Abstract
Background
Fragrances are widely used in scented products used in daily life with the potential to induce skin sensitization.
Objective
To evaluate exposure to scented products and to explore associations between exposure and fragrance contact allergy.
Methods
A cross‐sectional study on individuals from 18 to 74 years of age, who were randomly selected from the general population in five European countries. A random sample (N = 3119) was patch tested and interviewed on exposure to scented products.
Results
Female participants were strongly associated with exposure to scented products relative to male participants. Participants age 40 years and older showed an inverse association with exposure to scented products. Compared to Sweden, The Netherlands followed by Germany showed the highest overall exposure to scented products. Sensitive skin was associated with exposure to scented products and with fragrance allergy. In univariable regression analysis, exposure to leave‐on products and to specific scented product subgroups was significantly associated with fragrance allergy.
Conclusion
Exposure to scented products depends primarily on sex and age. Female sex and sensitive skin are relevant indicators for developing fragrance allergy. Because aggregate exposure, especially to scented leave‐on products, may enhance the prevalence of contact allergy to fragrances, further investigations into exposure amounts and frequencies is warranted.
Keywords: contact allergy, contact dermatitis, epidemiology, exposure, fragrances, household products, leave‐on products, rinse‐off products, scented products
1. INTRODUCTION
Fragrances are used widely in scented products used in daily life. These products can be intended to be left on the skin such as creams and perfumes, intended to be rinsed off the skin such as shampoos or shaving products, or intended to be used as household items. Scented products contain low‐molecular‐weight chemicals that may have the potency to induce skin sensitization and subsequently, at sufficient exposure levels, cause allergic contact dermatitis. 1 , 2 The most common allergen found at most centers after patch testing consecutive patients with the baseline series, after nickel, is Fragrance Mix I (FM I). 3 , 4 , 5 , 6 In the European clinical population the prevalence of contact allergy to FM I was 7.8%. 7 In the European general population, the prevalence of contact allergy to FM I was reported in a systematic review and meta‐analysis yielding a prevalence of 3.5%. 3 Another study from the general population showed that fragrance contact allergy was associated with self‐reported cosmetic dermatitis. 8 Multiple studies have evaluated the safety of chemicals in scented products and/or used quantitative risk assessment (QRA) to evaluate the induction of sensitization resulting from exposure to scented products. 9 , 10 , 11 , 12 These studies were carried out by calculating product use data using product information sheets or market information databases. Studies evaluating overall real life exposure to scented products are scarce.
The European Dermato‐Epidemiology Network (EDEN) Fragrance Study is a large cross‐sectional epidemiological study on a random sample of adults from the European general population. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 The aim of this study is to investigate the overall real life exposure to scented products used in daily life and to explore a relationship between exposure to scented products and fragrance contact allergy.
2. METHODS
2.1. Study design and population
The EDEN fragrance study is a cross‐sectional descriptive epidemiological study. Methods and study design have been published previously. 13 , 14 , 19 In summary, a random sample of individuals from 18 to 74 years of age were selected from the general population and invited to a face‐to‐face interview in which a questionnaire was administered. The full phase of the study was conducted between August 2008 and October 2011. Additional patch testing was offered to a random sample of the included participants. If a participant was enrolled in the patch‐test group, he or she was invited to patch testing after the interview had been completed. 14 , 19 We performed a retrospective data analysis on the collected patch test data. Participants were included from Sweden, Germany (test sites: Heidelberg, Jena), The Netherlands, Italy, and Portugal. The study was approved by the ethics committee of each center.
2.2. Patch testing
A detailed description of the patch‐testing procedures has been reported previously. 16 , 17 Participants were patch tested with TRUE Test panels 1, 2, 3, hydroxyisohexyl 3‐cyclohexene carboxaldehyde (HICC) 5% pet. and sesquiterpene lactone mix 0.1% pet. 17 Two preparations of FM I at 8% pet. were tested with the Finn chamber technique, one containing Evernia prunastri (oak moss) with a high content of atranol and chloratranol and the other containing Evernia prunastri (oak moss) with a low content of atranol and chloratranol. The corresponding test preparations with a low and high concentration of atranol and chloratranol were tested with the TRUE Test technique in panel 1 and as a Nixema test, respectively. In addition the eight separate ingredients of FM I were tested in pet. and FM II was tested in 14% pet., including its six separate ingredients. A detailed description hereof was published by Bruze et al. 20 Patch tests were applied on the back for 48 hours under occlusion. Patch‐test readings were performed at day (D)3. A reaction was considered positive if a weak (+), strong (++), or extreme (+++) reaction was seen. Irritant (ir) and doubtful (? +) reactions were considered as negative reactions.
2.3. Data collection and analysis
Face‐to‐face interviews were conducted by trained interviewers and consisted of three parts. 14 , 19 The sociodemographic characteristics included in the current analysis were sex, age (<40/≥40), physician‐diagnosed contact dermatitis (including allergic‐ and irritant contact dermatitis), physician‐diagnosed atopic dermatitis, self‐reported dry or sensitive skin, and country. The question regarding presence of dry or sensitive skin was asked by interviewer in two separate questions: “Do you think that you have a dry, respectively, sensitive skin.” A detailed history of exposure to different scented products in common use was reported. These products include the following: leave‐on products (toiletry items that remained on the skin), rinse‐off products (toiletry items that were rinsed off the skin), and household products (cleaning and freshener products). 14 In the questionnaire, the estimated frequency of exposure during the last 12 months was scored after completion as follows: (0) never exposure, (1) exposure less than once a month, (2) monthly but not weekly exposure, (3) weekly but not daily exposure, and (4) daily exposure. For leave‐on, rinse‐off, and household products, respectively, 10, 5, and 5 different product subgroups were included in the questionnaire. Therefore, the overall exposure to scented products could be expressed as a continuous outcome. To study exposure to the different subgroups, the exposure outcome was categorized in which the response options (3) weekly but not daily exposure and (4) daily exposure were counted as exposure and the response options (0) never exposure was counted as no exposure. The response options (1) exposure less than once a month and (2) monthly but not weekly exposure were not included in the analysis. Therefore, exposure to the different subgroups could be expressed as a binary score.
To study a possible association between exposure and sensitization, all the 21 patch‐tested fragrance allergens were included for analysis; FM I in pet. in high and low content of atranol and chloratranol, FM I with the TRUE Test and Nixema test, FM II, the eight and six separate components of FM I and FM II, respectively, Myroxylon pereirae, sesquiterpene lactone mix, and colophonium.
2.4. Statistics
Descriptive data are presented as numbers and percentages with 95% confidence intervals (95% CIs). For the overall exposure analysis, univariable linear regression analysis was performed and data are presented as standardized regression coefficients (beta [β]) with corresponding 95% CI. To compare countries, Sweden was taken as reference. For the exposure analysis of the product subgroups, univariable binary logistic regression was performed. Effects of different variables on exposures are presented as odds ratio (ORs) with 95% CIs. A multivariable regression analysis including all variables that were significant in the univariable model was performed to control for potential confounders in both the linear and binary regression model. For the sub‐analysis of associations between exposure and sensitization, univariable binary regression was performed and adjusted for age and sex. The Benjamini‐Hochberg procedure was performed to control the False Discovery Rate (FDR) in multiple testing. The FDR was set to 0.10. Statistical analyses were performed with SPSS v.23 (IBM) and Excel 2013 (Microsoft).
3. RESULTS
3.1. Sociodemographic characteristics
Table 1 presents the sociodemographic characteristics of the 3119 patch‐tested subjects included in the overall exposure analysis, together with the characteristics of the 224 subjects (7.2%) with at least one positive patch‐test reaction to a fragrance allergen. Female subjects (54.9%) outnumbered male subjects (45.1%) and being ≥40 years of age (58.9%) outnumbered being <40 years of age (41.2%). In the positive patch‐test subsample, the female/male ratio shifted toward a predominantly female subpopulation (67.7% female compared to 32.3% male). For age, the percentage of subjects ≥40 years of age increased from 58.9% toward 69.6%. The proportion of subjects with contact dermatitis, dry, and sensitive skin was higher in the fragrance contact allergy subsample (16.1%, 59.6%, and 47.9%, respectively) compared to the total patch‐tested population (8.1%, 52.1%, and 41.0%, respectively).
TABLE 1.
Sociodemographic characteristics of the patch‐tested subjects and a subsample of subjects with a positive patch‐test reaction to a fragrance allergen
| Total patch‐tested subjects (N = 3119) | Positive patch‐test reaction to a fragrance allergen (N = 224) | |||||
|---|---|---|---|---|---|---|
| Sex | N | % | 95% CI | N | % | 95% CI |
| Male | 1405 | 45.1 | 43.3–46.8 | 72 | 32.3 | 26.2–38.4 |
| Female | 1712 | 54.9 | 53.2–56.7 | 151 | 67.7 | 61.6–73.8 |
| Age (years) | ||||||
| <40 | 1279 | 41.1 | 39.3–52.8 | 68 | 30.4 | 24.3–36.4 |
| ≥40 | 1836 | 58.9 | 57.2–60.7 | 156 | 69.6 | 63.6–75.7 |
| Contact dermatitis | 253 | 8.1 | 7.2–9.1 | 36 | 16.1 | 11.3–20.9 |
| Atopic dermatitis | 190 | 6.5 | 5.6–7.4 | 12 | 5.7 | 2.6–8.9 |
| Dry skin | 1626 | 52.1 | 51.8–55.3 | 130 | 59.6 | 53.1–66.1 |
| Sensitive skin | 1252 | 41.0 | 39.3–42.7 | 105 | 47.9 | 41.3–54.6 |
Abbreviations: CI, confidence interval; N, number.
Note: Included fragrance allergens: fragrance mix (FM) I 8% pet. containing Evernia prunastri (oak moss) with a high content of atranol and chloratranol, FM I 8% pet. containing Evernia prunastri (oak moss) with a low content of atranol and chloratranol, FM I True test and Nixema, FM I separate components; FM II 14% pet, FM II separate components, Myroxylon pereirae, sesquiterpene lactone mix, and colophonium.
3.2. Overall exposure to scented products
Table 2 shows the results of the linear regression analysis with overall exposure to scented products as outcome. In a multivariable analysis, female subjects showed a significantly higher exposure to scented products (β 0.59, 95% CI 0.56–0.62) than male subjects. Subjects ages ≥40 years of age showed a significant inverse association with exposure to scented products (β −0.19, 95% CI −0.22 to −0.16). Small significant association effects were found between dry skin and overall exposure to scented products (β 0.04, 95% CI 0.01–0.07). Compared to Sweden, The Netherlands showed the highest overall exposure to scented products (β 0.14, 95% CI 0.11–0.17) followed by Germany (β 0.04, 95% CI 0.01–0.07).
TABLE 2.
Linear regression analysis with exposure to scented products as outcome
| Exposure to scented products | ||||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| Factors | Β | 95% CI | β | 95% CI |
| Female | 0.59 | 0.56–0.62 | 0.59 | 0.56–0.62 |
| ≥40 years | −0.18 | −0.21 to −0.14 | −0.19 | −0.22 to −0.16 |
| Contact dermatitis | 0.06 | 0.02–0.10 | 0.00 | −0.03–0.03 |
| Atopic dermatitis | 0.03 | −0.01–0.07 | ||
| Dry skin | 0.15 | 0.11–0.19 | 0.04 | 0.01–0.07 |
| Sensitive skin | 0.16 | 0.12–0.20 | 0.03 | −0.01–0.06 |
| Country (SE = ref) | ||||
| NL | 0.13 | 0.08–0.18 | 0.14 | 0.11–0.17 |
| DE | 0.06 | 0.01–0.11 | 0.04 | 0.01–0.07 |
| IT | 0.02 | −0.03–0.07 | ||
| PT | −0.01 | −0.06–0.04 | ||
Note: Data are presented as standardized regression coefficients with corresponding standardized 95% CIs. Bold values indicate significant difference after Benjamini‐Hochberg procedure for multiple testing with FDR = 0.10.
Abbreviations: β, standardized beta‐coefficient; CI, confidence interval; DE; Germany; IT, Italy; NL, the Netherlands; PT, Portugal; SE, Sweden.
3.3. Exposure to different scented products subgroups
In Figure 1, daily and/or weekly exposure to all scented product subgroups is presented. Mouthwashes or toothpastes, soaps, and shampoos are the most frequently used scented products, followed by deodorants and liquid detergents. Tables 3 and 4 show the results of the binary regression analysis of exposure to the different leave‐on (Table 3) and the different rinse‐off and household (Table 4) products subgroups. Only the significant results from the multivariable analysis will be reported in this section. Female subjects showed a strong significant associations in exposure to all scented product subgroups, except for exposure to men's cologne/aftershave and shaving products. As expected, male subjects were significantly associated with exposure to these two products subgroups. Age 40 years of age and older showed significant inverse associations for all leave‐on products, except for exposure to men's cologne/aftershave and hygiene products. An additional stratified analysis by sex was performed and showed no statistical significant interaction. For rinse‐off products, a significant inverse association for age 40 years of age and older was seen for exposure to bath oils, shaving products, and mouthwashes or toothpaste. Physician‐diagnosed contact dermatitis was only associated with exposure to eye make‐up (odds ratio [OR] 1.43, 95% CI 1.06–1.92). Atopic dermatitis was only associated with exposure to lipsticks and lip balms (OR 1.88, 95% CI 1.13–3.12). Self‐reported dry skin was associated with exposure to sunscreens (OR 1.30, 95% CI 0.96–1.75), skin creams (OR 2.69, 95% CI 2.11–3.44), eye make‐up (OR 1.39, 95% CI 1.17–1.67), and lipstick and lip balms (OR 1.45, 95% CI 1.13–1.87). For exposure to household products, dry skin was associated with exposure to laundry detergents (OR 1.38, 95% CI 1.12–1.71), dishwashing detergents (OR 1.37, 95% CI 1.04–1.81), and scouring powders (OR 1.26, 95% CI 1.01–1.57). The presence of a sensitive skin was associated with exposure to different leave‐on product subgroups: perfumes (OR 1.19, 95% CI 0.97–1.46), sunscreens (OR 1.62, 95% CI 1.20–2.18), skin creams (OR 1.31, 95% CI 1.01–1.70), and eye make‐up (OR 1.83, 95% CI 1.53–2.18).
FIGURE 1.
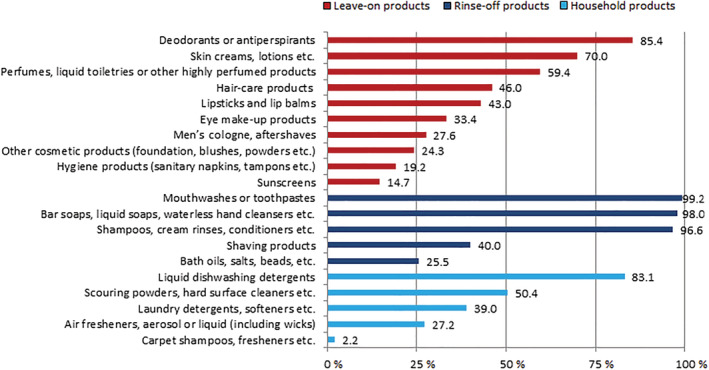
Proportion of subjects who report daily or weekly exposure to different scented product subgroups among 3119 patch‐tested subjects
TABLE 3.
Leave on products: Binary regression analysis with exposure to different subgroups as outcome for different subject characteristics
| Female, OR (95% CI) | ≥ 40 years, OR (95% CI) | Contact dermatitis, OR (95% CI) | Atopic dermatitis, OR (95% CI) | Dry skin, OR (95% CI) | Sensitive skin, OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Perfumes | UVA | 5.23 (4.34–6.29) | 0.29 (0.24–0.35) | 0.90 (0.66–1.21) | 1.18 (0.83–1.70) | 1.23 (1.04–1.46) | 1.65 (1.38–1.97) |
| MVA | 5.83 (4.77–7.13) | 0.23 (0.19–0.29) | 0.90 (0.74–1.11) | 1.19 (0.97–1.46) | |||
| Men's cologne/ aftershaves | UVA | 0.04 (0.03–0.05) | 0.99 (0.84–1.16) | 0.68 (0.49–0.93) | 0.57 (0.39–0.83) | 0.61 (0.51–0.71) | 0.52 (0.44–0.62) |
| MVA | 0.04 (0.03‐0.05) | 1.07 (0.69–1.65) | 0.73 (0.45–1.19) | 0.10 (0.88–1.39) | 0.85 (0.67–1.07) | ||
| Sunscreens | UVA | 4.26 (3.25–5.58) | 0.32 (0.26–0.43) | 1.46 (0.93–2.27) | 1.41 (0.83–2.38) | 1.83 (1.41–2.37) | 2.36 (1.82–3.06) |
| MVA | 3.54 (2.64–4.73) | 0.37 (0.27–0.50) | 1.30 (0.96–1.75) | 1.62 (1.20–2.18) | |||
| Skin creams, lotions, etc. | UVA | 10.63 (8.29–13.64) | 0.66 (0.54–0.81) | 1.25 (0.86–1.80) | 1.56 (0.99–2.58) | 3.57 (2.88–4.43) | 2.39 (1.92–2.98) |
| MVA | 9.74 (7.50–12.72) | 0.59 (0.47–0.75) | 0.79 (0.46–1.37) | 2.69 (2.11–3.44) | 1.31 (1.01–1.70) | ||
| Deodorants or antiperspirants | UVA | 2.05 (1.61–2.61) | 0.23 (0.17–0.32) | 1.16 (0.74–1.83) | 1.34 (0.78–2.30) | 1.21 (0.95–1.53) | 1.37 (1.07–1.76) |
| MVA | 2.16 (1.69–2.77) | 0.22 (0.16–0.30) | 1.16 (0.89–1.50) | ||||
| Hair‐care products | UVA | 4.16 (3.53–4.90) | 0.52 (0.44–0.61) | 1.18 (0.88–1.56) | 1.02 (0.74–1.41) | 1.26 (1.07–1.47) | 1.36 (1.16–1.59) |
| MVA | 4.50 (3.76–5.38) | 0.45 (0.38–0.54) | 0.97 (0.81–1.18) | 0.98 (0.81–1.18) | |||
| Eye make‐up | UVA | nt a | 0.67 (0.58–0.78) | 1.71 (1.30–2.25) | 1.49 (1.09–2.05) | 1.68 (1.43–1.96) | 2.09 (1.78–2.46) |
| MVA | 0.69 (0.58–0.82) | 1.43 (1.06–1.92) | 1.08 (0.77–1.51) | 1.39 (1.17–1.67) | 1.83 (1.53–2.18) | ||
| Lipsticks and lip balms | UVA | 27.76 (22.19–34.73) | 0.34 (0.54–0.75) | 1.70 (1.24–2.31) | 2.22 (1.53–3.23) | 2.13 (1.80–2.52) | 2.06 (1.74–2.45) |
| MVA | 28.96 (22.49–37.27) | 0.46 (0.35–0.59) | 1.01 (0.65–1.54) | 1.88 (1.13–3.12) | 1.45 (1.13–1.87) | 1.00 (0.78–1.30) | |
| Other cosmetic products | UVA | 82.06 (50.94–132.19) | 0.73 (0.62–0.87) | 1.59 (1.19–2.15) | 1.39 (0.99–1.94) | 1.61 (1.35–1.92) | 1.88 (1.58–2.23) |
| MVA | 89.03 (54.21–146.21) | 0.52 (0.42–0.65) | 1.14 (0.80–1.62) | 0.86 (0.56–1.30) | 0.96 (0.76–1.21) | 1.08 (0.86–1.36) | |
| Hygiene products | UVA | 84.21 (55.83–127.01) | 0.85 (0.70–1.03) | 1.57 (1.12–2.20) | 1.39 (0.93–2.08) | 1.82 (1.49–2.21) | 1.89 (1.56–2.30) |
| MVA | 84.56 (55.16‐129.65) | 1.05 (0.93–1.63) | 0.77 (0.57–1.03) | 1.24 (0.94–1.63) |
Note: Bold values indicate significant difference after Benjamini‐Hochberg procedure for multiple testing with FDR = 0.10. Multiple testing with FDR = 0.10.
Abbreviations: CI, confidence interval; MVA, multivariable analysis; nt, not tested; OR, odds ratio; UVA, univariable analysis.
Not tested because <10 males were exposed and analysis could therefore not be performed.
TABLE 4.
Rinse‐off and household products: Binary regression analysis with exposure to different subgroups as outcome for different subject characteristics
| Female, OR (95% CI) | ≥ 40 years, OR (95% CI) | Contact dermatitis, OR (95% CI) | Atopic dermatitis, OR (95% CI) | Dry skin, OR (95% CI) | Sensitive skin, OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Bath oils, salts, beads, etc. | UVA | 1.55 (1.30–1.83) | 0.76 (0.64–0.90) | 1.09 (0.80–1.48) | 1.18 (0.83–1.69) | 1.20 (1.01–1.42) | 1.19 (1.00–1.41) |
| MVA | 1.56 (1.32–1.85) | 0.74 (0.62–0.88) | 1.10 (0.92–1.33) | 1.08 (0.90–1.30) | |||
| Soaps, hand cleansers | UVA | 1.80 (0.89–3.65) | 0.33* (0.13–0.80) | 0.47 (0.18–1.24) | nt a | 1.23 (0.61–2.50) | 0.85 (0.42–1.72) |
| MVA | |||||||
| Shampoos, conditioners | UVA | 28.19 (8.85–89.87) | 0.71 (0.43–1.17) | 1.15 (0.46–2.87) | 0.51 (0.24–1.09) | 1.44 (0.88–2.37) | 2.00 (1.17–3.45) |
| MVA | 26.69 (8.33–85.53) | 1.24 (0.71–2.15) | |||||
| Shaving products | UVA | 0.10 (0.09–0.12) | 0.42 (0.36–0.50) | 0.69 (0.52–0.93) | 0.99 (0.71–1.37) | 0.73 (0.62–0.85) | 0.75 (0.64–0.88) |
| MVA | 0.09 (0.07–0.11) | 0.30 (0.25–0.37) | 1.05 (0.73–1.50) | 1.11 (0.91–1.36) | 1.13 (0.92–1.39) | ||
| Mouthwashes or toothpastes | UVA | 2.66 (1.00–7.01) | 0.27 (0.08–0.92) | 0.25 (0.09–0.68) | 1.26 (0.17–9.49) | 2.32 (0.87–6.21) | 1.51 (0.57–3.98) |
| MVA | 3.11 (1.17–8.32) | 0.28 (0.80–0.95) | 0.22 (0.08–0.63) | ||||
| Laundry detergents | UVA | 8.15 (6.70–9.93) | 1.22 (1.02–1.46) | 1.76 (1.25–2.46) | 1.24 (0.85–1.82) | 1.72 (1.44–2.05) | 1.44 (1.21–1.72) |
| MVA | 7.75 (6.30–9.52) | 1.14 (0.93–1.40) | 1.39 (0.94–2.07) | 1.38 (1.12–1.71) | 0.86 (0.67–1.07) | ||
| Dishwashing detergents | UVA | 4.95 (3.66–6.70) | 0.88 (0.68–1.16) | 1.63 (0.92–2.90) | 0.87 (0.51–1.49) | 1.77 (1.35–2.32) | 1.19 (0.90–1.56) |
| MVA | 4.57 (3.36–6.22) | 1.37 (1.04–1.81) | |||||
| Scouring powders | UVA | 4.74 (3.86–5.83) | 0.76 (0.62–0.93) | 1.51 (1.03–2.20) | 1.08 (0.73–1.63) | 1.60 (1.31–1.94) | 1.41 (1.16–1.73) |
| MVA | 4.51 (3.63–5.60) | 0.66 (0.53–0.82) | 1.30 (0.86–1.97) | 1.26 (1.01–1.57) | 1.98 (0.78–1.22) | ||
| Air fresheners | UVA | 1.21 (1.02–1.43) | 0.77 (0.65–0.91) | 0.79 (0.58–1.09) | 0.80 (0.60–1.21) | 1.00 (0.84–1.18) | 1.01 (0.85–1.20) |
| MVA | 1.20 (1.01–1.42) | 0.77* (0.65–0.91) | |||||
| Carpet shampoos, fresheners | UVA | 3.10 (1.74–5.54) | 1.19 (0.72–1.96) | 0.54 (0.17–1.74) | 1.18 (0.47–3.00) | 1.27 (0.77–2.10) | 1.25 (0.76–2.06) |
| MVA |
Note: Bold values indicate difference after Benjamini‐Hochberg procedure for multiple testing with FDR = 0.10.
Abbreviations: CI, confidence interval; MVA, multivariable analysis; nt, not tested; OR, odds ratio; UVA, univariable analysis.
Not tested because expected count in one cell <5.
3.4. Associations between exposure to scented products and fragrance contact allergy
Table 5 presents the results of the analysis of overall exposure to scented products and fragrance contact allergy stratified per scented product subgroup. In total, 224 subjects (7.2%) showed a positive patch‐test reaction to at least 1 of the 21 allergens included in the association analysis. Univariable regression analysis showed a significant association between overall exposure to scented products and contact allergy to one of the included scented allergens (OR 1.70, 95% CI 1.14–2.52). Overall exposure to leave‐on products showed a significant association with fragrance contact allergy (OR 1.79, 95% CI 1.21–2.64). Exposure to rinse‐off products showed an inverse significant association with fragrance contact allergy (OR 0.61, 95% CI 0.44–0.81). Exposure to household products was not significantly associated with fragrance contact allergy. Stratified by product subgroups, significant associations between exposure and fragrance contact allergy were seen for exposure to skin creams (OR 1.80, 95% CI 1.15–2.82), eye make‐up (OR 1.37, 95% CI 1.02–1.84), lipstick and lip balm (OR 1.41, 95% CI 1.03–1.93), other cosmetic products (OR 1.52, 95% CI 1.12–2.06), and hygiene products (OR 1.88, 95% CI1.34–2.63). Significant inverse associations were seen between exposure and fragrance contact allergy for men's cologne/aftershave (OR 0.66, 95% CI 0.48–0.93) and shaving products (OR 0.60, 95% CI 0.44–0.81). The presence of a sensitive skin was associated with an increased risk of fragrance contact allergy in univariable regression analysis (OR 1.36, 95% CI 1.03–1.79). After adjusting for age and sex the outcomes were no longer significant.
TABLE 5.
Binary regression analysis with fragrance contact allergy as outcome (N = 224)
| Exposure to | Crude OR (95% CI) | Adjusted a OR (95% CI) |
|---|---|---|
| Scented overall | 1.70 (1.14–2.52) | 1.25 (0.69–2.29) |
| Leave on (including all subgroups) | 1.79 (1.21–2.64) | 2.43 (1.20–4.90) |
| Perfumes, liquid toiletries, or other highly perfumed products | 0.96 (0.69–1.34) | 0 |
| Men's cologne/aftershaves | 0.66 (0.48–0.93) | 1.09 (0.70–1.69) |
| Sunscreens | 1.45 (0.90–2.35) | 1.55 (0.92–2.61) |
| Skin creams, lotions, etc. | 1.80 (1.15–2.82) | 1.47 (0.91–2.39) |
| Deodorants or antiperspirants | 1.07 (0.67–1.70) | 1.09 (0.68–1.76) |
| Hair‐care products (sprays, tonics, gels, mousses–not shampoos, conditioners or other rinse‐off products) | 1.32 (0.98–1.79) | 1.26 (0.91–1.75) |
| Eye make‐up products | 1.37 (1.02–1.84) | 0.90 (0.61–1.34) |
| Lipsticks and lip balms | 1.41 (1.03–1.93) | 1.19 (0.78–1.82) |
| Other cosmetic products (foundation, blushes, powders, etc.) | 1.52 (1.12–2.06) | 1.20 (0.84–1.74) |
| Hygiene products (sanitary napkins, tampons, etc.) | 1.88 (1.34–2.63) | 1.64 (1.03–2.62) |
| Rinse‐off (including all subgroups) | 0.61 (0.43–0.89) | 0.69 (0.46–1.04) |
| Bath oils, salts, beads, etc. | 1.18 (0.85–1.62) | 1.13 (0.82–1.57) |
| Bar soaps, liquid soaps, waterless hand cleansers, etc. | 0.42 (0.16–1.09) | 0.41 (0.16–1.09) |
| Shampoos, cream rinses, conditioners, etc. | 0.58 (0.27–1.22) | 0.41 (0.19–0.89) |
| Shaving products | 0.60 (0.44–0.81) | 0.86 (0.59–1.24) |
| Mouthwashes or toothpastes | 1.39 (0.19–10.49) | 1.36 (0.18–10.29) |
| Household (including all subgroups) | 1.43 (0.94–2.16) | 0.92 (0.56–1.52) |
| Laundry detergents, softeners, etc. | 1.20 (0.85–1.69) | 0.81 (0.55–1.22) |
| Liquid dishwashing detergents | 1.31 (0.75–2.29) | 1.06 (0.60–1.89) |
| Scouring powders, hard surface cleaners, etc. | 1.33 (0.90–1.97) | 1.09 (0.72–1.66) |
| Air fresheners, aerosol or liquid (including wicks) | 1.05 (0.77–1.44) | 1.03 (0.75–1.41) |
| Carpet shampoos, fresheners, etc. | 0 | 0 |
| Dry skin | 1.30 (0.99–1.73) | 1.16 (0.87–1.55) |
| Sensitive skin | 1.36 (1.03–1.79) | 1.23 (0.93–1.64) |
Note: Included fragrance allergens in sub‐analysis: fragrance mix (FM) I 8% pet. containing Evernia prunastri (oak moss) with a high content of atranol and chloratranol, FM I 8% pet. containing Evernia prunastri (oak moss) with a low content of atranol and chloratranol, FM I True Test and Nixema, FM I separate components; FM II 14% pet, FM II separate components, Myroxylon pereirae, sesquiterpene lactone mix, and colophonium. Bold values indicate significant difference after Benjamini‐Hochberg procedure for multiple testing with FDR = 0.10.
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for age and sex.
4. DISCUSSION
4.1. Scented product exposure and fragrance contact allergy
This European study evaluated self‐reported skin exposure to different scented products used in daily life. In addition, we explored associations between exposure to different leave‐on, rinse‐off, and household products and fragrance contact allergy using patch‐test results in adults from the general population.
In this study, exposure to scented products varied with sex, age, the presence of a skin condition, and country of residence. We showed that females are significantly more exposed to scented products used in daily life than males. Several studies have been published on the effect of sex on the development of contact allergy. 21 , 22 , 23 , 24 It was assumed that increased female sensitivity to contact allergens is most likely due to a higher exposure to contact allergens than males. 21 , 23 The current study shows that females are indeed more exposed to scented products and consequently may be more frequently sensitized to fragrance allergens than males. We also showed that subjects younger than the age of 40 years are more exposed to scented products than subjects 40 years of age and older, even though a positive patch‐test reaction to one of the included fragrance allergens in this analysis was more frequently observed in subjects age 40 years of age and older. It is important to note that our questionnaire only addressed exposure in the 12 months prior to the interview. Repeated exposure over a period longer than 12 months was therefore not included in the analysis. This could explain the discrepancy in which younger subjects are more exposed to scented products during the last 12 months but show less positive patch‐test reactions to fragrance allergens than older subjects, who may have a higher number of repeated exposures over a longer period. Diepgen et al reported the clinical relevance of contact allergy to fragrance allergens from the current EDEN cohort. 17 Clinical relevance was defined as a positive patch test in a subject who also reported a lifetime history of avoidance of any scented products because of any skin problem plus an itchy skin reaction lasting >3 days. The prevalence of clinically relevant fragrance allergy was 1.9% and increasing with age. Consequently, if older subjects have more clinically relevant reactions to fragrances, they might avoid contact with scented products and thus might be less exposed than subjects younger than 40 years of age.
In the current analysis, sensitive skin was associated with exposure to perfumes, sunscreens, skin creams and eye make‐up. In addition, reporting a sensitive skin was associated with fragrance contact allergy in univariable regression analysis. Subjects with presumed sensitive skin probably use more skin care products. This could be a reason they are more frequently sensitized to fragrance allergens. Subjects with self‐reported sensitive skin should therefore be advised to be cautious with exposure to scented products. Furthermore, a subdivision by country showed that The Netherlands followed by Germany were more exposed to scented products than Sweden. Diepgen et al, however, reported no statistically significant difference for contact allergy to the different fragrance allergens between countries. 17 Considering the study design, in which interviewers from the different countries were trained equally and different geographic areas from the different countries were taken into account (subjects participated from both rural and central areas), we could not find a clear explanation for the significant differences in exposure but not in the prevalence of fragrance contact allergy we found.
We found significant associations between overall exposure to scented products and fragrance contact allergy. For different leave‐on product subgroups we found a positive association between exposure and fragrance contact allergy. A model for assessing the risk of induction of skin sensitization to fragrances has been developed that resulted in Quantitative Risk Assessment (QRA) to determine safe levels of fragrance ingredients in consumer products. 25 , 26 The QRA methodology follows four fundamental steps: hazard identification, dose–response assessment or hazard quantification, exposure assessment, and risk characterization. 25 Recently QRA1 has been refined, termed QRA2, which includes aggregate exposure to fragrances from multiple product use. 12 The design of the current study did not allow us to perform an aggregate exposure analysis for specific fragrance ingredients. However, we aimed to calculate an aggregate exposure to scented products by calculating the total sum of the frequency of exposure to the included scented products, thereby creating a continuous outcome as described in the methods. Longitudinal clinical studies to evaluate the efficacy of QRA2 as a tool for prevention of fragrance contact allergy are required. 12
4.2. Limitations
The measurement of exposure recommended by the Scientific Committee on Consumer Safety (SCCS) to use in skin sensitization risk assessment for fragrance ingredients is dose/area (μg/cm2). 27 The measurement of exposure in the current study ranges between never exposure to daily exposure. The total dose applied to the skin including daily frequency, dose per unit, and application site was not reported and could therefore not be included in our analysis. In addition, another limitation is the cross‐sectional design of the study: The questionnaire included only exposure during the last 12 months; therefore data about lifetime exposure is lacking. Subjects with high exposure to scented products prior to this last 12 months that resulted in contact dermatitis could have become current avoiders of the same scented products.
To decrease the false discovery rate and correct for multiple testing we applied the Benjamini‐Hochberg procedure, which is less conservative than the family‐wise error rate controlling procedures such as the Bonferroni correction. Because this was an exploratory study, we decided to use the Benjamini‐Hochberg correction to identify possible associations to include for future research, which might otherwise not be found by applying more conservative correction procedures.
4.3. Conclusion
In the European general population, female subjects and subjects younger than 40 years of age are most exposed to scented products. In females, high exposure to scented products may lead to fragrance contact allergy. Exposure to leave‐on products by females can therefore be considered as a risk factor in developing fragrance contact allergy. Sensitive skin is associated with exposure to different leave‐on product subgroups and with contact allergy to fragrances. Overall, exposure to scented products, especially to leave‐on products, is associated with fragrance contact allergy. Because aggregate exposure, specifically to scented leave‐on products, may enhance the prevalence of contact allergy to fragrances, further investigations into exposure amounts and frequencies are warranted.
CONFLICTS OF INTEREST
This study was supported by the Research Institute for Fragrance Materials, Inc. Magnus Bruze is a member of an expert panel for fragrance safety: http://fragrancesafetypanel.org/
AUTHOR CONTRIBUTIONS
Cynthia van Amerongen: Conceptualization; data curation; formal analysis; investigation; methodology; software; validation; visualization; writing‐original draft; writing‐review & editing. Robert Ofenloch: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing‐review & editing. Simone Cazzaniga: Funding acquisition; project administration; software; supervision; writing‐review & editing. Peter Elsner: Funding acquisition; project administration; resources; writing‐review & editing. Margarida Gonçalo: Funding acquisition; project administration; resources; writing‐review & editing. Luigi Naldi: Funding acquisition; project administration; resources; supervision; writing‐review & editing. Åke Svensson: Funding acquisition; project administration; resources; writing‐review & editing. Magnus Bruze: Funding acquisition; project administration; resources; supervision; writing‐review & editing. Marie Schuttelaar: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing‐original draft; writing‐review & editing.
van Amerongen CCA, Ofenloch RF, Cazzaniga S, et al. Skin exposure to scented products used in daily life and fragrance contact allergy in the European general population ‐ The EDEN Fragrance Study. Contact Dermatitis. 2021;84:385–394. 10.1111/cod.13807
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. de Groot AC, Frosch PJ. Adverse reactions to fragrances. Contact Dermatitis. 1997;36(2):57‐86. [DOI] [PubMed] [Google Scholar]
- 2. Cheng J, Zug KA. Fragrance allergic contact dermatitis. Dermatitis. 2014;25(5):232‐245. [DOI] [PubMed] [Google Scholar]
- 3. Alinaghi F, Bennike NH, Egeberg A, Thyssen JP, Johansen JD. Prevalence of contact allergy in the general population: a systematic review and meta‐analysis. Contact Dermatitis. 2019;80(2):77‐85. [DOI] [PubMed] [Google Scholar]
- 4. Buckley DA, Wakelin SH, Seed PT, et al. The frequency of fragrance allergy in a patch‐test population over a 17‐ year period. Br J Dermatol. 2000;142(2):279‐283. [DOI] [PubMed] [Google Scholar]
- 5. Nardelli A, Carbonez A, Ottoy W, Drieghe J, Goossens A. Frequency of and trends in fragrance allergy over a 15‐year period. Contact Dermatitis. 2008;58(3):134‐141. [DOI] [PubMed] [Google Scholar]
- 6. Thyssen JP, Carlsen BC, Menné T, Johansen JD. Trends of contact allergy to fragrance mix I and Myroxylon pereirae among Danish eczema patients tested between 1985 and 2007. Contact Dermatitis. 2008;59(4):238‐244. [DOI] [PubMed] [Google Scholar]
- 7. Uter W, Amario‐Hita JC, Balato A, et al. European surveillance system on contact allergies (ESSCA): results with the European baseline series, 2013/14. J Eur Acad Dermatol Venereol. 2017;31(9):1516‐1525. [DOI] [PubMed] [Google Scholar]
- 8. Thyssen JP, Linneberg A, Menné T, Nielsen NH, Johansen JD. The prevalence and morbidity of sensitization to fragrance mix i in the general population. Br J Dermatol. 2009;161(1):95‐101. [DOI] [PubMed] [Google Scholar]
- 9. Hall B, Tozer S, Safford B, et al. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem Toxicol. 2007;45(11):2097‐2108. [DOI] [PubMed] [Google Scholar]
- 10. Drechsel DA, Towle KM, Fung ES, Novick RM, Paustenbach DJ, Monnot AD. Skin sensitization induction potential from daily exposure to fragrances in personal care products. Dermatitis. 2018;29(6):324‐331. [DOI] [PubMed] [Google Scholar]
- 11. Safford B, Api AM, Barratt C, et al. Use of an aggregate exposure model to estimate consumer exposure to fragrance ingredients in personal care and cosmetic products. Regul Toxicol Pharmacol. 2015;72(3):673‐682. [DOI] [PubMed] [Google Scholar]
- 12. Api AM, Basketter D, Bridges J, et al. Updating exposure assessment for skin sensitization quantitative risk assessment for fragrance materials. Regul Toxicol Pharmacol. 2020;118:104805. [DOI] [PubMed] [Google Scholar]
- 13. Rossi M, Coenraads PJ, Diepgen T, et al. Design and feasibility of an international study assessing the prevalence of contact allergy to fragrances in the general population: the European Dermato‐epidemiology network fragrance study. Dermatology. 2010;221(3):267‐275. [DOI] [PubMed] [Google Scholar]
- 14. Naldi L, Cazzaniga S, Gonçalo M, et al. Prevalence of self‐reported skin complaints and avoidance of common daily life consumer products in selected european regions. JAMA Dermatol. 2014;150(2):154‐162. [DOI] [PubMed] [Google Scholar]
- 15. Diepgen TL, Naldi L, Bruze M, et al. Prevalence of contact allergy to p‐Phenylenediamine in the European general population. J Invest Dermatol. 2016;136(2):409‐415. [DOI] [PubMed] [Google Scholar]
- 16. Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174(2):319‐329. [DOI] [PubMed] [Google Scholar]
- 17. Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross‐sectional study. Br J Dermatol. 2015;173(6):1411‐1419. [DOI] [PubMed] [Google Scholar]
- 18. Svensson A, Ofenloch RF, Bruze M, et al. Prevalence of skin disease in a population‐based sample of adults from five European countries. Br J Dermatol. 2018;178(5):1111‐1118. [DOI] [PubMed] [Google Scholar]
- 19. Schuttelaar MLA, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy to metals in the European general population with a focus on nickel and piercings: the EDEN fragrance study. Contact Dermatitis. 2018;79(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruze M, Mowitz M, Ofenloch RF, et al. The significance of batch and patch test method in establishing contact allergy to fragrance mix I—EDEN fragrance study group. Contact Dermatitis. 2019;81(2):104‐109. [DOI] [PubMed] [Google Scholar]
- 21. Kwangsukstith C, Maibach HI. Effect of age and sex on the induction and elicitation of allergic contact dermatitis. Contact Dermatitis. 1995;33(5):289‐298. [DOI] [PubMed] [Google Scholar]
- 22. Brasch J, Schnuch A, Uter W. The profile of patch test reactions to common contact allergens is related to sex. Contact Dermatitis. 2008;58(1):37‐41. [DOI] [PubMed] [Google Scholar]
- 23. Modjtahedi BS, Modjtahedi SP, Maibach HI. The sex of the individual as a factor in allergic contact dermatitis. Contact Dermatitis. 2004;50(2):53‐59. [DOI] [PubMed] [Google Scholar]
- 24. Garg S, McDonagh AJG, Gawkrodger DJ. Age‐ and sex‐related variations in allergic contact dermatitis to common allergens. Contact Dermatitis. 2009;61(1):46‐47. [DOI] [PubMed] [Google Scholar]
- 25. Api AM, Basketter DA, Cadby PA, et al. Dermal sensitization quantitative risk assessment (QRA) for fragrance ingredients. Regul Toxicol Pharmacol. 2008;52(1):3‐23. [DOI] [PubMed] [Google Scholar]
- 26. Api AM, Vey M. Implementation of the dermal sensitization quantitative risk assessment (QRA) for fragrance ingredients. Regul Toxicol Pharmacol. 2008;52(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 27. SCCS (Scientific Committee on Consumer Safety of the European Commission) , 2017. Preliminary Opinion on Skin Sensitisation Quantitative Risk Assessment for Fragrance Ingredients (QRA2) Submission I, Adopted by the SCCS at the Plenary Meeting on 24‐25 October 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


