Dear Editor,
Nail curing devices often use a source of artificial ultraviolet radiation (UVR) to dry, harden and cure finger and toe nails. 1 In the United States of America, the Food and Drug Administration (FDA) recommends a time limit for use of these devices of no more than 10 min per hand, per session for healthy individuals and restrictive use by sun‐sensitive individuals including people using some antibiotics, oral contraceptives, estrogens and dietary supplements. 2 The FDA also suggests specific protective measures such as using sunscreen or wearing UV‐absorbing gloves that expose only nails while drying/curing nail polish. However, in Australia, no guidelines for UVR‐emitting nail curing devices have been established.
In this study, we tested eight nail curing devices for use at home, which were commercially available in Australia and quantified the amount of UVR emitted from each device as well as performed hazard identification analysis of point of sale marketing material and product instructions. UVA was emitted by all UV nail lamp devices tested and the intensity ranged from 39.0 W/m2 to 184.9 W/m2 (Table 1, Fig. S1). UVA was the predominant irradiance emitted by devices, however, the compact fluorescent lamp (CFL) device emitted trace amounts of UVB (0.001 W/m2) and there was no UVC detected from any device (Fig. S2). To calculate the exposure hazard of each nail curing device, the unweighted spectral irradiances were weighted for effect on human skin and eyes using the International Radiation Protection Association (IRPA) and International Commission on Non‐Ionizing Radiation Protection (ICNIRP) relative spectral effectiveness weightings. The weighted spectral irradiances were then assessed against the IRPA/ICNIRP occupational exposure limits. 3 The permissible occupational exposure times were calculated for each device and are expressed as (Tmax) (Table 1). Hazard identification analysis revealed the labelling of safety information was poor with only one device providing a warning for UV light (Table 1, Fig. S3).
Table 1.
Characteristics of UV nail curing devices and measurements of spectral irradiance
| ID | Brand | UVA* (W/m2) | UVB* (W/m2) | Tmax † (min) | Bulb type | Safety information ‡ | Photograph of device | Price ¶ |
|---|---|---|---|---|---|---|---|---|
| 1 | Professional nail | 108.5 | 0.000 | 122 | LED | Limited |


|
$5–$10 |
| 2 | Salon Chic | 39.0 | 0.000 | 168 | LED | Limited |


|
$10–$25 |
| 3 | Salon Chic | 118.6 | 0.000 | 59 | LED | Limited |


|
$50–$80 |
| 4 | Jia Di | 57.5 | 0.000 | 178 | LED | Limited |


|
$5–$10 |
| 5 | SUNmini | 60.1 | 0.000 | 197 | LED | Moderate |


|
$10–$25 |
| 6 | Sunuvied | 184.9 | 0.000 | 38 | LED | Nil |


|
$5–$10 |
| 7 | Bevili | 105.5 | 0.000 | 96 | LED | Nil |


|
$10–$25 |
| 8 |
Glammar Professional |
62.8 | 0.001 | 88 | CFL | Nil |


|
$25–$50 |
CFL, compact fluorescent lamp; LED, light‐emitting diode.
Unweighted measurement with wavelength ranging; UVA = 315–400 nm, UVB = 280–315 nm, UVC = 100–280 nm.
The Tmax values reported are the permissible exposure time limits to the UVR hazard emitted from each device.
Safety Information; nil = no safety or warning labels on webpage or provided in packaging material; limited = safety or warning labels on webpage only, or included in packaging material but contained limited details; moderate = safety information included in packaging and contained relevant instructions; high = relevant safety material included on webpage and packaging.
Prices confirmed at 30 April 2019, quoted in Australian dollars.
The temperatures of all LED devices when they were initially turned on were similar ranging from 23‐31°C, while the CFL device was 68 °C. Thermal imaging revealed the light bulbs commonly increased in heat following 30 min of continuous operation (Fig. 1). The drying time for each device was estimated for one UV‐set nail polish product and ranged from 30 s for device 6, 60 s for devices 1–3, 90 s for device 5,7,8 and 150 s for device 4 (Fig. S4).
Figure 1.
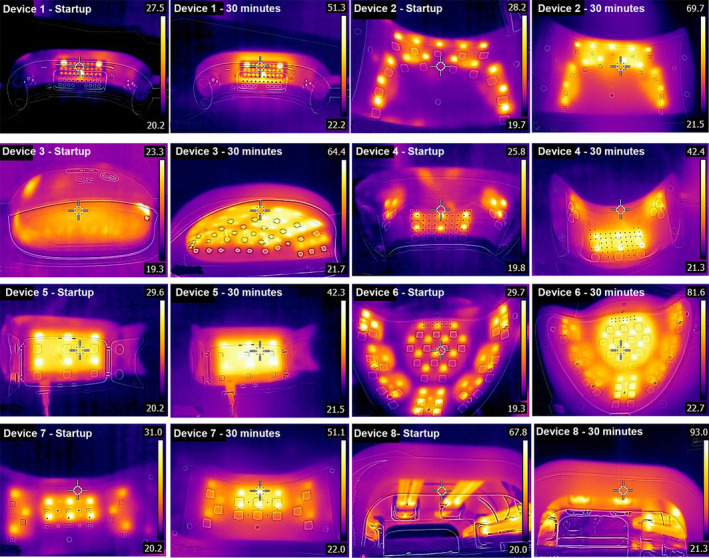
Temperature measurements and thermal images of nail curing devices. Temperature measurements were collected in a laboratory with a room temperature of 25°C using a hand‐held thermal camera (FLIR, United States). The temperature of the nail curing devices was recorded when they were initially turned on (1 min of continuous operation) and then again after 30 min of continuous operation.
This study found a large degree of variation between nail curing devices and the price of the device was not an indicator of reduced UVR irradiance or safer operational temperatures. There was limited safety information provided to consumers about the potential hazards of each device including the UVR light source and the high temperatures the devices may reach when operational. The spectral irradiance emitted by the tested devices was low with 7/8 devices requiring more than an hour of use before reaching the maximum time periods for safe exposure. Given the context that nail curing devices are typically used for between 1 and 10 min for each nail polish treatment the UVR dose received was low. However, the physiological impact of this accumulated UVA exposure to the hands remains unknown and further studies are needed to evaluate any long‐term effect.
To promote better protection for the consumer guidelines could be introduced in Australia with similar recommendations as the FDA including a 10‐min time limit per hand, use of protective measures and restrictions for UV‐sensitive individuals. Currently, the regulation of cosmetic devices emitting non‐ionising radiation is inconsistent in Australia. The Therapeutic Goods Administration (TGA), who have oversight of devices used for medical purposes (e.g. UV phototherapy devices for treating psoriasis and vitiligo) do not regulate devices used for cosmetic purposes.
Following 30 min of continuous operation, the temperature increased for all nail curing devices with 6 out of 8 devices recording temperatures over 50°C. The degree of tissue destruction depends on the temperature and duration of exposure as well as other factors, which influence the body’s ability to resist burn injury. 4 However, it is likely a thermal burn injury would develop in several seconds if the exposure temperature is at least 70°C. 4 The thermal imaging results of this study highlighted the potential risk of thermal burns from nail curing devices and future studies should explore the frequency and severity of thermal burns among users of these devices.
The recommendations from this study include providing further safety information for consumers regarding safe operating conditions and potential hazards of using a UVR‐emitting nail curing device.
Conflict of interest
The authors declare that they have no competing interests.
Authors' contributions
EH contributed to conceptualisation, funding acquisition, investigation, methodology, project administration, resources, data curation, formal analysis, supervision, visualisation, and writing, reviewing and editing the manuscript. CH contributed to project administration, investigation, writing, reviewing and editing the manuscript. HF contributed to project administration, investigation, writing, reviewing and editing the manuscript. ARPANSA authors RT contributed to reviewing and editing the manuscript. DU contributed to investigation and writing, reviewing and editing the manuscript.
Funding
The sponsors of the study (Queensland Government Advance Queensland fund) had no role in the study design, collection, analysis and interpretation of data; in the writing of this manuscript; and in the decision to submit the paper for publication. The corresponding author had full access to all data in the study and final responsibility for the decision to submit for publication.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article and its supplementary information files (Additional File [Link], [Link], [Link], [Link]).
Supporting information
Figure S1. UVR exposure assessment and measurement.
Figure S2. Spectral UVA Irradiance of Nail Lamp Devices.
Figure S3. Hazard identification analysis of safety information provided with device.
Figure S4. Nail polish drying time estimation.
Acknowledgement
The authors would like to thank all the project staff (Kerryn King, Lydiawati Tjong, Anindita Das) for their contributions to the UVR measurements which were collected and reported by the Australian Radiation Protection and Nuclear Safety Agency (ARPANSA).
References
- 1. Shihab N, Lim H. Potential cutaneous carcinogenic risk of exposure to UV nail lamp: A review. Photodermatol. Photoimmunol. Photomed. 2018; 34: 362–5. [DOI] [PubMed] [Google Scholar]
- 2. US Food & Drug Administration (FDA) . How to Safely Use Nail Care Products [cited 2020 August 5]. Available from: https://www.fda.gov/consumers/consumer‐updates/how‐safely‐use‐nail‐care‐products.
- 3. Agency ARPaNS . Radiation Protection Series No. 12 Radiation Protection Standard Standard for Occupational Exposure to Ultraviolet Radiation (6) 2006. [Available from: https://www.arpansa.gov.au/regulation‐and‐licensing/regulatory‐publications/radiation‐protection‐series/codes‐and‐standards/rps12.
- 4. Andrews CJ, Kimble RM, Kempf M et al. Evidence‐based injury prediction data for the water temperature and duration of exposure for clinically relevant deep dermal scald injuries. Wound Repair Regen. 2017; 25: 792–804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. UVR exposure assessment and measurement.
Figure S2. Spectral UVA Irradiance of Nail Lamp Devices.
Figure S3. Hazard identification analysis of safety information provided with device.
Figure S4. Nail polish drying time estimation.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files (Additional File [Link], [Link], [Link], [Link]).


