Abstract
Background
Hepatitis B virus (HBV) was found to exist in semen and male germ cells of patients with chronic HBV infection. Our previous studies demonstrated that HBV surface protein (HBs) could induce sperm dysfunction by activating a calcium signaling cascade and triggering caspase‐dependent apoptosis. However, the relationship between sperm dysfunction caused by HBs and caspase‐independent apoptosis has not been investigated.
Objectives
To evaluate the effects of HBs exposure on sperm dysfunction by activating caspase‐independent apoptosis.
Materials and methods
Spermatozoa were exposed to HBs at concentrations of 0, 25, 50, and 100 μg/mL for 3 h. Flow cytometry, qRT‐PCR, immunofluorescence assay, ELISA, and zona‐free hamster oocyte penetration assays were performed.
Results
With increasing concentrations of HBs, various parameters of the spermatozoa changed. The number of Bcl2‐positive cells declined and that of both Bax‐positive cells and Apaf‐1–positive cells increased. The transcription level of Bcl2 increased and that of both Bax and Apaf‐1 declined. The average levels of AIF and Endo G declined in mitochondria and increased in the cytoplasm and nucleus. The sperm DNA fragmentation index increased. The mean percentages of live spermatozoa declined and that of both injured and dead spermatozoa increased; and the sperm penetration rate declined. For the aforementioned parameters, the differences between the test and the control groups were statistically significant.
Conclusion
HBs exposure can activate the Bax/Bcl2 signaling cascade that triggers AIF/Endo G–mediated apoptosis, resulting in sperm DNA fragmentation, sperm injury, and death, and a decrease in the sperm fertilizing capacity. This new knowledge will help to evaluate the negative impact of HBV on male fertility in HBV‐infected patients.
Keywords: apoptosis, Bcl2 family, HBs protein, mitochondria, sperm function
1. INTRODUCTION
Hepatitis B is a potentially life‐threatening liver infection caused by hepatitis B virus (HBV) and is a major global health problem. An estimated 257 million people are hepatitis B surface antigen–positive. 1 HBV can cause chronic infection and puts people at high risk of death from cirrhosis and liver cancer. In 2015, hepatitis B resulted in 887,000 deaths. 1
HBV is one of the smallest viruses known to infect humans and belongs to the hepadnavirus family. 2 The HBV genome contains four long open reading frames: preS/S, preC/C, P, and X. The preS/S region of the genome encodes the three hepatitis B viral surface antigens (HBsAgs): large HBsAg, middle HBsAg, and small HBsAg. Small HBsAg is very hydrophobic, containing four transmembrane spanning regions; it is the primary constituent of all hepatitis B particle forms and appears to be manufactured by the virus in high quantities. 3 The guidelines from the World Health Organization (WHO) reported that the immune‐tolerant phase of chronic HBV infection occurs most commonly in HBsAg‐positive children and young adults infected in the perinatal or early childhood period. This phase usually persists into young adulthood and might last for 10–30 years after perinatal infection. 2 Young adults of 20–30 years of age are at the optimal age for reproduction; thus, the effect of HBV infection on their fertility might have grave consequences.
The subviral particles of HBV, which predominantly comprise HBs, are produced in vast excess over HBV virions into the circulation where concentrations reach 50–300 µg/mL. 4 However, HBsAg concentration evaluation in HBV carrier semen was never reported in epidemiological studies because of the difficulty to obtain semen and the semen volume is far smaller than blood samples. Previous studies have shown that HBV can enter male germ cells through the blood‐testis barrier and integrate into their genome, inducing sperm chromosomal abnormalities. 5 , 6 , 7 Thus, HBV infection may compromise sperm function and viability and play a role in reducing male fertility. 8 , 9 , 10 , 11 However, little is known about the mechanism underlying these effects.
A recent report showed that apoptosis might play a major role in causing male infertility. 12 Apoptosis is induced via two main routes involving either mitochondria (the intrinsic pathway) or the activation of death receptors (the extrinsic pathway). Mitochondria control cell death via caspase‐dependent and caspase‐independent mechanisms. 13 , 14 Our previous studies demonstrated that HBV surface protein (HBs) could induce sperm dysfunction by activating a calcium signaling cascade and triggering caspase‐dependent apoptosis, resulting in reduced sperm motility, loss of sperm membrane integrity, sperm dysfunction, decreased fertility, and sperm death. 15 , 16 , 17 To date, however, the relationship between HBs‐induced sperm dysfunction and caspase‐independent apoptosis has not been investigated. This study was undertaken to examine whether HBs induces sperm dysfunction through the activation of a B‐cell lymphoma 2 (Bcl2)/Bcl2‐associated X (Bax) signaling cascade that triggers apoptosis‐inducing factor (AIF)/endonuclease G (Endo G)–mediated caspase‐independent apoptosis.
2. MATERIALS AND METHODS
2.1. Ethical Approval
All protocols used in this study involving human subjects were approved by the Ethics Committee of Shantou University Medical College (approval no. SUMC‐00–0031) according to the World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. 18 Written informed consent was obtained from all study subjects for the use of their specimen for research.
All studies involving animals were reviewed and approved by the Medical Animal Care & Welfare Committee of Shantou University Medical College (IACUC protocol no. SUMC2015‐152) according to the recommended guideline for the care and use of laboratory animals published by the National Research Council (USA). 19
2.2. Sperm preparation
2.2.1. Subjects
The ten healthy donors were strictly selected and met the following criteria: (a) They had no systemic diseases or severe organic diseases such as heart disease, diabetes, tuberculosis, liver disease, urogenital system disease, blood system diseases, hypertension, psychosis, and leprosy; (b) they had no long‐term exposure to radiation and toxic and harmful substances, and no drug, alcohol, smoking, and other bad habits and homosexual history; (c) they had no history of sexually transmitted diseases, such as chlamydia, mycoplasma, gonorrhea, syphilis, condyloma acuminatum, soft wart, genital herpes, AIDS, hepatitis B, and hepatitis C, and exclude sexual partners of sexually transmitted diseases, vaginal trichomoniasis, and other diseases; and (d) their parameters of semen routine examination had to meet the standard of normal value in the WHO laboratory manual. 20 Their toluidine blue–stained micrographs of pooled seminal smear are shown in Figure 1.
Figure 1.
The toluidine blue–stained micrographs of pooled seminal smear of the ten donors.
2.2.2. Sperm preparation
Semen samples were obtained from ten healthy donors and were then incubated in a humidified incubator (37 ºC, 5% CO2 in air) for 30 min to allow liquefaction. Routine examination of the semen from the ten donors showed normal parameters according to the criteria of the WHO laboratory manual. 20 Motile spermatozoa were selected using the swim‐up method. 21 The supernatant collected from the tubes was centrifuged at 300 g for 5 min. The pellet of motile sperm was washed once and was then resuspended in 0.5 mL of medium. The motile sperm samples not contaminated with other components (eg, round sperm cells; autoagglutinated, dead, or immotile spermatozoa; leukocyte; and other seminal debris) were pooled and adjusted to a final concentration of 1 × 106 sperm/mL in Biggers‐Whitten‐Whittingham (BWW) medium supplemented with 0.3% BSA (Sigma‐Aldrich) for use in subsequent experiments.
2.2.3. Exposure of sperm cells to HBs
HBsAg, a recombinant HBV small surface (S) protein (NCPC Gene Tech Biotechnology Co., Ltd.), was made by Recombinant DNA Techniques in CHO (Chinese hamster ovary, CHO) cells. HBsAg secreted in the culture medium through cell proliferation and purified by high‐performance liquid chromatography (HPLC). The motile sperm sample was randomly divided into 4 groups, and 3 of the groups were incubated with HBs at concentrations of 25, 50, and 100 μg/mL in BWW medium in a humidified incubator (37 ºC, 5% CO2) for 3 h as the test groups. The remaining group, without exposure to HBs, was established as the control group. All groups were washed twice for subsequent experiments.
2.3. Detection of Bcl2, Bax, and Apoptotic Peptidase Activating Factor 1(Apaf‐1) activation
The sperm sample was randomly divided into 4 groups: A, B, C, and N. Each group was divided into 4 subgroups, among which subgroups A2‐4, B2‐4, and C2‐4 were exposed to HBs at final concentrations of 25, 50, and 100 μg/mL, respectively, as the test groups; subgroups A1, B1, and C1 were not exposed to HBs and were established as the blank control groups. Groups A, B, and C were incubated first with specific rabbit monoclonal antibodies against Bcl2, Bax, and Apaf‐1 (the primary antibodies), respectively, and then with FITC‐labeled goat anti‐rabbit IgG (the secondary antibody). The four subgroups of group N (N1‐4) were exposed to HBs at final concentrations of 0, 25, 50, and 100 μg/mL, respectively, and incubated only with the secondary antibody as the negative control. Fixation, permeabilization, and staining of sperm cells were performed with a BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit (BD Biosciences) according to the manufacturer's instructions. Approximately 10,000 events per sample were analyzed.
2.4. Transcription of the Bcl2, Bax, and Apaf‐1 genes
Real‐time quantitative polymerase chain reaction (RT‐qPCR was carried out to detect the effects of various concentration of HBs on transcriptional levels of the Bcl2, Bax, and Apaf‐1 genes in the sperm cells. The housekeeping gene glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was co‐amplified as an internal control. Total RNA was extracted from sperm cells with TRIzol reagent (Life Technologies), and cDNA was synthesized with a PrimeScriptTM RT reagent kit and gDNA Eraser (Catalog #RR047A) (Takara Biotech) according to the manufacturer's instructions. PCR was performed with a QuantiFast SYBR® Green PCR Kit (TaKaRa) according to the manufacturer's instructions. The primer pairs used to amplify the Bcl2, Bax, and Apaf‐1 genes were designed with Primer Premier 5.0 software, and the sequences are shown in Table 1. The thermal cycling conditions were as follows: 5 min at 95°C, followed by 40 cycles of 15 seconds at 95°C and 31 seconds at 60°C. The data were analyzed quantitatively with the 2−△△Ct method.
Table 1.
Primers used to amplify the apoptosis‐related and internal control genes
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Apaf−1 | 5’AGAAGGTTGTTTCCCAGGGGGAGTG3’ | 5’GCGAAGCATCAGAATGCGGAGACGG3’ |
| Bcl2 | 5’TCCAGGATAACGGAGGCTGGGATGC3’ | 5’CCAGGGCCAAACTGAGCAGAGTCTT3’ |
| Bax | 5’GGGTTTCATCCAGGATCGAGCAGGG3’ | 5’GCGGCAATCATCCTCTGCAGCTCCA3’ |
| GAPDH | 5’GGTGGTCTCCTCTGACTTCAACA3’ | 5’GGTGCTGTAGCCAAATTCGTTGT3’ |
2.5. Expression of the Bcl2, Bax, Apaf‐1, AIF, and Endo G proteins
Immunofluorescence assay (IFA) was employed to detect the effects of HBs exposure on expression of Bcl2, Bax, Apaf‐1, AIF, and Endo G proteins in the sperm cells. Sperm cells were mounted onto polylysine‐pretreated glass slides and fixed with 4% paraformaldehyde for 20 min at room temperature (RT). Cells were washed three times with phosphate‐buffered solution (PBS) at RT for 5 min (the same below) and then permeabilized by exposure to 0.1% Triton X‐100 for 10 min. After washing, the cells were placed in blocking solution (10% horse serum) for 30 min and then incubated first with the specific rabbit monoclonal antibodies against Bcl2, Bax, Apaf‐1, AIF, and Endo G (the primary antibodies), respectively, overnight at 4°C. After washing, the cells were incubated with FITC‐labeled goat anti‐rabbit IgG (the secondary antibody) for 30 min at RT in the dark. The slides were washed and then counterstained with 4'6‐diamidino‐2‐phenylindole (DAPI) (Invitrogen). Images of the stained cells were acquired by fluorescence microscopy (Olympus BX‐53, Optical).
2.6. Isolation of the mitochondrial,cytosolic, and nuclear fractions
Each sample was divided into two equal parts: One part was used to isolate the mitochondrial fraction (MF) and cytosolic fraction (CF) from the cells using a Mitochondria/Cytosol Fractionation Kit (Catalog #K256, BioVision), and the other part was used to isolate the nuclear fraction (NF) and CF with a Nuclear/Cytosol Fractionation Kit (Catalog #K266, BioVision) according to the manufacturer's instructions.
2.7. In vitro assays of AIF and Endo G translocation
The levels of AIF and Endo G in the CF, MF, and NF were measured using a Human Apoptosis‐Inducing Factor ELISA Kit and Human Mitochondrial Endonuclease G ELISA Kit (CUSABIO) according to the manufacturer's instructions. Enzyme‐linked immunosorbent assay (ELISA) was performed to calculate the average levels of AIF and Endo G in the CF, MF, and NF, which were expressed as picograms per milligram of protein (pg/mg of protein). The absorbance reading at 450 nm (a measure of the AIF or Endo G concentration) was corrected by subtracting the background reading at 570 nm. The AIF and Endo G concentrations were calculated by interpolating these values on a standard curve constructed for each plate.
2.8. Flow cytometry (FCM) analysis
FCM analyses were performed with a FACSCantoTM II flow cytometer (BD Biosciences). Sperm cells were isolated from debris by gating on the forward and side scatter signals, and cells were then identified and analyzed according to their relative fluorescence intensities compared with those of unstained cells. A minimum of 10,000 events were acquired and analyzed in each sample at a rate of 100–1000 events per second, and data analysis was performed with FlowJo software (Tree Star Inc.).
2.9. Sperm chromatin structure assay (SCSA)
An SCSA was performed to detect sperm cells with a high degree of DNA fragmentation and to distinguish them from normal sperm cells. Sperm cells in the test and control groups were adjusted to a final concentration of 2 × 106 sperm cells/mL in Tris‐sodium chloride‐EDTA (TNE) buffer. Immediately, 400 μL of acid detergent solution (0.08 M HCl, 0.15 M NaCl, and 0.1% (v/v) Triton X‐100; pH 1.2) was admixed with 200 μL of the adjusted sample in a Falcon tube (BD Biosciences). After 30 sec, sperm cells were stained by adding 1.2 mL of acridine orange (AO)–staining solution containing 6 μg of AO (chromatographically purified; Polysciences) per milliliter of buffer (0.037 M citric acid, 0.126 M Na2HPO4, 1.1 mM EDTA disodium, 0.15 M NaCl; pH 6.0). Three minutes later, approximately 10,000 events per sample were analyzed. Sperm chromatin damage was quantified by FCM measurements of the metachromatic shift from green (native double‐stranded DNA) to red (denatured single‐stranded DNA) fluorescence and displayed as red versus green fluorescence intensity cytogram patterns. The extent of DNA denaturation is expressed as the sperm DNA fragmentation index (DFI).
2.10. Assessment of sperm viability
A BDTM Cell Viability Kit (BD Biosciences) containing thiazole orange (TO) solution for staining all cells and propidium iodide (PI) for staining dead cells was used to distinguish live and dead sperm cells via FCM analysis according to the manufacturer's instructions. Approximately 10,000 events per sample were analyzed.
2.11. Assessment of sperm fertilizing capacity
Sperm penetration assays including hamster oocyte penetration assays were performed according to the WHO laboratory manual. 22 Briefly, after swim‐up, motile sperm cells were incubated with 10 μM Ca2+ ionophore (Sigma‐Aldrich Corp.) at 37 ºC for 10 min. These sperm cells were then washed twice and adjusted to a final concentration of 3.5 × 106 cells/mL. Zona‐free golden hamster oocytes were fertilized by the sperm cells and were then incubated in a humidified incubator (37ºC, 5% CO2) for 3 h. The preparations were examined with phase‐contrast microscopy at 200× magnification. The decondensed sperm heads with an attached or closely associated tail were counted, and the percentage of eggs penetrated by at least one spermatozoon was recorded.
2.12. Statistical analysis
Data are presented as the mean values ±standard deviations (SDs). One‐way analysis of variance (ANOVA) and the least significant difference (LSD) post hoc test were performed with SPSS version 16.0 (SPSS Inc.) to evaluate the impact of HBs exposure. In the 2−△△Ct analysis, individual data were converted to a linear form using the 2−Ct calculation method 23 and were then subjected to ANOVA using SPSS version 16.0 to determine whether the average transcription levels of the Bcl2, Bax, and Apaf‐1 genes differed significantly between the test and control groups. A P‐value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Effect of HBs exposure on Bcl2, Bax, and Apaf‐1 activation
Bcl2, Bax, and Apaf‐1 activation was measured with FCM analysis. The average percentages of Bcl2‐positive cells were declined with increasing concentrations of HBs and were 10.57 ± 0.21%, 9.47 ± 0.23%, 8.53 ± 0.50%, and 7.24 ± 0.89% in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, respectively. Significant differences in the average percentages of Bcl2‐positive cells between the test and control groups were observed (P < 0.01 or P < 0.05) (Figure 2A, Figure 3). In contrast, the average percentages of Bax‐ and Apaf‐1–positive cells increased with increasing concentrations of HBs. This percentages were 9.20 ± 0.53%, 10.17 ± 0.93%, 11.13 ± 0.91% and 13.40 ± 1.3% for Bax and 13.23 ± 0.47%, 14.60 ± 0.70%, 15.70 ± 0.70%, and 17.33 ± 1.64% for Apaf‐1 in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, respectively. After 3 h of exposure to 50 and 100 µg/mL HBs, significant differences in average percentages of Bax‐positive cells were observed as compared to that in the control. In parallel, significant differences in average percentages of Apaf‐1–positive cells were observed in 50 and 100 µg/mL HBs‐treated groups, as compared to that in control group (P < 0.05 or P < 0.01) (Figure 2A, Figure 3). The percentages of positive cells were very low in the negative control groups treated with increasing concentrations of HBs and were 0.17 ± 0.06%, 0.13 ± 0.06%, 0.17 ± 0.06%, and 0.23 ± 0.06% in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, respectively. No significant change was observed with increasing concentrations of HBs (Figure 3).
Figure 2.
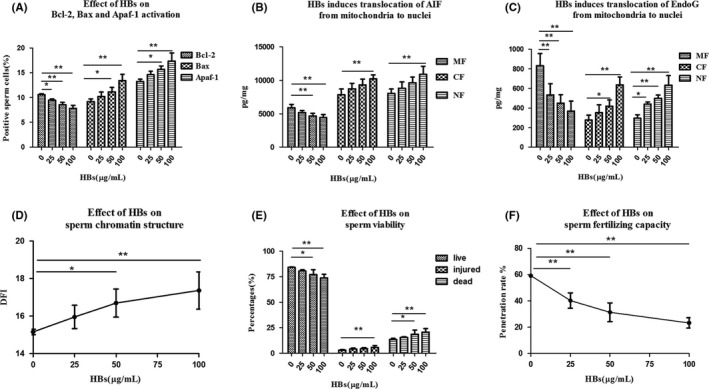
HBs‐induced apoptotic events resulting in sperm dysfunction. (A) Effect of HBs exposure on Bcl2, Bax, and Apaf‐1 activation in sperm cells. (B) Effect of HBs exposure on the translocation of AIF from mitochondria to nuclei in sperm cells. (C) Effect of HBs exposure on the translocation of Endo G from mitochondria to nuclei in sperm cells. (D) Effect of HBs on the sperm chromatin structure. (E) Effect of HBs on sperm viability. (F) Effect of HBs on the sperm fertilizing capacity. *P < 0.05; **P < 0.01.
Figure 3.
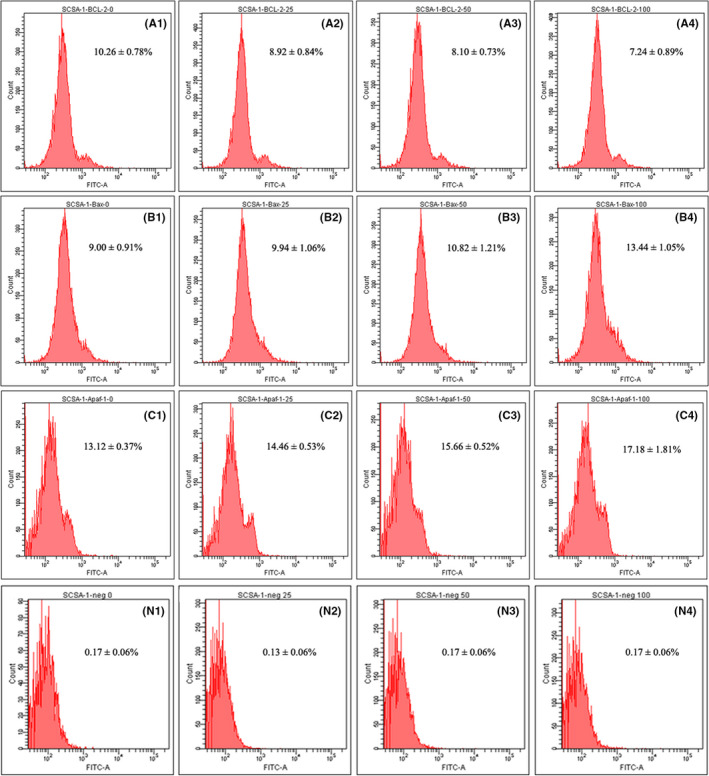
Effects of HBs exposure on Bcl2, Bax, and Apaf‐1 activation in sperm cells. The sperm sample were randomly divided into 4 groups: A, B, C, and N. Each group was divided into 4 subgroups, among which subgroups A2‐4, B2‐4, and C2‐4 were exposed to HBs at the final concentrations of 25, 50, and 100 μg/mL, respectively, as the test groups; subgroups A1, B1, and C1 were not exposed to HBs and were established as the blank control groups. Groups A, B, and C were incubated first with specific rabbit monoclonal antibodies against Bcl2, Bax, and Apaf‐1 (the primary antibodies), respectively, and then incubated with FITC‐labeled goat anti‐rabbit IgG (the secondary antibody). The four subgroups of group N (N1‐4) were exposed to HBs at final concentration of 0, 25, 50, and 100 μg/mL, respectively, and incubated only with the secondary antibody as the negative control.
3.2. Effect of HBs exposure on the transcription and translation of Bcl2, Bax, and Apaf‐1 genes in sperm cells
The transcription levels of Bcl2,Bax, and Apaf‐1 genes were measured with qRT‐PCR and the 2−ΔΔCT method. The transcription levels of the Bcl2 gene was declined with increasing concentrations of HBs; the fold change values compared to the levels in the control group were −3.45, −5.56, and −10 in the 25, 50, and 100 µg/mL HBs‐treated groups, respectively. In contrast, the transcription levels of the Bax and Apaf‐1 genes were increased with increasing concentrations of HBs; the fold changes compared to the levels in the control group were 1.28, 1.52, and 1.8 for Bax, and 1.26, 2, and 2.68 for Apaf‐1 in the 25, 50, and 100 µg/mL HBs‐treated groups, respectively (Table 2). The average transcription level of Bcl2 differed significantly among the groups. In parallel, significant differences in the average transcription levels of Bax and Apaf‐1 genes were observed in 50 and 100 µg/mL HBs‐treated groups as compared to the control group (P < 0.05) (Table 2).
Table 2.
Effect of HBs exposure on the transcription levels of Bcl2, Bax, and Apaf‐1 genes in sperm cells
| HBs‐treated groups (µg/mL) | Bcl2 | Bax | Apaf−1 | |||
|---|---|---|---|---|---|---|
| 2−△△CT | FCV | 2−△△CT | FCV | 2−△△CT | FCV | |
| 0 | 1.00 | 1.00 | 1.00 | |||
| 25 | 0.29* | −3.45 | 1.28 | 1.28 | 1.26 | 1.26 |
| 50 | 0.18* | −5.56 | 1.52* | 1.52 | 2.00* | 2.00 |
| 100 | 0.10* | −10.00 | 1.80* | 1.80 | 2.68* | 2.68 |
Total RNA was extracted from sperm cells in the HBs (0, 25, 50, and 100 µg/mL)‐treated groups and subjected to RT‐qPCR to detect the transcription levels of the Bcl2, Bax, and Apaf‐1 genes, The data are presented as a fold change values (FCVs) for the transcription levels of the Bcl2, Bax, and Apaf‐1 genes in the test groups normalized to the transcription level of the internal control gene (GAPDH) and relative to those in the control group. Individual data were converted to a linear form using 2−Ct method 23 and were then subjected to ANOVA to determine whether the average transcription levels of the Bcl2, Bax, and Apaf‐1 genes differed significantly between the test and control groups.
P < 0.05.
The effects of HBs exposure on expression of Bcl2, Bax, and Apaf‐1 proteins in the sperm cells were detected using IFA, and the results are shown in Figure 4. Each sperm sample was divided into two groups. One group was exposed to HBs at concentration of 25 μg/mL as the test group and one was not exposed to HBs as the control group. For Bcl2, the fluorescence intensity in the test group was weaker than that in the control group, indicating that HBs decreased Bcl2 expression. For Bax and Apaf‐1, the fluorescence intensity in the test groups was stronger than those in the control groups, indicating that HBs increased Bax and Apaf‐1 expression.
Figure 4.
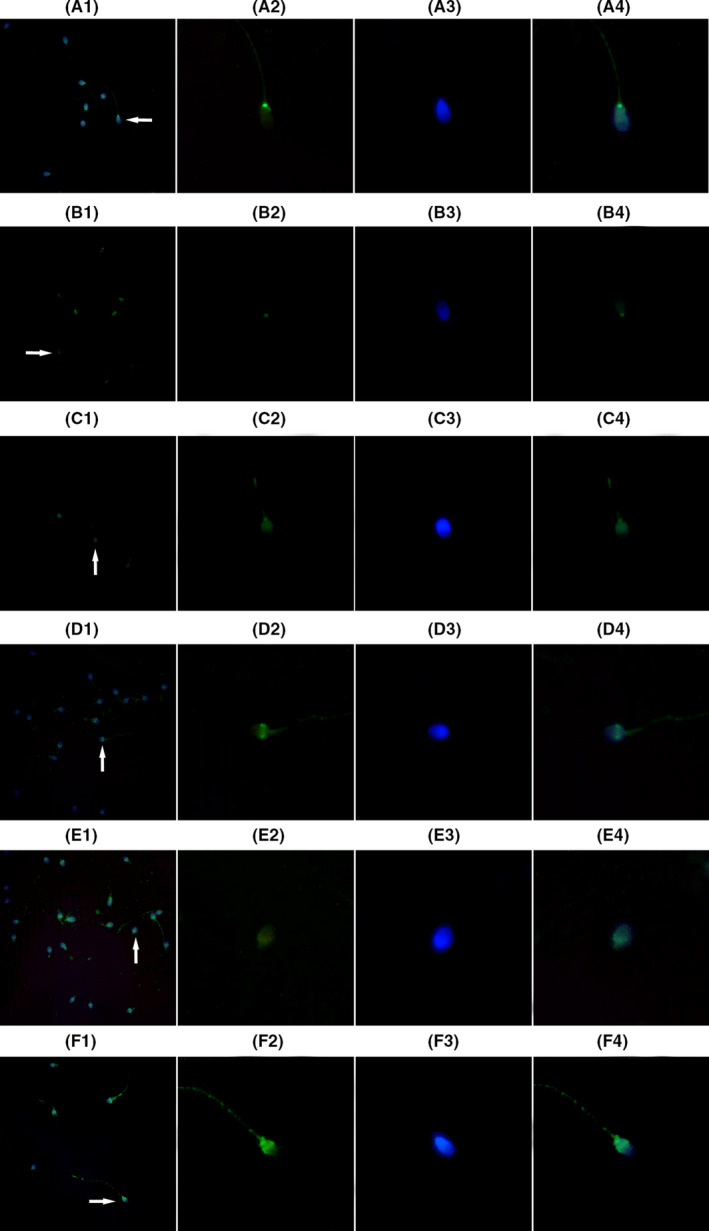
The effects of HBs exposure on expression of Bcl2, Bax, and Apaf‐1 proteins in the sperm cells. A1‐4, C1‐4, and E1‐4 showed expression of Bcl2, Bax, and Apaf‐1 proteins in the sperm cells not exposed to HBs, respectively (the control group); B1‐4, D1‐4, and F1‐4 showed expression of Bcl2, Bax, and Apaf‐1 proteins in the sperm cells exposed to HBs at concentration of 25 μg/mL, respectively (the test group). 1. Low‐magnification micrographs of seminal smears. 2. A signal of the fluorescence‐labeled protein (green) in a single sperm cell. 3. DAPI‐stained nucleus (blue) in a single sperm cell. 4. A merged figure of 2 and 3. The fluorescence intensity in the test group B was weaker than that in the control group A, indicating that HBs decreased Bcl2 expression. The fluorescence intensity in the test groups D and F was stronger than those in the control groups C and E, indicating that HBs increased Bax and Apaf‐1 expression.
3.3. HBs exposure induced the translocation of AIF and Endo G from mitochondria to nuclei
The levels of AIF in the MF, CF, and NF were measured with ELISA. The average levels of AIF were declined in the MF and increased in the CF and NF with increasing concentrations of HBs: in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, these respective levels were 5905.6 ± 484.2, 5142.4 ± 320.4, 4674.3 ± 367.1, and 4421.4 ± 447.3 pg/mg in the MF; 7834.4 ± 856.5, 8723.0 ± 814.3, 9299.3 ± 868.3, and 10178.9 ± 632.5 pg/mg in the CF; and 8001.2 ± 683.3, 8811.9 ± 938.3, 9631.2 ± 834.1, and 10884.2 ± 1212.6 pg/mg in the NF. After 3 h of exposure to 50 and 100 µg/mL HBs, the average levels of AIF in the MF differed significantly as compared to that in the control. In parallel, significant differences in the CF and NF were observed between the 100 µg/mL HBs‐treated groups and the control group (P < 0.01) (Figure 2B).
The levels of Endo G in the MF, CF, and NF were measured with ELISA. The average levels of Endo G were declined in the MF and increased in the CF and NF with increasing concentrations of HBs: in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, these respective levels were 832.4 ± 123.8, 523.7 ± 115.1, 449.1 ± 86.5, and 368.5 ± 101.3 pg/mg in the MF; 276.5 ± 50.6, 351.6 ± 80.4, 418.6 ± 63.7, and 636.6 ± 80.1 pg/mg in the CF; and 296.1 ± 35.2, 440.4 ± 21.5, 499.7 ± 32.1, and 631.9 ± 98.5 pg/mg in the NF. The average levels of Endo G in the MF and NF differed significantly among groups. In parallel, significant differences in the CF were observed in the 50, 100 µg/mL HBs‐treated groups, as compared to that in the control (P < 0.05 or P < 0.01) (Figure 2C).
IFA was performed to detect directly that HBs exposure induced the translocation of AIF and Endo G from mitochondria to nuclei. The results are shown in Figure 5. Each sperm sample was divided into two groups: One group was exposed to HBs at concentration of 25 μg/mL as the test group and one was not exposed to HBs as the control group. The fluorescence intensity, either for AIF or for Endo G, on the nucleus in the test groups was greatly stronger than those in the control groups. The results indicate that HBs exposure has induced the translocation of AIF and Endo G from mitochondria to the nuclei of sperm cells.
Figure 5.
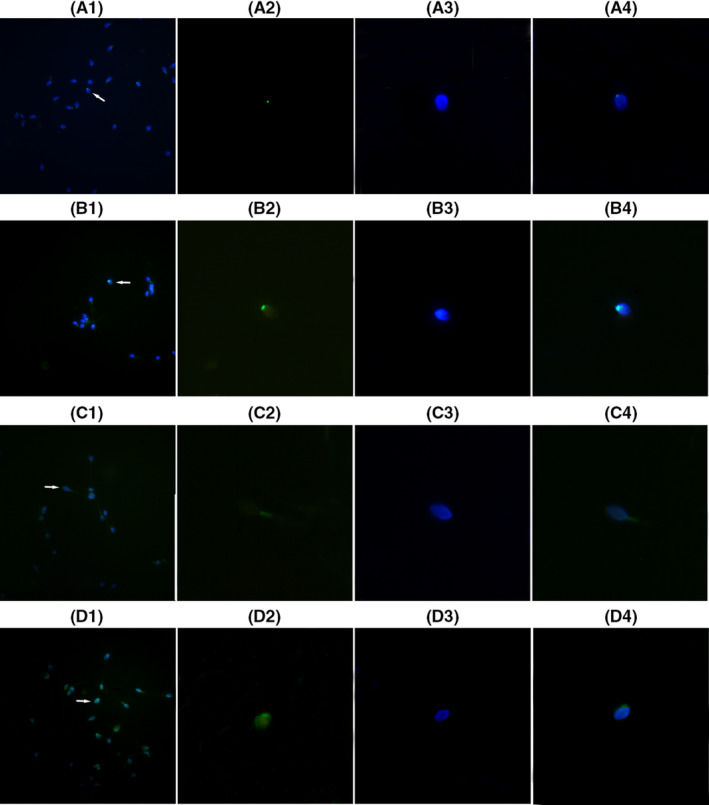
HBs exposure induced the translocation of AIF and Endo G from mitochondria to nuclei. A1‐4 and C1‐4 showed expression of AIF and Endo G protein in the sperm cells not exposed to HBs, respectively (the control group); B1‐4 and D1‐4 showed expression of AIF and Endo G proteins in the sperm cells exposed to HBs at concentration of 25 μg/mL, respectively (the test group). 1. Low‐magnification micrographs of seminal smears. 2. A signal of the fluorescence‐labeled protein (green) in a single sperm cell. 3. DAPI‐stained nucleus (blue) in a single sperm cell. 4. A merged figure of 2 and 3. The fluorescence intensity, for AIF and Endo G, on the nucleus in the test groups B and D was greatly stronger than those in the control groups A and C, indicating that AIF and Endo G proteins increased in the nuclei of the sperm cells after exposure to HBs.
3.4. Effects of HBs exposure on the sperm chromatin structure
The sperm DFI was measured with FCM analysis after AO staining. The sperm DFI increased with increasing concentrations of HBs and was 15.16 ± 0.15, 15.96 ± 0.62, 16.70 ± 0.75, and 17.37 ± 0.99 in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, respectively (Figure 2D). After 3 h of exposure to 50 and 100 µg/mL HBs, significant differences in the sperm DFI were observed between the test and the control groups (P < 0.05 or P < 0.01).
3.5. Effect of HBs exposure on sperm viability
Sperm viability was measured with FCM after TO and PI staining. The mean percentages of live sperm declined with increasing concentrations of HBs and were 84.23 ± 0.28, 80.54 ± 1.16, 77.04 ± 4.82, and 73.68 ± 3.64 in the 0, 25, 50, and 100 µg/mL HBs‐exposed treated groups, respectively. In contrast, the mean percentages of injured and dead sperm increased with increasing concentrations of HBs and were as follows: 2.94 ± 0.50, 4.06 ± 0.98, 4.37 ± 1.12, and 5.58 ± 1.95 for injured spermatozoa; and 13.49 ± 0.96, 15.41 ± 0.75, 18.60 ± 3.94, and 20.74 ± 3.36 for dead spermatozoa in the aforementioned groups, respectively. In parallel, the mean percentage of apoptosis spermatozoa increased with increasing concentrations of HBs was as follows: 16.33 ± 0.88, 19.47 ± 1.16, 22.96 ± 4.82, 26.32 ± 3.64 in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, respectively. After 3 h of exposure to 50 and 100 µg/mL HBs, significant differences in the mean percentages of live and dead spermatozoa as compared to that in the control were observed. In parallel, significant differences in the mean percentages of injured spermatozoa were detected between the 100 µg/mL HBs‐treated groups and the control group (P < 0.05 or P < 0.01) (Figure 2E).
3.6. Effects of HBs exposure on sperm fertilizing capacity
The sperm fertilizing capacity was measured with the zona‐free hamster oocyte penetration assay. The penetration rates were declined with increasing concentrations of HBs and were 59.14 ± 0.84, 40.31 ± 5.84, 31.38 ± 7.16, and 23.28 ± 3.96 in the 0, 25, 50, and 100 µg/mL HBs‐treated groups, respectively (Figure 2F). Significant differences in the penetration rate were detected between the test groups and the control (P < 0.01).
4. DISCUSSION
The presence of apoptotic signals in sperm cells has long been controversial. Historically, it was generally accepted that RNA is lost or degraded during spermiogenesis, when nuclear condensation is accompanied by the loss of most of the cytoplasm. Sperm cells were thought to be transcriptionally silent, based on the observation that nucleosomal histones, which are present in spermatogonia, were replaced by protamine in elongating spermatids and sperm cells and that these proteins were responsible for highly stable chromatin condensation, leading to a sperm genome that was transcriptionally inactive. 24 , 25 Thus, early works denied the existence of apoptosis in human ejaculated sperm cells. 26 However, an increasing number of studies have reported the existence of RNA in human ejaculated sperm cells. 27 , 28 , 29 Microarray analysis showed that a human sperm RNA profile contains approximately 3000–7000 types of coding transcripts. 30 Although most DNA is packaged by protamine, some (up to 15%) is present as histone‐bound chromatin scattered throughout the paternal genome, largely at gene promoter regions 31 , 32 ; these structures maintain features of active chromatin, mainly acetylated histones. 31 , 32 , 33 Therefore, in contrast to the traditional view, detectable levels of transcription in sperm cells persist, 34 and the presence and activation of apoptotic signals in human ejaculated sperm cells in response to various stimuli are presently widely accepted. 12 The RNA sequencing (RNA‐Seq) technique provides a more complete picture of the transcript population, including apoptotic transcripts, in mature spermatozoa. 35 Recent literature reported that 1469 proteins were identified in human ejaculated spermatozoa by proteomic analysis. 36 The aforementioned findings constitute the basis of our study.
Members of the Bcl2 superfamily have been reported to be key regulators of mitochondrial apoptosis. 37 This superfamily can be divided into pro‐ and antiapoptotic members. Proapoptotic Bcl2 members such as Bax and Bak are located primarily in the cytoplasm and can insert into the outer mitochondrial membrane (OMM) on demand; antiapoptotic members such as Bcl2 itself and Bcl‐xL are located in the OMM and inhibit apoptosis by preventing the opening of voltage‐dependent anion channels (VDACs) in association with proapoptotic Bcl2 members or by inhibiting the assembly of supramolecular openings formed by proapoptotic Bcl2 members. 24 The large number of mitochondria in the sperm midpiece provides a possible platform for apoptosis in response to intracellular stress. 38 Recent literature reported that the expression of apoptosis‐related proteins, including Bcl2 and Bax, was detected in mammalian spermatozoa. 39 , 40 In the present study, the average percentages of Bcl2‐positive cells declined dramatically with increasing concentrations of HBs. In contrast, the average percentages of Bax‐positive cells increased with increasing concentrations of HBs. Moreover, the transcription levels of Bax dramatically increased with increasing concentrations of HBs and HBs also increased the expression of Bax protein, and conversely, the transcription levels of Bcl2 dramatically decreased with increasing concentrations of HBs and HBs decreased the expression of Bcl2 protein, suggesting that HBs exposure activated Bax and suppressed Bcl2 transcription and translation in the sperm cells.
Mitochondrial apoptosis is regulated not only by the activation of Bcl2 superfamily members but also by the activation of certain membrane channels and the release of apoptogenic proteins through the permeability transition pore (PT) from the mitochondria into the cytosol. This event could activate the terminal elements of a protease cascade pathway through the PT and induce nuclear DNA fragmentation. 37 The main components of the PT are the VDAC located at the OMM, and the adenine nucleotide translocator (ANT) located at the inner mitochondrial membrane (IMM). Many apoptogenic proteins such as cytochrome c (Cyto C), AIF, and Endo G are located at the PT. 24
AIF is a 57 kD flavoprotein encoded by the apoptosis‐inducing factor mitochondria‐associated 1 (AIFM1) gene, 41 and Endo G is a 30 kD protein encoded by a nuclear gene. Both are translated in the cytosol and are then imported into mitochondria, where they are anchored to the IMM 42 and not to the nucleus. In the present study, the average levels of AIF and Endo G declined dramatically in the MF, increased in the CF and NF with increasing concentrations of HBs, and the HBs increased the expression of AIF and Endo G in the nucleus, suggesting that after exposure of sperm cells to HBs, AIF and Endo G were released from the intermembrane space and translocated into the cytoplasm and further into the nucleus.
Under normal conditions, antiapoptotic Bcl2 family members can simply act as ion channels to dissipate a potential across the OMM, which would permit VDACs to remain open under stress and perform their physiological function. Following the reception of stress signals, for example, HBs exposure in this study, mitochondrial apoptosis is activated, causing VDAC closure by Bax, thus leading to ANT malfunction followed by an inward flux of protons and ions through ANT. The increasing matrix osmolarity causes water attraction and mitochondrial swelling, along with the release of AIF and Endo G from intercristal storage into the cytosol through the ruptured OMM and further into the nucleus, where AIF cooperates with Endo G in a DNA degradation complex to provoke caspase‐independent chromatin condensation and subsequent DNA fragmentation. 24 This mechanism might explain why the average sperm DFI increased dramatically with increasing concentrations of HBs in the present study.
Herein, the mean percentages of live sperm declined dramatically with increasing concentrations of HBs; conversely, the mean percentages of injured and dead sperm increased markedly with increasing concentrations of HBs. In addition, the penetration rate of spermatozoa declined significantly with increasing concentrations of HBs. These results suggest that HBs exposure can induce sperm injury and death and reduce the sperm fertilizing capacity through the activation of a Bcl2/Bax signaling cascade that triggers the AIF/Endo G–mediated caspase‐independent apoptosis.
Apaf‐1 is a cytoplasmic protein that initiates apoptosis 25 and is an essential component of the vertebrate “apoptosome.” This apoptosome is composed of Cyto C, Apaf‐1, and procaspase‐9. Cyto C activates caspases by binding to Apaf‐1, inducing it to associate with procaspase‐9, thereby triggering caspase‐9 activation and initiating the proteolytic cascade that culminates in apoptosis. 43 In our previous studies, we found that HBs exposure could induce the release of Cyto C from the mitochondrial intermembrane space into the cytoplasm and induce the activation of caspase‐9 and caspase‐3. However, the data on Apaf‐1 were missing. 15 , 16 , 17 In this study, the average percentages of Apaf‐1–positive cells and the Apaf‐1 transcription levels increased with increasing concentrations of HBs, and HBs increased the expression of Apaf‐1 protein, suggesting that HBs exposure activated Apaf‐1 to participate in caspase‐dependent apoptosis, adding to our understanding of the mechanism.
During coevolution with their hosts, viruses have developed multiple strategies to manipulate biological processes in infected cells. Viruses can regulate cell proliferation, differentiation, and death. 44 Thus, many viruses induce the apoptosis of either infected cells or immunologically relevant cells, which increases viral spread or subverting the host's immune response. 44 , 45 In addition, viruses may inhibit apoptosis, a strategy that subverts one of the most ancient (non‐immune) antiviral mechanisms, thereby allowing the virus to replicate before its host cell dies. 46 , 47 Our study demonstrated that HBs induces apoptosis in sperm cells by triggering both caspase‐dependent and caspase‐independent pathways. Clarification of the mechanisms by which viral modulators of mitochondrial apoptosis exert their local action would contribute to preventing the negative impact of HBV infection on the fertilizing capacity of sperm cells in HBV‐infected patients.
COMPETING INTERESTS
The authors declare no competing interest.
AUTHORS’ CONTRIBUTIONS
TTH and THH carried out the study design and execution. TTH, JG, YZ, and THH performed result analysis and interpretation, manuscript preparation, and critical discussion. TTH, JHH, and QDX performed the experiments and analyzed the data. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors sincerely thank Professor Junhui Bian and Dr. Jianjun Zhang and colleagues of Shantou University Medical College who generously supported this study.
Funding information
This work was supported by the National Natural Science Foundation of China (No. 30972526) and by the Applied Basic Research Programs of Sichuan Province (No. 2014JY0110). The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Contributor Information
Ying Zhong, Email: yzhong08@126.com.
Tian‐Hua Huang, Email: yzhong08@126.com, Email: thhuang@stu.edu.cn.
REFERENCES
- 1. World Health Organization . Hepatitis B. Fact sheet. http://www.who.int/mediacentre/factsheets/fs204/en/ Accessed Aug 31, 2019.
- 2. World Health Organization . Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. WHO Press. Geneva, Switzerland:2015,166p. [PubMed]
- 3. Scuderi MA.DNA Vaccines for the Prevention and Treatment of Hepatitis B Virus. http://biology.kenyon.edu/slonc/bio38/scuderi/index.html/ Accessed Sep 16, 2019.
- 4. Ganem D.Hepadnaviridae and their replication. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology, 3rd ed. Philadelphia:Lippincott‐Raven, 2703–2738;1996.
- 5. Hadchouel M, Scotto J, Huret JL, et al. Presence of hbv DNA in spermatozoa: A possible vertical transmission of hbv via the germ line. J Med Virol. 1985;16(1):61‐66. [DOI] [PubMed] [Google Scholar]
- 6. Lang ZW. Distribution of hepatitis b virus in testicle tissue in patients with hepatitis b infection. Zhonghua Yi Xue Za Zhi. 1993;73(6):329‐331. [PubMed] [Google Scholar]
- 7. Huang JM, Huang TH, Qiu HY, et al. Effects of hepatitis b virus infection on human sperm chromosomes. World J Gastroenterol. 2003;9(4):736‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lorusso F, Palmisano M, Chironna M, et al. Impact of chronic viral diseases on semen parameters. Andrologia. 2010;42(2):121‐126. [DOI] [PubMed] [Google Scholar]
- 9. Moretti E, Federico MG, Giannerini V, Collodel G. Sperm ultrastructure and meiotic segregation in a group of patients with chronic hepatitis b and c. Andrologia. 2008;40(5):286‐291. [DOI] [PubMed] [Google Scholar]
- 10. Oger P, Yazbeck C, Gervais A, et al. Adverse effects of hepatitis b virus on sperm motility and fertilization ability during ivf. Reprod Biomed Online. 2011;23(2):207‐212. [DOI] [PubMed] [Google Scholar]
- 11. Shi L, Liu S, Zhao W, Zhou H, Ren W, Shi J. Hepatitis b virus infection reduces fertilization ability during in vitro fertilization and embryo transfer. J Med Virol. 2014;86(7):1099‐1104. [DOI] [PubMed] [Google Scholar]
- 12. Bejarano I, Espino J, Paredes SD, et al. Apoptosis, ROS and Calcium Signaling in Human Spermatozoa: Relationship to Infertility. Male infertility. InTech‐Open Access Publisher:Bashamboo A, McElreavey KD;2012, 51‐76p.
- 13. Sevrioukova IF. Apoptosis‐inducing factor: Structure, function, and redox regulation. Antioxid Redox Signal. 2011;14(12):2545‐2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pradelli LA, Beneteau M, Ricci JE. Mitochondrial control of caspase‐dependent and ‐independent cell death. Cell Mol Life Sci. 2010;67(10):1589‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou XL, Sun PN, Huang TH, Xie QD, Kang XJ, Liu LM. Effects of hepatitis b virus s protein on human sperm function. Hum Reprod. 2009;24(7):1575‐1583. [DOI] [PubMed] [Google Scholar]
- 16. Kang XiangJin, Xie QingDong, Zhou XiaoLing, et al. Effects of hepatitis b virus s protein exposure on sperm membrane integrity and functions. PLoS One. 2012;7(3):e33471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang JiHua, Zhong Y, Fang XiaoWu, et al. Hepatitis b virus s protein enhances sperm apoptosis and reduces sperm fertilizing capacity in vitro. PLoS One. 2013;8(7):e68688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. [DOI] [PubMed] [Google Scholar]
- 19. National Research Council . Guide for the Care and use of Laboratory Animals. 8th edn. Washington DC: National Academies Press; 2011. [Google Scholar]
- 20. World Health Organization . Semen analysis. WHO Press. WHO Laboratory Manual for the Examination and Processing of Human Semen 5th ed. Geneva, Switzerland:2010, 7–114p.
- 21. World Health Organization . Direct swim‐up. WHO Press. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland:2010, 164‐165p.
- 22. World Health Organization . Zona‐free hamster oocyte penetration test. WHO Press. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: 2010, 152‐157p..
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative pcr and the 2(‐delta delta c(t)) method. Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 24. Grootegoed JA, Siep M, Baarends WM. Molecular and cellular mechanisms in spermatogenesis. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(3):331‐343. [DOI] [PubMed] [Google Scholar]
- 25. Lambard S, Galeraud‐Denis I, Martin G, Levy R, Chocat A, Carreau S. Analysis and significance of mrna in human ejaculated sperm from normozoospermic donors: Relationship to sperm motility and capacitation. Mol Hum Reprod. 2004;10(7):535‐541. [DOI] [PubMed] [Google Scholar]
- 26. Weil M, Jacobson MD, Raff MC. Are caspases involved in the death of cells with a transcriptionally inactive nucleus? Sperm and chicken erythrocytes. J Cell Sci. 1998;111(Pt 18):2707‐2715. [DOI] [PubMed] [Google Scholar]
- 27. Miller D, Briggs D, Snowden H, et al. A complex population of rnas exists in human ejaculate spermatozoa: Implications for understanding molecular aspects of spermiogenesis. Gene. 1999;237(2):385‐392. [DOI] [PubMed] [Google Scholar]
- 28. Kumar G, Patel D, Naz RK. c‐MYC mRNA is present in human sperm cells. Cell Mol Biol Res. 1993;39(2):111‐117. [PubMed] [Google Scholar]
- 29. Wykes SM, Visscher DW, Krawetz SA. Haploid transcripts persist in mature human spermatozoa. Mol Hum Reprod. 1997;3(1):15‐19. [DOI] [PubMed] [Google Scholar]
- 30. Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360(9335):772‐777. [DOI] [PubMed] [Google Scholar]
- 31. Arpanahi A, Brinkworth M, Iles D, et al. Endonuclease‐sensitive regions of human spermatozoal chromatin are highly enriched in promoter and ctcf binding sequences. Genome Res. 2009;19(8):1338‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2010;16(1):30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gatewood JM, Cook GR, Balhorn R, Schmid CW, Bradbury EM. Isolation of four core histones from human sperm chromatin representing a minor subset of somatic histones. J Biol Chem. 1990;265(33):20662‐20666. [PubMed] [Google Scholar]
- 34. Miteva K, Valkov N, Goncharova‐Peinoval J, et al. Electron microscopic data for the presence of post‐meiotic gene expression in isolated ram sperm chromatin. Cytobios. 1995;83(333):85‐90. [PubMed] [Google Scholar]
- 35. Sendler E, Johnson GD, Mao S, et al. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res. 2013;41(7):4104‐4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agarwal A, Cui Z, Sharma R. Proteomic Analysis of Mature and Immature Ejaculated Spermatozoa from Fertile Men. Asian J Androl. 2015;18(5):735‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayer B, Oberbauer R. Mitochondrial regulation of apoptosis. News Physiol Sci. 2003;18:89‐94. [DOI] [PubMed] [Google Scholar]
- 38. Paasch U, Grunewald S, Dathe S, Glander HJ. Mitochondria of human spermatozoa are preferentially susceptible to apoptosis. Ann N Y Acad Sci. 2004;1030:403‐409. [DOI] [PubMed] [Google Scholar]
- 39. Liu Q, Si T, Xu X, Liang FQ, Wang LF, Pan SY. Electromagnetic radiation at 900 MHz induces sperm apoptosis through bcl‐2, bax and caspase‐3 signaling pathways in rats. Reprod Health. 2015;12(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dogan S, Mason MC, Govindaraju A, et al. Interrelationships Between Apoptosis and Fertility in Bull Sperm. J Reprod Dev. 2013;59(1):18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Human Gene Database . Gene Cards. http://www.genecards.org// Accessed Mar 06 2019.
- 42. Mohamad N, Gutierrez A, Nunez M, et al. Mitochondrial apoptotic pathways. Biocell. 2005;29(2):149‐161. [PubMed] [Google Scholar]
- 43. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309‐1312. [DOI] [PubMed] [Google Scholar]
- 44. Yoshida M. Multiple viral strategies of htlv‐1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475‐496. [DOI] [PubMed] [Google Scholar]
- 45. Seet BT, Johnston JB, Brunetti CR, et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377‐423. [DOI] [PubMed] [Google Scholar]
- 46. Benedict CA, Norris PS, Ware CF. To kill or be killed: Viral evasion of apoptosis. Nat Immunol. 2002;3(11):1013‐1018. [DOI] [PubMed] [Google Scholar]
- 47. Boya P, Pauleau AL, Poncet D, Gonzalez‐Polo RA, Zamzami N, Kroemer G. Viral proteins targeting mitochondria: Controlling cell death. Biochim Biophys Acta. 2004;1659(2–3):178‐189. [DOI] [PubMed] [Google Scholar]


