Abstract
Bee pollinators are an important guild delivering a fundamental input to European agriculture due to the ecological service they provide to crops in addition to the direct economic revenues from apiculture. Bee populations are declining in Europe as a result of the effects of several environmental stressors, both natural and of anthropic origin. Efforts are ongoing in the European Union (EU) to improve monitoring and management of pollinator populations to arrest further declines. Genetically modified (GM) crops are currently cultivated in a limited area in Europe, and an environmental risk assessment (ERA) is required prior to their authorization for cultivation. The possible impacts of GM crops on pollinators are deemed relevant for the ERA. Existing ecotoxicological studies indicate that traits currently expressed in insect‐resistant GM plants are unlikely to represent a risk for pollinators. However, new mechanisms of insect resistance are being introduced into GM plants, including novel combinations of Cry toxins and double strand RNA (dsRNA), and an ERA is required to consider lethal and sublethal effects of these new products on nontarget species, including insect pollinators. The evaluation of indirect effects linked to the changes in management practices (e.g. for herbicide‐tolerant GM crops) is an important component of EU regulations and a requirement for ERA. This paper reviews current approaches used to test the sensitivity of pollinators to GM plants and their products to determine whether sufficient data are being provided on novel GM plants to satisfy EU risk assessment requirements. © 2021 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: biosafety, genetically modified plants, apiculture, pollination, nontarget organisms
There is concern that genetically modified (GM) plants might induce adverse effects on pollinators either directly or indirectly via reduced food availability. Biosafety regulation in the EU aims to assess these potential risks to ensure the biosafety of GM crops.

1. INTRODUCTION
Apiculture in Europe has a long tradition based on the production of honey for consumption and, although commercial activities have become more important, a high proportion of amateur beekeepers are still actively rearing honey bee colonies. In 2019, 18.5 million colonies were maintained in the European Union (EU) by 650 000 beekeepers (https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/animals_and_animal_products/presentations/market-presentation-honey_en.pdf). The overall gross production of honey in the EU in 2019 was c. 240 000 t.
In addition to the production of honey and other beehive products (royal jelly, propolis, wax) the main socio‐economic and environmental benefits produced by honeybees is pollination of cropped and wild plants. Together with several other insect taxa (and some bats and birds), pollination is considered a valuable ecosystem service 1 and it is endorsed as an important protection goal by several European regulatory bodies. Therefore, pollination, bee diversity and provisioning of food (honey and other beehive products for honeybees) were identified as ecosystem services to be protected. 2 According to Gallai et al. 3 the production of 80% of the 264 crop species cultivated in the EU depends directly on the activity of insect pollinators (among which social bees and solitary bees are the most abundant), and the economic value of insect pollination services is estimated to be €14 billion per year. 4 The estimate of the economic value of pollination services in natural ecosystems is more difficult to establish, but there are well‐known reports of many cases of populations of wild plant species declining due to the diminished number of pollinators active in an area. 5 , 6 Indeed, specific protection goals have also been proposed for wild bees (i.e. bumblebees and solitary bees). However, because data on mortality rates is scarce and it is not possible to clearly define the magnitude of effects based on background mortality, their definition remains a challenge. 7
Pollinators are declining in many regions of the world 8 , 9 , 10 and Europe is by no means an exception. 10 , 11 Data collection in Europe is fragmented, but evidence of decline has been collected in some countries for honeybees 11 and bumblebees. 12 , 13
A great number of studies have addressed the possible causes of pollinator decline, 9 , 12 moreover estimates concerning the future effect of climate change on biodiversity indicate alarming consequences. 14 Diminished abundance and diversity of floral resources, exposure to agrochemicals, the action of parasites (e.g. Varroa destructor, Nosema spp.) and change of climate and land use are considered the main drivers of pollinator decline. 10 Moreover, there is data showing that in nature stressors do not act in isolation. 12 A major potential cause of decline is considered to be the interaction between environmental stressors, particularly between exposure to pesticides and pathogens. 15 For instance, sublethal effects caused by pesticides can damage detoxification mechanisms and weaken immune responses in bees, 16 which could then become more susceptible to parasites. This cascade effect, however, has so far only been observed at very high doses of toxicants. 17
Given the relevant ecological and economic value of pollinators and pollination services in agriculture and nature conservation, protection of pollinating species has become a widely recognized issue. In this respect, many areas of Europe are considered to have higher risk of pollinator decline, 9 and therefore management measures are urgently needed. Dicks et al. 18 proposed 10 possible policies to safeguard pollination services and suggested including the evaluation of direct, indirect and sublethal effects on pollinators in genetically modified (GM) crop risk assessments. More specifically, the authors stated that ‘GM crops pose potential risks to pollinators through poorly understood sub‐lethal and indirect effects’ and indicated as an example of such lack of information the case of herbicide‐tolerant (HT) GM crops which could impair pollinators' possibilities of finding food sources with possible consequences at population and landscape scale. The authors further clarified that for both pesticides and GM crops, indirect effects should be considered during risk assessment, for instance by examining what changes to agricultural management arise from the adoption of these products in farming practices. 19
Following this line of thought, in 2018 the Conference of the parties to the Convention on Biological Diversity adopted a decision on the conservation and sustainable use of pollinators (https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-06-en.pdf). The document urges the parties to ‘develop, enhance and implement on a regular basis risk assessment procedures for pesticides, pesticide‐coated seeds and living modified organisms to take into account possible impacts and cumulative effects, including sub‐lethal and indirect effects, on wild and managed pollinators.’
The aim of this review is to illustrate the main pillars of environmental risk assessment (ERA) for GM plants concerning pollinators in the EU according to the guidelines of the European Food Safety Authority (EFSA) 20 and underline if the current ERA approach for GM plants and pollinators adequately addresses safety concerns. We then summarize the most relevant evidence earned during many years of biosafety studies on pollinators and GM crops, including newly obtained GM events whose commercialization is expected to increase in the next few years.
2. ERA FOR POLLINATORS IN THE EFSA GUIDANCE DOCUMENT
The EFSA guidance document (GD) on the ERA of GM plants 20 provides directions for assessing potential effects of GM plants on the environment and the rationale for data requirements. Among the seven specific areas of concern considered in the GD, the interactions of the GM plant with nontarget organisms (NTOs) need to be evaluated by estimating potential hazards for each specific GM event and the expected exposure for selected categories of NTOs. As a first step in selecting focal species to start the assessment, it is necessary to identify the ecosystem functions and services provided in the agro‐ecosystem and the guild of species involved. Pollination is considered in the GD to be an essential ecosystem service, and the potential impacts of the cultivation of a GM plant should be assessed. The first step of the process requires that within each ecosystem function identified, the main species in each functional group should be listed, considering the GM plant and the organisms associated in its receiving environment(s). From the main list of species, applicants should then prioritize NTOs from each relevant functional group according to ecological criteria and practicability of laboratory and field experiments for hazard characterization. Once these focal species have been selected, measurement endpoints for assessing adverse effects need to be indicated. Both lethal and sublethal effects are considered relevant in the assessment of a possible hazard for a given NTO. Testing for sublethal effects is important since it can also give indications of possible long‐term effects of the stressor. It is therefore required that NTO tests consider both toxic effects (short‐term mortality, longevity) and sublethal effects. The GD indicates that sublethal effects can be assessed through growth, development and reproduction parameters. Moreover, the phenotypic characteristics of GM plants need to be reported, describing their interactions with NTOs in the environment (e.g. as support of the food web).
In addition to the safeguarding of service‐providing NTOs, the EFSA GD also indicates the need to consider other existing protection goals, such as biodiversity conservation. In this respect, species of cultural/conservation importance may need to be selected on a case‐by‐case basis. This indicates that, in addition to the most relevant species providing pollination services (e.g. honeybees, bumblebees, mason bees), in some receiving environments other pollinating species might become the subject of specific ERAs.
The EU legislation for the introduction of GM plants contains a requirement to assess the environmental impacts of the specific cultivation and management of GM plants. In the case of GM HT plants, this means evaluating the environmental impacts of the specific cultivation practices, including the change in herbicide use associated with these plants, along with the environmental impacts directly associated with the GM plant itself.
The specific changes in farming practices linked to the use of HT plants are discussed at length in the EFSA GD and it is specifically indicated that the ERA should consider whether uses of the herbicide could result in reductions in biodiversity leading to environmental damage.
Previously, the environmental impacts of changes in the herbicide management of GM HT crops were governed by EC2001/18, and therefore the EFSA GMO panel and the GMO authorities of EU Member States conducted risk assessments. Since March 2018, the direct and indirect effects of herbicide use on GM HT crops have been assessed under directive EU 2018/35 for plant protection products.
Currently, the EFSA GD does not address directly the issue of combined stressors on bees. However, EFSA has produced specific documents to consolidate the transition towards an integrated ERA of multiple stressors on bees as a framework for the ERA of these pollinators relevant for different types of environmental stressors. 7 , 21 Knowledge gaps have been identified in this area, and efforts are ongoing to define research needs to improve both exposure assessment and hazard characterization for multiple stressors acting on bees. Two main activities are currently conducted in Europe with the aim of increasing available data on exposure of honeybees to pathogens and pesticides, and reviewing the scientific literature on interactions between various stress factors. Under the umbrella of a EU Bee Partnership, initiatives are ongoing for harmonizing data collection, sharing and processing. 22 Based on an updated database, specific protocols will need to be developed to refine risk assessment in more realistic scenarios. The overall risk analysis will need the support of population dynamics and predictive modelling approaches taking into account co‐exposures and cumulative/synergistic interactions between stressors in bees.
3. DATA SUPPORTING ERA OF POLLINATORS IN GM CROPS
The issue of the possible impacts of GM plants on pollinators has been the subject of several studies, with a majority addressing the possible effects on honeybees of Bacillus thuringiensis (Bt)‐derived Cry toxins, which are currently expressed in a large proportion of the GM crops cultivated worldwide. 23 GM plants expressing a wide range of pathogen and virus resistances have been produced, although risk assessment for these GM events has focused mostly on different nontarget taxa, especially soil microorganisms. 24 , 25 In the EU the only authorized GM crop in cultivation is the MON810 maize, which expresses a Cry1Ab toxin targeting two lepidopteran pests, Ostrinia nubilalis and Sesamia nonagroides. In 2019, MON810 was cultivated mostly in Spain (107 127 ha), where MON 810 maize represents c. 35% of the total maize area. The adoption rate of this Bt maize in some regions with a high incidence of corn borer infestations (e.g. Aragon, Catalonia) can be higher than 60%. Mon810 in 2019 was also cultivated in Portugal (4718 ha), accounting for c. 10% of the total area cropped with maize. 26 This paper focuses on the main GM traits that are most likely to produce direct or indirect hazards for pollinators, although it is stressed that in all cases risk assessment needs to consider impacts on nontarget species exposed to GM plants.
3.1. Exposure of pollinator bees to maize pollen in field conditions
Field studies regarding the activity of pollinators in GM agroecosystems have mostly been conducted to evaluate the potential for gene flow and consequent possible introgression of the transgene into relatives. However, possible exposure of pollinators to newly expressed proteins/molecules in pollen or nectar is an important issue, particularly when insecticidal proteins/molecules are expressed.
Maize pollen expressing Cry1Ab toxin can be collected by foragers, stored in the hive and consumed by both adult and immature honeybees, 27 providing the main exposure route for bees via direct feeding. Odoux et al. 28 performed a field study to investigate the flower range exploited in an agrarian environment in western France. Their palynological analyses showed the importance of maize among crop pollens. Danner et al. 29 conducted field observations in northern Bavaria, Germany, at a landscape scale. During maize flowering, observation hives were rotated between 11 different landscapes, which covered a gradient from low to high maize acreage. The authors observed intracolonial dance communication to gather information about the location of utilized pollen resources. A higher frequency of dances was detected for foraging locations on maize fields compared to other land use types, indicating that maize was an intensively used pollen resource for honeybee colonies in summer when other pollen resources become scarce. The proportion of grassland area providing alternative pollen sources did not reduce the percentage of maize pollen foragers. Maize plants release pollen for up to 3–4+ weeks in long day length situations. In some parts of Europe maize flowers in the ‘summer gap’ (e.g. the ‘June gap’ in the UK) when few other plants are flowering and then it provides an important source of pollen. This importance/significance varies with climates/seasons and may shift in relation to climate change.
However, a study on pollen consumption conducted with field cages 30 containing only flowering maize plants discovered that the amounts of maize pollen detected in fully grown bee larvae constituted only a small part (c. 5%) of the total amount of protein necessary for a complete larval development.
During the activities of the EU‐funded AMIGA project, a comparative study of pollen samples was conducted by collecting every 2 h from a bumblebee and a honeybee colony placed in the same landscape in Germany using pollen traps developed by Würzburg University. Honeybees collected more maize pollen, and when investigating the diversity of pollen food sources using the Simpson index, the bumblebee Bombus terrestris reached on average higher values, which might be due to their lower flower constancy (Fig. 1). All these results confirm that maize is a highly relevant pollen source for honeybee colonies, which may be actively collecting maize pollen during the flowering season in different landscapes.
Figure 1.
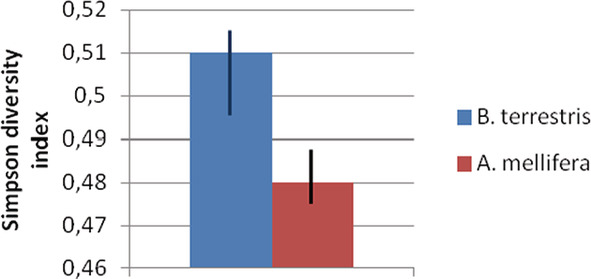
Diversity of pollen origin collected by honeybees (Apis mellifera) and bumblebees (Bombus terrestris) in field experiments in Germany (data from AMIGA project, http://www.amiga.enea.it/web/wp‐content/uploads/Deliverables/D6.2.pdf).
3.2. Possible hazard of Cry toxins to pollinators
Experience with cultivation of Bt crops for more than two decades has enabled the collection of relevant information on this pest management approach. GM crops may indirectly provide benefits to NTOs due to reductions in pesticide use. The global insecticide savings from using insect‐resistant GM maize and cotton in 2016 were 8.7 million kg (−82% of insecticides) in maize and 18.9 million kg (−56% of all insecticides) in cotton of active ingredient use. 31
Cry toxins currently expressed in GM crops have a spectrum of activity generally limited to a single insect order, though cross‐activity has been demonstrated for some of them, e.g. Cry1Ac, Cry3Aa and Vip1A/Vip2A. 32 The only Bt toxin known to be specifically active against Hymenoptera is Cry5, 33 , 34 but currently no GM plants expressing this toxin have been produced.
The scientific opinion issued by the EFSA GMO panel which supported the renewal of the permit for cultivation of MON810 maize in the EU made a specific assessment of potential risks for pollinating species and concluded that the likelihood of adverse effects on honeybees caused by the cultivation of maize MON810 is expected to be very low. 35 To produce this positive opinion the GMO panel reviewed existing literature available at that time. Duan et al. 36 used the approach of a meta‐analysis considering 25 independent laboratory studies in which Cry1, Cry2, Cry3 or Cry9 classes were tested on honeybees and concluded that these Cry toxins do not negatively affect the survival of either honey bee larvae or adults in laboratory settings. The authors, however, highlighted a knowledge gap, as the studies used in the review did not consider possible interactions of the ingestion of the toxin with other environmental stressors. Malone and Burgess 37 included in a further review experiments conducted with bees in (semi)field conditions and reported that insecticidal traits used in commercially available GM crops (Cry and Vip toxins) did not induce any deleterious effects on pollinators. Indeed, the only molecules that caused adverse effects in laboratory experiments were some serine protease inhibitors and the snowdrop lectins, none of which has been used in commercial GM plants, mainly due to their effects on a wider range of nontarget species.
Babendreier et al. 38 observed specifically the hypopharyngeal gland development in honeybees, considered an important indicator of bee life history. No negative effects were identified after feeding young adult bees for 10 days with Bt maize pollen expressing Cry1Ab protein or with purified Cry1Ab protein solubilized in sugar solutions.
More recent studies were conducted in Germany by Hendriksma et al. 39 exposing honeybee colonies in flight cages to pollen from GM maize expressing three different insecticidal Cry proteins (Cry1A.105, Cry2Ab2 and Cry3Bb1 in the GM maize hybrid MON 89034 × MON 88017). The consumption of Bt maize pollen had no effect on the survival rate, body weight and rates of pollen digestion of nurse bees compared to those foraging on conventional maize varieties. The authors also considered additional endpoints, and found that more than 98% of the recombinant proteins were degraded in the gut of the insects. In addition, bacterial population sizes in the gut were not affected by the genetic modification. 40 Han et al. 41 examined behavioral responses of NTOs, including pollinators, when exposed to GM plants. The authors indicated that behavioral effects of GM crops on arthropod pollinators were relatively limited. Results were quite variable among the different studies reviewed by the authors, who claimed that more studies are required to better understand the ecological relevance of these results.
Although insect‐resistant crops based on the expression of Bt‐derived proteins have remained effective against most pest populations after many years of commercial cultivation, cases of resistance with consequent increased damage to Bt crops have been reported in some populations of major target insect pests. 42 , 43 , 44 GM maize MON 863 harboring the Cry3Bb1 toxin was developed with the aim of controlling soil‐dwelling larvae of the western corn rootworm Diabrotica virgifera virgifera. 45 However, this pest quickly developed resistance to the cry3Bb toxin, probably due to the low levels of expression in some maize root tissues, 46 triggering the need for additional control measures.
3.3. GM plants expressing dsRNA
There is considerable literature on the effects of Cry and related toxins on a wide range of insect species. More recently, plants expressing double strand RNA (dsRNA) for pest and disease resistance, and to change plant physiology, composition and metabolism, have been produced. One of the most appealing aspects of this toxic mechanism for controlling agricultural pests is the potentially very high selectivity of the newly expressed molecules in GM plants, since they can be specifically designed to silence a selected gene by coupling to a short (c. 20–25 nucleotides) sequence of the target gene. The use of dsRNA in GM plants is therefore expected to have little or no activity in NTOs. However, some examples of adverse effects on nontarget insects have been reported in laboratory conditions. 47 , 48 This pest control approach is based on a completely new mechanism of action against target organisms, therefore information on the possible nontarget and off‐target effects needs to be proactively collected. 49 This paper considers potential hazards and their risk assessment in more detail.
The RNA interference (RNAi) mechanism was discovered as a natural defense mechanism in the nematode Caenorhabditis elegans, 50 and more recent research has identified the genetics of essential physiological and metabolic functions of RNAi in a range of insect pests. 51 RNAi efficiency in insects is characterized by a remarkable variability between taxa. Certain coleopteran species, including D. virgifera virgifera, are known to be highly sensitive to dsRNA on ingestion. Some of these dsRNAs have now been introduced into plants to confer protection against the targeted pest. The first available commercial application is a maize conferring resistance to the western corn rootworm D. virgifera virgifera and the northern corn rootworm Diabrotica barberi through silencing of the Snf7 gene. 52 The SmartStax PRO hybrid maize targets Coleoptera pests through the expression of several Bt‐derived toxins (Cry3Bb1, Cry34Ab1/Cry35Ab1) together with the dsRNA aimed at silencing the DvSnf7 gene. The multiple toxins approach should guarantee a higher toxicity against D. virgifera virgifera and at the same time provide more lasting insect resistance features.
Insects feeding on dsRNA expressing GM plants will ingest the dsRNA, resulting in exposure to the dsRNA in their gut, if it is not inactivated in the gastrointestinal tract. Moreover, on ingestion, dsRNA may also be systemically transported to other organs so that genes expressed in these organs are also RNAi targets. Different molecular and physiological aspects are involved in cases of nontarget or off‐target effects (e.g. homology of nucleotide sequences, need for matches of more than 19 mer with nontarget genes, etc.). 51 It is therefore paramount the design of appropriate dsRNA sequences as a first step for ensuring the biosafety of a specific RNAi approach.
Honeybees have been used as surrogate species during ERA of different dsRNA. During the development of the MON 87411 maize, Bachman et al. 53 evaluated the possible ecological risks of this GM event harboring a dsRNA targeting the DvSnf7 gene. A number of NTOs representative of the main ecological functions in the maize agro‐ecosystems were selected for testing, including honeybees (A. mellifera). Two‐ or 3‐day‐old larvae were fed with an artificial diet containing 1000 ng of dsRNA per gram of diet for 17 days. The same diet was offered to 1‐ or 2‐day‐old adults for 14 days. At the end of the tests, no differences in survival of either larvae or adults, in comparison with the control specimen, were detected. For immatures, additional endpoints were measured (time to adult emergence, percentage of capped brood) and again no differences were recorded. Concentration of dsRNA in pollen ranged between 0.056 × 10−3 and 0.224 × 10−3 μg g−1 of fresh weight tissue, therefore the authors considered the laboratory bioassays with very high doses of dsRNA as a reliable stress test. Similar outcomes were obtained by Chen et al., 54 who investigated the effects of dsRNA targeting rpl19 gene from Bactrocera dorsalis. dsRNA was supplied via feeding on a honey‐based artificial diet to adult bees at concentrations of 500 and 1000 ng μL−1 of a liquid diet for 6 h. While A. mellifera‐specific dsRNA was ingested, gene expression was reduced by 76% at the higher dose and by 50% at the low dose. On the contrary, when B. dorsalis‐specific dsRNA was fed to honeybees no effects on the expression of the target gene were detected.
This lack of sensitivity of honeybees to dsRNA was confirmed in another experiment conducted by Garbian et al., 55 who investigated bidirectional transfer of dsRNA between honeybees and the parasite spider mite, Varroa destructor. A dsRNA targeting V. destructor was supplied to a bee colony, which was then infested with Varroa mites. After this treatment, the population of the parasite was reduced while the bee colony did not suffer any adverse effects. This experiment demonstrated that movement of dsRNA along the food chain did not impair biological activity of dsRNA, and that honeybees proved to be quite insensitive to this dsRNA on ingestion. This particular example indicated a possible beneficial use of dsRNA for beekeepers, for controlling Varroa destructor infestations.
Unlike the case of Bt‐expressing GM plants, most of the relevant knowledge for biosafety purposes is currently based on the single case of MON 87411. For instance, for estimating the exposure to pollinators and flower visitors, an important parameter is the expression of dsRNA in pollen tissues, which has not been measured in other GM events. Further insight on how dsRNA is stabilized and consequences thereof would also be needed. The availability of the full genome of A. mellifera and two bumblebee species 56 may facilitate the design of dsRNA‐targeting genes not present in the genome of bees, limiting the possibility of nontarget silencing.
3.4. HT GM plants
A range of other traits has been expressed in GM plants and these events are subject to the same assessment during their authorization procedure for cultivation. GM plants tolerant to herbicides are assessed for indirect effects on biodiversity via changes in management practices, including changes in herbicide regime. In relation to pollinating insects, the risk scenario comes from the consideration that the change in herbicide use may change or reduce the botanical diversity in treated fields and hence the availability of nectar and/or pollen. The diversity of weeds in and around cropped fields provides important resources for pollinators in areas of intensive land management, therefore significant changes in weed management can have impacts on pollinator activity and success in these landscapes. 57 The largest field experiment conducted in Europe addressing this question was the farm‐scale evaluation in the UK, 58 which demonstrated that changes in weed management may indeed affect biodiversity (flora and fauna) in and around fields. In detail, Hawes et al. 59 reported that numbers of pollinators (including bees, butterflies and moths) tended to be lower in fields with GM HT sugar beet and spring oilseed rape, while they were slightly more abundant in HT maize. The extent of these differences was variable along the cropping season. When detailed analyses of the field experiments were referred to each taxonomic group and the three different crops used in the trials (maize and oilseed rape varieties tolerant to glufosinate‐ammonium and sugar beet tolerant to glyphosate), it was concluded that the most significant effects were observed on bees (particularly honeybees) foraging in GM HT beet. 60 Since beets were not flowering, the effect might be attributed to the difference in flowering weeds present in cropped areas and their margins. These field studies overall demonstrated that results might be different case by case and that local management practices and agronomic conditions are expected to be relevant drivers toward possible harm. To this aim, Bürger et al. 61 used a spatially explicit simulation model to estimate the effects of the introduction of Bt maize and HT maize cultivation into different agrarian landscapes in France (Aquitaine) and Spain (Catalonia). The model allows quantification of the effects of cropping systems on weed dynamics as well as indicators of weed‐related biodiversity (i.e. species richness and equitability, trophic resources for birds, insects and specifically pollinators). Eleven most probable scenarios (three Bt and eight HT) were simulated over a time span of 28 years for each of the two regions, and repeated with 10 different regional random weather series. Resources for pollinators are determined in the model, i.e. weed flower density from March to November, weighted by the relevance of the plant species as food source for honeybees. The results of the simulations clearly indicate that the availability of food for bees is expected to be affected substantially in Catalonia with the introduction of HT maize, while considering local conditions only slight changes could be expected in Aquitaine (Table 1). The expected negative impacts will be exacerbated when the shift to HT varieties is accompanied by changes in agronomic practices (i.e. simplified rotation, maize monoculture).
Table 1.
Impact of switching from control to GM HT cultivars and associated practice changes on availability of bee food
| Availability of food for bees (no unit, [0, +∞[) | ||
|---|---|---|
| Farm practice | Catalonia (ES) | Aquitaine (FR) |
| Control | 1.24 | 0.63 |
| Impact of change in practices relatively to control | ||
| Switch to HT maize | −0.03 ns | −0.04 ns |
| Simplified rotation | −0.35 | +0.04 |
| Maize monoculture | −0.58 | −0.36 |
| No plough | −0.84 | −0.20 |
| No till | +0.46 | +1.01 |
| Earlier sowing | +0.54 | +1.03 |
| Winter catch crop | +0.46 | +0.18 |
ES, Spain; FR, France; ns, not significant.
Numbers indicate the effect (±) as compared to control scenarios (conventional maize). Modified after Bürger et al. 61
These consequences are also induced by changes in rotation or tillage in the cropping systems, made possible by the GM HT varieties, in addition to changes associated with the herbicide (glyphosate) treatments themselves. The changes in weed‐related biodiversity are different between regions, and the results can thus not be simply extrapolated to other maize‐growing regions. These simulations are in line with the outcomes of the farm‐scale evaluation, with the additional value of a larger analysis of different scenarios and different possible cropping practices over a longer time series.
In its opinion on the first GM HT crop assessed for cultivation in the EU, 62 the EFSA GMO panel stated ‘… the potential environmental impacts of the specific cultivation, management and harvesting techniques of maize NK603 are indirect effects entirely associated with the use of the complimentary herbicide regimes’. Thus, the EFSA GMO panel concludes that maize NK603 plants are unlikely to cause any direct adverse effects, but that potential adverse environmental effects of the cultivation of maize NK603 associated with the use of the complimentary glyphosate herbicide have been identified. The EFSA GMO panel recommends that the potential adverse effects of the glyphosate should be evaluated for the specific use on maize NK603 during the national registration by Member States. Thus, EFSA makes it clear that the changes in management associated with this GM HT maize could cause environmental harm and this could include changes in flora and effects on NTOs linked to this flora.
A range of mitigation measures and agricultural practices have been developed for improving the biodiversity of farming systems. In the UK, Pidgeon et al. 63 showed that various measures could be adopted to maintain or increase botanical diversity in farming systems. Drossart and Gérard 64 described measures that can be used to support insect pollinator populations, specifically bees. Measures include increasing crop diversity, growing wildflower mixtures and uncultivated refuge areas, and reducing pesticide exposure by specific targeting and timing of pesticide applications.
4. CONSIDERATIONS FOR ERA OF GM CROPS FOR POLLINATORS
The EFSA GD on ERA of GM plants requires that environmental protection goals, including the protection of ecological functions and the species which provide them, should be duly considered in the ERA. 20 Thus in the EU, pollinators are one of the taxa for which an ERA for GM plants should be conducted. Particular attention is suggested for selecting measurement endpoints that enable sublethal as well as lethal, and chronic as well as acute effects to be addressed.
To this end, the AMIGA project developed several protocols to make the EFSA GD practically applicable for ERA. In the area of pollinators, efforts were devoted to creating easily reproducible experimental setups for hazard characterization 39 , 65 and exposure assessment. 29 Validated protocols are available for field monitoring in newly cultivated regions that may be used to evaluate the ecological function of pollination under field conditions at landscape level. 66 , 67
Protocols for measuring pollination service are recommended for establishing baseline data and for estimating possible impacts of GM cultivation both in experimental conditions and in a commercial phase (during post market environmental monitoring). These methods proved to be effective and amenable to use with the support of basic taxonomic expertise, supporting the value of the ERA GD approach to assess environmental impacts of GM plants on pollinators.
5. FUTURE PERSPECTIVES
The ongoing decline of populations of pollinators in many regions is a major threat to nature conservation but also to the sustainability of human managed landscapes where agriculture is a dominant factor. 68 The maintenance of ecosystem services is deemed fundamental in this effort of strengthening the sustainability of agriculture while maintaining levels of primary production in view of a growing human population.
The spread of the so‐called CCD in the USA and the colony losses over winter reported in Europe has triggered governments to implement measures aimed at ensuring pollinator conservation in natural and human‐managed landscapes. Halting and reversing the decline of pollinators in Europe is considered one of the key elements of the EU biodiversity strategy for 2030, one of the pillars of the European Green Deal (https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en), which aims to make Europe the first climate‐neutral continent. A recent executive decision (UE 2019/974) has increased the support to the apicultural sector by additional 12 million euros (10% increase in the available budget for 2020–2022). This action comes from the consideration that apiculture constitutes a fundamental part of the EU agrifood sector, as it contributes to employment in rural areas and because bees are essential for the sustainability of agriculture and ecosystems.
As indicated above, the only GM crop authorized in Europe is the insect‐resistant maize MON810, which is currently cultivated only in the Iberian peninsula (Spain and Portugal) and the situation is not expected to change in the near future. However, in other geographic areas the majority of commodity crops such as soybean, canola, maize and cotton are GM plants. 23
Most of the existing research on the safety of GM crops for pollinators has been focused on honeybees or bumblebees as focal species; existing evidence from scientific literature and two decades of cultivation of GM crops support the belief that there are no negative effects of currently cultivated insect‐resistant GM crops for insect pollination services. An interesting option is the possible use of the newly available RNAi technology (developed as genetically induced host resistance in GM plants or as a sprayable pesticide product) for managing agricultural pests or to directly control pests of honeybee colonies, such as the ravaging Varroa mites. dsRNA‐expressing plants can be designed with a high specificity to target particular genes expressed in a few closely related species and may represent very selective tools for pest control in agriculture.
The assessment report on pollinators, pollination and food production 10 notes that risk assessments required for the approval of GM crops in most countries worldwide do not adequately address the direct sublethal effects of insect‐resistant crops or the indirect effects of HT and insect‐resistant crops. The EFSA GD considers both these aspects. Sublethal measurement endpoints are routinely evaluated in the scientific opinions of the EFSA GMO panel concerning cultivation dossiers. 20 The issue of indirect effects due to changes in agricultural practices are also specifically assessed via a scenario analysis. We contend that the current safety assessment of GM crops in the EU has proven to be effective in evaluating GM crops with a first generation of inserted traits (i.e. Bt or HT GM crops) and can be safely adopted for newly developed traits (e.g. RNAi). 69
Experience from the USA has shown that large‐scale and long‐term use of HT crops can change the patterns of herbicide use and the adoption of different rotation schemes. 70 Modelling studies have indicated that local conditions constitute the major driver in determining a possible harm to pollinators under a scenario of reduced food availability. Further attention should be given to estimate the possibility of indirect effects to pollinators due to the impact of changes in agricultural practices linked to the adoption of GM crops. From the regulatory point of view, this involves the harmonization of different legislations, i.e. legislation on the use of plant protection products and on GMOs.
From the spread of cases of loss of honeybee colonies in many geographic areas, the primary lesson to be learnt is the need to consider the complexity of the system and try to understand interactions between different environmental stressors that are most likely the cause of this adversity, which is threatening honeybee populations. Goulson et al. 12 clarified that the combined action of parasites, pesticides and lack of adequate flower resources in fragmented habitats is the cause of the decline of managed honeybee stocks. Regarding GM crops, reported cases of pollinator decline cannot be directly linked to their cultivation, since events of colony losses were reported in several areas of the EU where they are not being cultivated. In this respect, field studies aimed at disentangling the role of single components involved in undermining the health of bees under different local conditions are necessary to increase our understanding of the system and implement appropriate, science‐based, management measures. Härtel and Steffan‐Dewenter 71 contend that future studies should unveil the relationships between foraging landscapes and colony development and mortality to support the preparation of agri‐environmental schemes, and guide pesticide applications and the deployment of GM plants to assess important questions in the context of ERA. This approach, however, goes beyond the scope and possibilities of an ERA framed to the preparation of requests for commercial approval of biotech products and highlights the need to generate ecological information from different geographic areas. The engagement of the scientific community has led to the launch by EFSA of a major project to develop a holistic approach to the risk assessment of multiple stressors in honeybees. 72 Central to this initiative are the development of a model for bee risk assessment and the creation of a Europe‐wide database where the outcomes of systematic monitoring activities based on common protocols are expected to overcome the fragmented nature of available data. A model could solve the complexity of the variety and number of interactions of factors when determining the exposure to bee foragers in the landscape. A spatially explicit honeybee model is available 73 which integrates colony dynamics, the population dynamics of the Varroa mite and the epidemiology of Varroa‐transmitted viruses. A prototype of a new model (ApisRAM) was designed by EFSA to incorporate input information to individual bees, including the communication between bees. These modelling approaches together with ongoing territorial surveys will allow an increase in data availability and harmonization to support better risk assessments and ultimately improve bee health in Europe. It is advisable that large research frameworks (e.g. Horizon Europe, New Green Deal) take into consideration such an approach to increase available knowledge to support the protection of an economically important and ecologically relevant arthropod guild.
ACKNOWLEDGEMENTS
This article is based on work from COST Action iPLANTA CA 15223, supported by COST (European Cooperation in Science and Technology). We are indebted to Nathalie Colbach for her advice in the production of Table 1 of this manuscript.
REFERENCES
- 1. Millennium Ecosystem Assessment , Ecosystems and Human Well‐Being: Synthesis. Island Press, Washington, DC, p. 160 (2005). [Google Scholar]
- 2. EFSA panel on plant protection products and their residues (PPR) , Scientific opinion on the science behind the development of a risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J 10:2668 (2012). [Google Scholar]
- 3. Gallai N, Salles JM, Settele J and Vaissière B, Economic valuation of the vulnerability of word agriculture confronted with pollinator decline. Ecol Econ 68:810–821 (2009). [Google Scholar]
- 4. European Union , The Economic Benefits of Natura 2000 (2013). Available: http://ec.europa.eu/environment/nature/info/pubs/docs/factsheets/economic/en.pdf [7 October 2020].
- 5. Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR et al., Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evolut Syst 36:467–497 (2005). [Google Scholar]
- 6. Biesmeijer JC, Roberts SP, Reemer M, Ohlemüller R, Edwards M, Peeters T et al., Parallel declines in pollinators and insect‐pollinated plants in Britain and The Netherlands. Science 313:351–354 (2006). [DOI] [PubMed] [Google Scholar]
- 7. European Food Safety Authority (EFSA) , Towards an integrated environmental risk assessment of multiple stressors on bees: review of research projects in Europe, knowledge gaps and recommendations. EFSA J 12:3594 (2014). [Google Scholar]
- 8. Neumann P and Carreck C, Honey bee colony losses: a global perspective. J. Apicult Res 49:1–6 (2010). [Google Scholar]
- 9. Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O and Kunin WE, Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353 (2010). [DOI] [PubMed] [Google Scholar]
- 10. IPBES , in The Assessment Report of the Intergovernmental Science‐Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production, ed. by Potts S, Imperatriz‐Fonseca VL and Ngo HT. Secretariat of the Intergovernmental Science‐Policy Platform on Biodiversity and Ecosystem Services, Bonn, p. 552 (2016). [Google Scholar]
- 11. Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, Jones R et al., Declines of managed honeybees and beekeepers in Europe. J Apic Res 49:15–22 (2010). [Google Scholar]
- 12. Goulson D, Nicholls E, Botías C and Rotheray EL, Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:6229 (2015). [DOI] [PubMed] [Google Scholar]
- 13. Williams PH and Osborne JL, Bumblebee vulnerability and conservation world‐wide. Apidologie 40:367–387 (2009). [Google Scholar]
- 14. Bellard C, Bertelsmeier C, Leadley P, Thuiller W and Courchamp F, Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacques A, Laurent M, Ribiere‐Chabert M, Saussac M, Bougeard S, Budge GE et al., A pan‐European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS One 12:e0172591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tarek H, Hamiduzzaman MM, Morfin N and Guzman‐Novoa E, Sub‐lethal doses of neonicotinoid and carbamate insecticides reduce the lifespan and alter the expression of immune health and detoxification related genes of honey bees (Apis mellifera). Gen Mol Res 17:gmr16039908 (2018). [Google Scholar]
- 17. Collison E, Hird H, Cresswell J and Tyler C, Interactive effects of pesticide exposure and pathogen infection on bee health–a critical analysis. Biol Rev 91:1006–1019 (2016). [DOI] [PubMed] [Google Scholar]
- 18. Dicks LW, Viana B, Bommarco R, Brosi B, del Coro Arizmendi M, Cunningham SA et al., Ten policies for pollinators. Science 354:975–976 (2016). [DOI] [PubMed] [Google Scholar]
- 19. Dicks LW, Viana B, Bommarco R, Brosi B, del Coro Arizmendi M, Cunningham SA, et al., Response to Tagliabue et al (2016). https://science.sciencemag.org/content/re-response-tagliabue-et-al-treatment-gm-crops-ten-policies-pollinators. [7 October 2020].
- 20. EFSA Panel on Genetically Modified Organisms (GMO) , Guidance on the environmental risk assessment of genetically modified plants. EFSA J 8:1879 (2010). [Google Scholar]
- 21. European Food Safety Authority (EFSA) , A mechanistic model to assess risks to honeybee colonies from exposure to pesticides under different scenarios of combined stressors and factors. EFSA Supp Publ 13:1069E (2016). [Google Scholar]
- 22. EFSA (European Food Safety Authority) , Terms of reference for an EU bee partnership. EFSA Supp Publ:EN‐1423. 18 (2018). 10.2903/sp.efsa.2018.EN1423. [DOI] [Google Scholar]
- 23. ISAAA , Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change. ISAAA Brief No. 54. ISAAA, Ithaca, NY: (2018). [Google Scholar]
- 24. Hsieh YT and Pan TM, Influence of planting papaya ringspot virus resistant transgenic papaya on soil microbial biodiversity. J Agric Food Chem 54:130–137 (2006). [DOI] [PubMed] [Google Scholar]
- 25. Sheu C, Wu CY, Chen SC and Lo CC, Extraction of DNA from soil for analysis of bacterial diversity in transgenic and nontransgenic papaya sites. J Agric Food Chem 56:11969–11975 (2008). [DOI] [PubMed] [Google Scholar]
- 26. Brookes G, Twenty‐one years of using insect resistant (GM) maize in Spain and Portugal: farm‐level economic and environmental contributions. GM Crops Food 10:90–101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keller I, Fluri P and Imdorf A, Pollen nutrition and colony development in honey bees: part I(review). Bee World 86:3–10 (2005). [Google Scholar]
- 28. Odoux JF, Feuillet D, Aupinel P, Loublier Y, Tasei JN and Mateescu C, Territorial biodiversity and consequences on physico‐chemical characteristics of pollen collected by honey bee colonies. Apidologie 43:561–575 (2012). [Google Scholar]
- 29. Danner N, Härtel S and Steffan‐Dewenter I, Maize pollen foraging by honey bees in relation to crop area and landscape context. Basic Appl Ecol 15:677–684 (2014). [Google Scholar]
- 30. Babendreier D, Kalberer NM, Romeis J, Fluri P and Bigler F, Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants. Apidologie 35:293–300 (2004). [Google Scholar]
- 31. Brookes G and Barfoot P, Environmental impacts of genetically modified (GM) crop use 1996–2016: impacts on pesticide use and carbon emissions. GM Crops Food 9:109–139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Frankenhuyzen K, Specificity and cross‐order activity of Bacillus thuringiensis pesticidal proteins, in Bacillus thuringiensis and Lysinibacillus sphaericus. Springer, Cham, pp. 127–172 (2017). [Google Scholar]
- 33. Porcar M, Gómez F, Gruppe A, Gómez‐Pajuelo A, Segura I and Schröder R, Hymenopteran specificity of Bacillus thuringiensis strain PS86Q3. Biol Contr 45:427–432 (2008). [Google Scholar]
- 34. Bachman PM, Ahmad A, Ahrens JE, Akbar W, Baum JA, Brown S et al., Characterization of the activity spectrum of MON88702 and the plant‐incorporated protectant Cry51Aa2.834_16. PLoS One 12:e0169409 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. EFSA Panel on Genetically Modified Organisms (GMO) , Applications (EFSA‐GMO‐RX‐MON810) for renewal of authorisation for the continued marketing of (1) existing food and food ingredients produced from genetically modified insect resistant maize MON810; (2) feed consisting of and/or containing maize MON810, including the use of seed for cultivation; and of (3) food and feed additives, and feed materials produced from maize MON810, all under Regulation (EC) No 1829/2003 from Monsanto. EFSA J 1149:38–85 (2009). [Google Scholar]
- 36. Duan JJ, Marvier M, Huesing J, Dively G and Huang ZY, A meta‐analysis of effects of Bt crops on honey bees (Hymenoptera: Apidae). PLoS One 3:e1415 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malone LA and Burgess EPJ, Impact of genetically modified crops on pollinators, in Environmental Impact of Genetically Modified Crops, ed. by Fery N and AMR G. CAB International, Wallingford, pp. 199–222 (2009). [Google Scholar]
- 38. Babendreier D, Kalberer NM, Romeis J, Fluri P, Mulligan E and Bigler F, Influence of Bt‐transgenic pollen, Bt‐toxin and protease inhibitor (SBTI) ingestion on development of the hypopharyngeal glands in honeybees. Apidologie 36:585–594 (2005). [Google Scholar]
- 39. Hendriksma HP, Küting M, Härtel S, Näther A, Dohrmann AB, Steffan‐Dewenter I et al., Effect of stacked insecticidal cry proteins from maize pollen on nurse bees (Apis mellifera carnica) and their gut bacteria. PLoS One 8:e59589 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jia HR, Geng LL, Li YH, Wang Q, Diao QY, Zhou T et al., The effects of Bt Cry1Ie toxin on bacterial diversity in the midgut of Apis mellifera ligustica (Hymenoptera: Apidae). Sci Rep 6:24664 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han P, Velasco‐Hernández MC, Ramirez‐Romero R and Desneux N, Behavioral effects of insect‐resistant genetically modified crops on phytophagous and beneficial arthropods: a review. J Pest Sci 89:859–883 (2016). [Google Scholar]
- 42. Tabashnik BE, Brévault T and Carrière Y, Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510 (2013). [DOI] [PubMed] [Google Scholar]
- 43. Tabashnik BE and Carrière Y, Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol 35:926 (2017). [DOI] [PubMed] [Google Scholar]
- 44. US EPA , FIFRA Scientific Advisory Panel. Meeting Minutes and Final Report No. 2018‐06 July 17–19, 2018, (2018). Available: https://www.regulations.gov/document?D=EPA-HQ-OPP-2017-0617-0032.
- 45. Vaughn T, Cavato T, Brar G, Coombe T, DeGooyer T, Ford S et al., A method of controlling corn rootworm feeding using a Bacillus thuringiensis protein expressed in transgenic maize. Crop Sci 45:931–938 (2005). [Google Scholar]
- 46. Gassmann AJ, Petzold‐Maxwell JL, Keweshan RS and Dunbar MW, Field‐evolved resistance to Bt maize by western corn rootworm. PLoS One 6:e22629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haller S, Widmer F, Siegfried BD, Zhuo X and Romeis J, Responses of two ladybird beetle species (Coleoptera: Coccinellidae) to dietary RNAi. Pest Manag Sci 75:2652–2662 (2019). [DOI] [PubMed] [Google Scholar]
- 48. Pan H, Yang X, Romeis J, Siegfried BD and Zhou X, Dietary RNAi toxicity assay exhibits differential responses to ingested dsRNAs among lady beetles. Pest Manag Sci 76:3606–3614 (2020). 10.1002/ps.5894. [DOI] [PubMed] [Google Scholar]
- 49. Taning CNT, Gui S, de Schutter K, Jahani M, Castellanos NL, Christiaens O et al., A sequence complementarity‐based approach for evaluating off‐target transcript knockdown in Bombus terrestris, following ingestion of pest‐specific dsRNA. J. Pest Sci:1–17 (2020). [Google Scholar]
- 50. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE and Mello CC, Potent and specific genetic interference by double‐stranded RNA in Caenorhabditis elegans . Nature 391:806 (1998). [DOI] [PubMed] [Google Scholar]
- 51. Christiaens O, Dzhambazova T, Kostov K, Arpaia S, Joga MR, Urru I et al., Literature review of baseline information on RNAi to support the environmental risk assessment of RNAi‐based GM plants. EFSA Suppl Publ:EN‐1424. 173 (2018). [Google Scholar]
- 52. Head GP, Carroll MW, Evans SP, Rule DM, Willse AR, Clark TL et al., Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: efficacy and resistance management. Pest Manag Sci 73:1883–1899 (2017). [DOI] [PubMed] [Google Scholar]
- 53. Bachman PM, Huizinga KM, Jensen PD, Mueller G, Tan J, Uffman JP et al., Ecological risk assessment for DvSnf7 RNA: a plant‐incorporated protectant with targeted activity against western corn rootworm. Regulat Toxicol Pharmacol 81:77–88 (2016). [DOI] [PubMed] [Google Scholar]
- 54. Chen A, Zheng W, Zheng W and Zhang H, The effects of RNA interference targeting Bactrocera dorsalis ds‐Bdrpl19 on the gene expression of rpl19 in non‐target insects. Ecotoxicology 24:595–603 (2015). [DOI] [PubMed] [Google Scholar]
- 55. Garbian Y, Maori E, Kalev H, Shafir S and Sela I, Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PLoS Pathog 8:e1003035 (2012). 10.1371/journal.ppat.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sadd BM, Barribeau SM, Bloch G et al., The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol 16:76 (2015). 10.1186/s13059-015-0623-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. New TR, Invertebrate Conservation and Agricultural Ecosystems. Cambridge University Press, Cambridge, p. 174 (2005). [Google Scholar]
- 58. Firbank LG, Heard MS, Woiwod IP, Hawes C, Haughton AJ, Champion GT et al., An introduction to the farm‐scale evaluations of genetically modified herbicide‐tolerant crops. J Appl Ecol 40:2–16 (2003). [Google Scholar]
- 59. Hawes C, Haughton AJ, Osborne JL, Roy DB, Clark SJ, Perry JN et al., Responses of plants and invertebrate trophic groups to contrasting herbicide regimes in the farm scale evaluations of genetically modified herbicide‐tolerant crops. Phil Trans R Soc London B 358:1899–1913 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haughton AJ, Champion GT, Hawes C, Heard MS, Brooks DR, Bohan DA et al., Invertebrate responses to the management of genetically modified herbicide−tolerant and conventional spring crops. II. Within‐field epigeal and aerial arthropods. Phil Trans R Soc London B 358:1863–1877 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bürger J, Darmency H, Granger S, Guyot SH, Messéan A and Colbach N, Simulation study of the impact of changed cropping practices in conventional and GM maize on weeds and associated biodiversity. Agric Syst 137:51–63 (2015). [Google Scholar]
- 62. European Food Safety Authority (EFSA) , Applications (references EFSA‐GMO‐NL‐2005‐22, EFSA‐GMO‐RX‐NK603) for the placing on the market of the genetically modified glyphosate tolerant maize NK603 for cultivation, food and feed uses, import and processing and for renewal of the authorisation of maize NK603 as existing products, both under regulation (EC) No 1829/2003 from Monsanto. EFSA J 7:1137 (2009). [Google Scholar]
- 63. Pidgeon JD, May MJ, Perry JN and Poppy GM, Mitigation of indirect environmental effects of GM crops. Proc R Soc B 274:1475–1479 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Drossart M and Gérard M, Beyond the decline of wild bees: optimizing conservation measures and bringing together the actors. Insects 11:649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hendriksma HP, Härtel S, Babendreier D, von der Ohe W and Steffan‐Dewenter I, Effects of multiple Bt proteins and GNA lectin on in vitro‐reared honey bee larvae. Apidologie 43:549–560 (2012). [Google Scholar]
- 66. Westphal C, Bommarco R, Carre' G, Lamborn E, Morison N, Petanidou T et al., Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78:653–671 (2008). [Google Scholar]
- 67. Meyer ST, Koch C and Weisser WW, Towards a standardized rapid ecosystem function assessment (REFA). Trends Ecol Evolut 30:390–397 (2015) http://www.sciencedirect.com/science/article/pii/S0169534715000968-fn1. [DOI] [PubMed] [Google Scholar]
- 68. Goulson D, Lye GC and Darvill B, Decline and conservation of bumble bees. Ann Rev Entomol 53:191–208 (2008). [DOI] [PubMed] [Google Scholar]
- 69. Arpaia S, Christiaens O, Giddings K, Jones H, Mezzetti B, Moronta F et al., Biosafety of GM crop plants expressing dsRNA: data requirements and EU regulatory considerations. Front Plant Sci 11:940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gibson DJ, Gage KL, Matthews JL, Young BG, Owen MDK, Wilson RG et al., The effect of weed management systems on weed species communities over 5 years in glyphosate‐resistant cropping systems. Appl Vegetat Sci 16:676–687 (2013). [Google Scholar]
- 71. Härtel S and Steffan‐Dewenter I, Ecology: honey bee foraging in human‐modified landscapes. Curr Biol 24:R524–R526 (2014). [DOI] [PubMed] [Google Scholar]
- 72. Rortais A, Arnold G, Dorne JL, More SJ, Sperandio G, Streissl F et al., Risk assessment of pesticides and other stressors in bees: principles, data gaps and perspectives from the European Food Safety Authority. Sci Tot Environ 587:524–537 (2017). [DOI] [PubMed] [Google Scholar]
- 73. Becher MA, Grimm V, Thorbek P, Horn J, Kennedy PJ and Osborne JL, BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J Appl Ecol 51:470–482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


