasker JJ. Outcome after 40 years with rheumatoid arthritis: a prospective study of function, disease activity, and mortality. J Rheumatol Suppl 2004;69:3–8. [PubMed] [Google Scholar]
- 9. Shinomiya F, Mima N, Nanba K, Tani K, Nakano S, Egawa H, et al. Life expectancies of Japanese patients with rheumatoid arthritis: a review of deaths over a 20‐year period. Mod Rheumatol 2008;18:165–9. [DOI] [PubMed] [Google Scholar]
- 10. Mok CC, Kwok CL, Ho LY, Chan PT, Yip SF. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum 2011;63:1182–9. [DOI] [PubMed] [Google Scholar]
- 11. Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis [review]. Pharmacoeconomics 2004;22 Suppl 1:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Rosery H, Bergemann R, Maxion‐Bergemann S. International variation in resource utilisation and treatment costs for rheumatoid arthritis: a systematic literature review. Pharmacoeconomics 2005;23:243–57. [DOI] [PubMed] [Google Scholar]
- 13. Stone C. The lifetime economic costs of rheumatoid arthritis. J Rheumatol 1984;11:819. [PubMed] [Google Scholar]
- 14. Hwang J. Monte Carlo estimation of extrapolation of quality‐adjusted survival for follow‐up studies. Stat Med 1999;18:1627–40. [DOI] [PubMed] [Google Scholar]
- 15. Hwang JS, Hu TH, Lee LJ, Wang JD. Estimating lifetime medical costs from censored claims data. Health Econ 2017;26:e332–44. [DOI] [PubMed] [Google Scholar]
- 16. Chung CP, Rohan P, Krishnaswami S, McPheeters ML. A systematic review of validated methods for identifying patients with rheumatoid arthritis using administrative or claims data [review]. Vaccine 2013;31:K41–61. [DOI] [PubMed] [Google Scholar]
- 17. Nathan NO, Mørch LS, Wu CS, Olsen J, Hetland ML, Li J, et al. Rheumatoid arthritis and risk of spontaneous abortion: a Danish nationwide cohort study. Rheumatology (Oxford) 2020;59:1984–91. [DOI] [PubMed] [Google Scholar]
- 18. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 19. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 20. Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 2006;36:182–8. [DOI] [PubMed] [Google Scholar]
- 21. Boyle P, Parkin DM. Statistical methods for registries. In: Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet RG, editors. Cancer registration: principles and methods. Lyon: International Agency for Research on Cancer; 1991. p. 147–8. [Google Scholar]
- 22. Hansen SN, Overgaard M, Andersen PK, Parner ET. Estimating a population cumulative incidence under calendar time trends. BMC Med Res Methodol 2017;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult‐onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum 2011;63:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang C, Chang Y, Hsu H, Twu S, Chen KT, Lin C, et al. Life expectancy of patients with newly‐diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM 2007;100:97–105. [DOI] [PubMed] [Google Scholar]
- 25. Chu PC, Wang JD, Hwang JS, Chang YY. Estimation of life expectancy and the expected years of life lost in patients with major cancers: extrapolation of survival curves under high‐censored rates. Value Health 2008;11:1102–9. [DOI] [PubMed] [Google Scholar]
- 26. Hung MC, Liu MT, Cheng YM, Wang JD. Estimation of savings of life‐years and cost from early detection of cervical cancer: a follow‐up study using nationwide databases for the period 2002–2009. BMC Cancer 2014;14:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu TY, Chung CH, Lin CN, Hwang JS, Wang JD. Lifetime risks, loss of life expectancy, and health care expenditures for 19 types of cancer in Taiwan. Clin Epidemiol 2018;10:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ou HT, Yang CY, Wang JD, Hwang JS, Wu JS. Life expectancy and lifetime health care expenditures for type 1 diabetes: a nationwide longitudinal cohort of incident cases followed for 14 years. Value Health 2016;19:976–84. [DOI] [PubMed] [Google Scholar]
- 29. Kao TW, Huang JW, Hung KY, Chang YY, Chen PC, Yen CJ, et al. Life expectancy, expected years of life lost and survival of hemodialysis and peritoneal dialysis patients. J Nephrol 2010;23:677–82. [PubMed] [Google Scholar]
- 30. Lêng CH, Chou MH, Lin SH, Yang YK, Wang JD. Estimation of life expectancy, loss‐of‐life expectancy, and lifetime healthcare expenditures for schizophrenia in Taiwan. Schizophr Res 2016;171:97–102. [DOI] [PubMed] [Google Scholar]
- 31. Rothman KJ, Greenland S. Measures of disease frequency. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 37–40. [Google Scholar]
- 32. Minichiello E, Semerano L, Boissier MC. Time trends in the incidence, prevalence, and severity of rheumatoid arthritis: a systematic literature review. Joint Bone Spine 2016;83:625–30. [DOI] [PubMed] [Google Scholar]
- 33. Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis 2019;78:1463–71. [DOI] [PubMed] [Google Scholar]
- 34. Health Promotion Administration. Ministry of Health and Welfare . Health education and tobacco control. URL: https://www.hpa.gov.tw/EngPages/List.aspx?nodeid=1054.
- 35. Yu HC, Su NY, Huang JY, Lee SS, Chang YC. Trends in the prevalence of periodontitis in Taiwan from 1997 to 2013: a nationwide population‐based retrospective study. Medicine (Baltimore) 2017;96:e8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeh CJ, Chang HY, Pan WH. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac J Clin Nutr 2011;20:292. [PubMed] [Google Scholar]
- 37. Wu TY, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. London J Prim Care (Abingdon) 2010;3:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Puolakka K, Kautiainen H, Pohjolainen T, Virta L. No increased mortality in incident cases of rheumatoid arthritis during the new millennium [letter]. Ann Rheum Dis 2010;69:2057–8. [DOI] [PubMed] [Google Scholar]
- 39. Naz SM, Farragher TM, Bunn DK, Symmons DP, Bruce IN. The influence of age at symptom onset and length of followup on mortality in patients with recent‐onset inflammatory polyarthritis. Arthritis Rheum 2008;58:985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 2006;65:1608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong JB, Ramey DR, Singh G. Long‐term morbidity, mortality, and economics of rheumatoid arthritis. Arthritis Rheum 2001;44:2746–9. [DOI] [PubMed] [Google Scholar]
- 42. Yazici Y, Paget SA. Elderly‐onset rheumatoid arthritis [review]. Rheum Dis Clin North Am 2000;26:517–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


