Abstract
Fishes show remarkably diverse aggressive behaviour. Aggression is expressed to secure resources; adjusting aggression levels according to context is key to avoid negative consequences for fitness and survival. Nonetheless, despite its importance, the physiological basis of aggression in fishes is still poorly understood. Several reports suggest hormonal modulation of aggression, particularly by androgens, but contradictory studies have been published. Studies exploring the role of chemical communication in aggressive behaviour are also scant, and the pheromones involved remain to be unequivocally characterized. This is surprising as chemical communication is the most ancient form of information exchange and plays a variety of other roles in fishes. Furthermore, the study of chemical communication and aggression is relevant at the evolutionary, ecological and economic levels. A few pioneering studies support the hypothesis that aggressive behaviour, at least in some teleosts, is modulated by “dominance pheromones” that reflect the social status of the sender, but there is little information on the identity of the compounds involved. This review aims to provide a global view of aggressive behaviour in fishes and its underlying physiological mechanisms including the involvement of chemical communication, and discusses the potential use of dominance pheromones to improve fish welfare. Methodological considerations and future research directions are also outlined.
Keywords: aggression, communication, dominance, endocrinology, pheromones, physiology
1. AGGRESSIVE BEHAVIOUR IN FISHES
Aggression is a widespread social feature which can be defined as any behaviour associated with threats, attacks and/or defensive strategies among individuals or groups (Nelson, 2005; Siegel et al., 2009). Most, if not all, vertebrates express some type of aggressive behaviour, and fishes are no exception (Frommen, 2020; Magnhagen et al., 2008). Having radiated to virtually all aquatic habitats, fishes show remarkably diverse aggressive displays, paralleling their wide variety of social systems and modes of reproduction (Magnhagen et al., 2008). Nonetheless, despite its importance, the physiological basis of aggression has been poorly studied. Although most studies of aggression assume vision as the sensory channel through which opponents assess each other, recent studies have highlighted the importance of chemical communication in the establishment of dominance hierarchies in fishes (Barata et al., 2007; Gonçalves‐de‐Freitas et al., 2008; Keller‐Costa et al., 2016). This review provides an overview of aggression in fishes. It explores the role of chemical communication in such behaviour, how the identification of potential dominance pheromones – chemical cues released to inform conspecifics of the high social rank of the emitter – can be relevant to fish welfare and some methodological considerations and future directions to improve studies in this field.
1.1. Characteristics of aggressive behaviour
Aggressive behaviour is expressed to secure resources such as food, territories and/or mates (Hardy & Briffa, 2013; Magnhagen et al., 2008). Therefore, aggression is higher during periods of social instability or when resources become scarce (Almeida et al., 2014b; Grossman, 1980). For example, aggression of in‐group‐living species is higher during the initial phase of group formation, tending to decrease as social hierarchies stabilize (Collias, 1944). Aggression can have negative consequences, being energetically costly and potentially injurious (Haller, 1995). Dominant individuals, i.e., those winning more aggressive encounters against conspecifics (Oliveira et al., 2011), have an advantage in accessing resources, whereas subordinates have more limited access to food, territory and/or mates (Bruce & White, 1995; Koebele, 1985). Thus, aggression is intrinsically associated with life‐history traits such as growth and reproduction, thereby having a direct bearing on fitness (Bruce & White, 1995; Hofmann & Fernald, 2000; Koebele, 1985; Nicieza & Metcalfe, 1999; Smith & Blumstein, 2008). Therefore, answering the question “can we predict which individual will win an aggressive encounter?” is relevant both at a proximate (function, mechanism) and ultimate (evolutionary) level, as it would give insights about the evolution of this conserved behaviour and a better understanding of its underlying mechanisms. The answer to this question was conceptualized by Parker (1974) under the term “Resource Holding Potential” (RHP), i.e., the capacity of an animal to win a dyadic encounter if one were to take place (Parker, 1974). In fishes, RHP depends on a number of variables, body size in particular, with larger animals usually being stronger and able to inflict greater damage upon their rivals (Barlow et al., 1986; Huntingford et al., 2001). Nonetheless, in one cichlid species at least, relative gonadal weight is a stronger predictor of victory than body size (Neat et al., 1998). It was proposed that males with larger gonads fight harder to defend their territory, possibly because the value of a territory correlates with the gonad maturity state of the individual (Neat et al., 1998). Evidence has also been accumulating that the outcomes of previous aggressive encounters affect RHP and thus those of subsequent interactions. The so‐called “winner‐loser” effect reflects the tendency of a winner or a loser, respectively, to remain so in future aggressive challenges (Hsu et al., 2006; Hsu & Wolf, 1999). This has now been described in several fishes, such as the three‐spined sticklebacks (Gasterosteus aculeatus) (Bakker et al., 1989), mangrove killifish (Kryptolebias marmoratus) (Hsu et al., 2014), pumpkin seed sunfish (Lepomis gibbosus) (Chase et al., 1994), blue gourami (Trichogaster trichopterus) (Frey & Miller, 1972), paradise fish (Macropodus opercularis) (Francis, 1983), Siamese fighting fish (Betta splendens) (Wallen & Wojciechowski‐Metzlar, 1985) and Mozambique tilapia (Oreochromis mossambicus) (Oliveira et al., 2009). It has been proposed that individuals that experience these effects could assess their fighting ability and consequently their fighting costs in subsequent contests (Hsu & Wolf, 1999). Others have proposed that prior winning or losing experience can affect an individual's fighting performance (Beacham, 1988), possibly mediated by androgens (Oliveira et al., 2009). Nonetheless, a correlation between androgen levels and aggression has not always been found (Almeida et al., 2014a; Smith, 1970; Weiss & Coughlin, 1979). Furthermore, in social species, dyadic aggressive interactions often occur in the presence of other conspecifics (Bertucci et al., 2014). During fights, non‐participants can “eavesdrop” on contestants, extracting information about their intention and RHP to their advantage (Hsu et al., 2006). For example, zebrafish (Danio rerio) are more attentive towards interacting (fighting context) than towards non‐interacting pairs of conspecifics (Abril‐de‐Abreu et al., 2015). Conversely, contestants can also adjust their aggressive behaviour according to the nature of the audience (Peake & McGregor, 2004). For instance, male B. splendens increase biting frequency in the presence of a male audience, but when females are present, they increase their tail beats and decrease bites (Doutrelant et al., 2001; Matos & McGregor, 2002).
1.2. Endocrine modulation of fish aggression
The review of the role of hormones in chemical communication and aggression is important, since these compounds and their metabolites have been found to also act as potential pheromones, at least in fishes (see Section 2.1).
The organization of the neuroendocrine system in teleosts is similar to that in other vertebrates; the hypothalamus controls the activity of the anterior pituitary gland which, in turn, controls the function of several peripheral endocrine glands (Oliveira & Gonçalves, 2008). In fishes, as in other vertebrates, the pituitary gland is composed of two tissue types: the adenohypophysis and the neurohypophysis (Oliveira & Gonçalves, 2008). The adenohypophysis is the site of synthesis, storage and release into the blood stream of several hormones and is under the direct control of releasing factors produced by hypothalamic neurons and through feedback of peripheral hormones (Oliveira & Gonçalves, 2008). The neurohypophysis is an aggregate of axons with their endings storing and releasing neuropeptides synthesized in their hypothalamic cell bodies (Ball & Baker, 1969). The hypothalamic–hypophysial portal vascular system that transmits the releasing factors from the hypothalamus to the pituitary is present in higher vertebrates but considered to be generally absent in teleosts as, in this taxon, the adenohypophysis receives direct innervation from the hypothalamus (Peter et al., 1990). Nonetheless, a portal system or vascular contribution has been suggested (Baskaran & Sathyanesan, 1992).
Because aggression usually differs between the sexes and is generally higher in males (Huntingford & Turner, 1987), hormones of the hypothalamus–pituitary–gonadal (HPG) axis, androgens in particular (Figure 1), have been suggested as modulators of aggression (Villars, 1983). The primary roles of androgens in male fishes are the stimulation of testes growth and maturation, the development of secondary sexual characteristics and the expression of reproductive behaviour (Borg, 1994; Patrão et al., 2009). Furthermore, administration of 11‐ketotestosterone (11KT), the most potent androgen in fishes, can promote aggressive behaviour (Ogino et al., 2016). Elevated circulating 11KT levels may also reflect aggressive encounters. For example, several cichlids (Neolamprologus pulcher, Lamprologus callipterus, Tropheus moorii, Pseudosimochromis curvifrons and O. mossambicus) subjected to a simulated territorial intruder protocol had elevated levels of circulating 11KT and aggressively defended their territory against an intruder (Desjardins et al., 2005; Hirschenhauser et al., 2004). High levels of androgens are thought to adjust aggressive motivation in response to an agonistic social challenge, as formulated in the “challenge hypothesis” (Antunes & Oliveira, 2009; Oliveira et al., 2001; Wingfield et al., 1990). This, in turn, further increases aggression and the likelihood of winning; this could be why winners keep winning (Fernald, 1976; Oliveira et al., 2009). Territorial aggression may also be regulated by androgens, and aggression itself can modulate androgen levels (Desjardins et al., 2005). Nonetheless, high androgen levels can have long‐term negative physiological consequences, such as immunosuppression (Gubbels & Jorgensen, 2018). Furthermore, cortisol and gonadotropin‐releasing hormone 1 mRNA levels (gnrh1) are increased after dominant status suppression (after 24 h), suggesting that individuals mount a neural defence against loss of status (Parikh et al., 2006). Nevertheless, alternatives to androgens as the key modulators of aggressive motivation have emerged (Oliveira et al., 2002; Villars, 1983). For example, castration (which dramatically reduces circulating androgen levels) fails to reduce aggression in B. splendens (Weiss & Coughlin, 1979), O. mossambicus (Almeida et al., 2014a) and L. gibbosus (Smith, 1970). This suggests that, at least in some species, androgens are not necessary to maintain high aggression.
FIGURE 1.
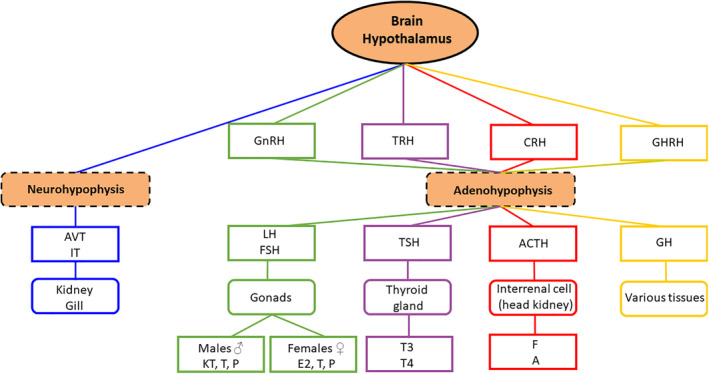
Schematic representation of the hierarchical organization of the neuroendocrine system in teleost fishes with major hypothalamic‐hypophysial axis for the control of peripheral glands and tissues (each colour represents a different set of hormones related to hypothalamic–hypophysial–peripheral gland/tissues stimulation). GnRH: gonadotropin‐releasing hormone; TRH: thyrotropin releasing hormone; CRH: corticotropin releasing hormone; GHRH: growth hormone‐releasing hormone; LH: luteinizing hormone; FSH: follicle stimulating hormone; TSH: thyroid stimulating hormone; ACTH: adrenocorticotropic hormone; F: cortisol; A: adrenaline; GH: growth hormone; AVT: arginine–vasotocin; IT: isotocin; KT: 11‐ketotestosterone; T: testosterone; P: 17,20β‐dihydroxypregn‐4‐en‐3‐one; E2: 17β‐estradiol; T3: triiodothyronine; T4: thyroxine. Adapted from Oliveira and Gonçalves (2008)
Hormones of the hypothalamus–pituitary–interrenal (HPI) axis have also been implicated in modulation of aggression. Corticotrophin releasing factor (Crf) stimulates the release of adrenocorticotropic hormone (Acth) from the anterior pituitary gland (Carpenter et al., 2014), which in turn stimulates the interrenal tissue to produce cortisol, the main corticosteroid in fishes (Figure 1) (Ganga et al., 2006). Subordinate fishes show increased HPI activity and, in general, have higher levels of circulating cortisol (Doyon et al., 2003). Cortisol levels are lowered, and crf and crf1 receptor mRNA are rapidly down‐regulated in ascending male Astatotilapia burtoni, further supporting that the HPI axis is involved in the physiological changes associated with shifts in dominance (Carpenter et al., 2014). In the rainbow trout (Oncorhynchus mykiss), administration of cortisol affects aggressive behaviour in a time‐dependent way; although short‐term exposure had no effect, exposure of up to 2 days led to an inhibition of aggression (Øverli et al., 2002).
The neuropeptides arginine‐vasotocin (Avt) and isotocin (It) have also been implicated in aggressive behaviour in fishes, although their role is still unclear (reviewed in Godwin & Thompson, 2012). In D. rerio, AVT‐related genes are associated with the expression of aggression in dominant males and females, and dominant males overexpress It mRNA (Filby et al., 2010). Levels of It mRNA levels are higher in the brains of dominant male G. aculeatus defending their territory (Kleszczyńska et al., 2012). Dominant D. rerio express Avt in one to three pairs of large cells in the magnocellular preoptic area, whereas in subordinate individuals it is expressed in 7–11 pairs of small cells in the parvocellular preoptic area (Larson et al., 2006), indicating that the vasotocinergic system may play a role in shaping dominant–subordinate relationships. In the same species, agonistic interactions are more directly associated with changes in brain Avt than It (Teles et al., 2016). Avt injections in territorial male bluehead wrasse (Thalassoma bifasciatum) increase courtship and decrease chases (Semsar et al., 2001). Furthermore, Avt injections in non‐territorial males increase courtship, chases and territorial behaviour, suggesting that increased levels of Avt promote the expression of territorial behaviours (Semsar et al., 2001). In Beaugregory damselfish (Stegastes leucostictus), injection of Avt also increases aggressive behaviour towards intruders in a dose‐dependent manner, and injection with Manning compound (an Avt receptor antagonist) reduces such behaviour, whereas It administration has no effect (Santangelo & Bass, 2006). A. burtoni individuals ascending from subordinate to dominant, and treated with Manning compound, also show a reduction in aggressive behaviour (Huffman et al., 2015). Other studies offer contradictory evidence. For instance, O. mykiss receiving 200 ng Avt become subordinate, whereas a dose of 20 ng has no effect on the outcome of fights for social dominance (Backström & Winberg, 2009). In goldfish (Carassius auratus), Avt inhibits approach responses towards the visual stimuli of conspecifics, whereas Manning compound stimulates such responses (Thompson & Walton, 2004).
A few studies have investigated the role of metabolic hormones in aggression. Thyroxine (T4) administration in salmonids reduces aggression (Hutchison & Iwata, 1998), whereas low and high doses of triiodothyronine (T3) reduce and increase aggression, respectively (Godin et al., 2011). Growth hormone (Gh) administration, which increases metabolic demands and feeding motivation in teleosts, increases aggression in juvenile O. mykiss, although it does not change their social status (Jönsson et al., 1998). It was proposed that Gh influences aggression indirectly by increasing swimming activity and/or by inducing defence of a larger territory, thus increasing the probability of encounter between opponents. In addition, salmonids strains selected for higher growth rates have a negative cost in the generation of more aggressive behaviour (Nicieza & Metcalfe, 1999). Somatostatin (Sst) is a hypothalamic polypeptide that regulates Gh release from the pituitary gland (Brazeau et al., 1973). In A. burtoni, dominant males show reduced somatic growth and increased Sst neuron size in the preoptic area of the brain. Sst antagonists increase aggressive behaviour in a dose‐dependent manner, and the potent Sst agonist octreotide decreases aggression (Hofmann & Trainor, 2006). It was proposed that Sst may reduce energetically costly processes such as somatic growth and aggressive behaviour in dominant males (Hofmann & Trainor, 2006).
A study in D. rerio explored gene expression linked to different levels of aggression (Filby et al., 2010). The authors identified several genes differentially expressed in relation to aggression; these genes belonged to seven functional pathways occurring in the hypothalamus and telencephalon, suggesting a multi‐factorial control of aggression similar in several aspects to mammalian neurophysiology. A recent study in B. splendens explored gene expression in the brain of animals in fight context and concluded that a synchronization occurs in the opponent pairs, suggesting that this physiological phenomenon can be the basis for the behavioural synchronization (Vu et al., 2020).
Overall, evidence indicates a role for the endocrine system in modulating aggressive behaviour in fishes, but a clear picture of the specific function of the different hormones and potential interactions among them is far from complete. Several steroidal hormones and their derivatives can be secreted during aggressive interactions, which are then released to the water (Almeida et al., 2005; Barata et al., 2007, 2008a; Martinovic‐Weigelt et al., 2012; Poling et al., 2001; Sorensen et al., 2005). In a similar way to some reproductive hormones, they could act as hormonal pheromones mediating aggressive interactions (Stacey & Sorensen, 2009).
1.3. Communication and aggression
Most interactions between animals involve communication; thus, understanding this phenomenon is crucial to fully appreciate animal behaviour (Frommen, 2020). Communication can be simply defined as the act of transferring information intentionally from a sender to a receiver, eliciting a response in the latter (Bradbury & Vehrencamp, 2015). Aggressive communication in aquatic environments has been recently reviewed (Frommen, 2020); aggressive signals can be conveyed through different sensorial modalities – visual, auditory, mechanosensory, electrical and chemical, in isolation or combined multimodal communication (Butler & Maruska, 2015; Chabrolles et al., 2017; Frommen, 2020; Ladich & Myrberg, 2006). Visual communication has been the most widely studied form of aggressive signalling in fishes. Aggressive behaviour can be divided into threat displays, where animals increase their apparent body size, e.g., by erecting their fins and expanding their gill covers (Huntingford & Turner, 1987; Simpson, 1968) or by changing colour or body patterns to signal dominance (Dawkins & Guilford, 1993), and attacks, where animals overtly charge, chase or bite an opponent (Pitcher, 1993; Reebs, 2001). The frequency of these aggressive displays varies among individuals of the same species, allowing an observer to distinguish the dominant/submissive relationship among them (McDonald et al., 1968). It is well established that many fishes use sonorous signals in aggressive contexts, with these usually being expressed after the visual detection of an opponent and/or intruder (Ladich, 1997). Sounds can be used during agonistic encounters and may affect the outcome of contests (Ladich & Myrberg, 2006). Some fishes can produce electrical discharges, with electrical pulses in the millivolt range proposed to act as electrical signals for communication (Hopkins, 1974; Kramer, 1990; Tricas & Carlson, 2012); higher voltages may be used to evade predators or stun prey. Electroreception appeared early in vertebrate evolution (Bullock et al., 1983); nonetheless, it seems that it has been subsequently largely lost in teleosts (Tricas & Carlson, 2012). Among the few species that have developed the capacity to communicate by electrical signals are the glass knifefish (Eigenmannia virescens) (Hopkins, 1974; Hupé & Lewis, 2008); the brown ghost knifefish (Apteronotus leptorhynchus) (Hupé & Lewis, 2008; Zakon et al., 2002; Zupanc, 2002) and some mormyrids: Petrocephalus catostoma, Cyphomyrus discorhynchus, Hippopotamyrus sp. (Scheffel & Kramer, 2000), Gnathonemus petersii (Bell et al., 1974) and Pollimyrus isidori (Bratton & Kramer, 1989). Electric organ discharges can be produced during fighting contexts, and their characteristics can convey dominance and transmit information to an audience (Dunlap & Larkins‐Ford, 2003; Westby, 1975). The lateral line system can be involved in agonistic interactions and has been suggested to facilitate noncontact assessment and fight behaviours as a protective mechanism against physical injury in A. burtoni (Butler & Maruska, 2015). The lateral line is also hypothesized to be involved in acoustic communication, as the beating of fins and tail creates a stream of water towards the opponent (Ladich & Myrberg, 2006).
The role of chemical signalling during agonistic encounters has received little attention. A few pioneering studies suggest that chemical signalling is used to transfer information during agonistic encounters, and evidence exists for a dominance pheromone(s) used by fishes to signal their social rank (Barata et al., 2007; Gonçalves‐de‐Freitas et al., 2008; Keller‐Costa et al., 2016; Maruska & Fernald, 2012).
1.4. Relevance of aggression in fish welfare
Aquaculture is one of the most rapidly evolving and technically innovative sectors of food production. It constitutes an alternative to fisheries, and the decrease in wild populations, associated with increasing demand for fish products, will drive a rapid expansion in the coming decades (Béné et al., 2015, 2016; Gentry et al., 2017; Longo et al., 2019). Fish welfare in aquaculture is an important issue for public perception, marketing and product acceptance, and also for the industry itself to promote efficiency, quality and quantity (Kupsala et al., 2013; Oka et al., 2012). Nonetheless, behavioural interactions occur between farmed fishes, and high stocking densities may cause chronic stress by preventing normal social behaviour (Ashley, 2007; Ejike & Schreck, 1980; Wedemeyer, 1997) leading to suppression of growth in low‐ranking fish (Abbott & Dill, 1989). Nevertheless, there are exceptions; in some species, high stocking densities may lead to reduced aggression and increased growth (e.g., Tilapia rendalli) (Torrezani et al., 2013). Social stress and uneven sizes promote aggressive behaviour (Ashley, 2007). Aggressive interactions cause injuries to the eyes, tails and pectoral fins, which facilitates secondary infections and can lead to death (Ashley, 2007). In addition, intra‐cohort cannibalism – an extreme form of aggression – because of size heterogeneity, may occur in farmed fish and lead to severe losses (Naumowicz et al., 2017). Aggressive behaviour also results in greater energy expenditure and higher metabolic rates, which decrease food conversion to biomass (Sloman et al., 2000). It is clear, therefore, that aggressive behaviour in aquaculture is of economic importance, and innovative solutions are needed to avoid compromise of welfare. Furthermore, several reports have suggested that application of pheromones may improve aquatic animal cultivation (Bardach et al., 1980; Barki et al., 2011; Wuertz, 1997) and help to control non‐indigenous fish species (Kupsala et al., 2013; Siefkes, 2017). It has been proposed that if a certain pheromone has a desirable effect, the aquaculture producers can supplement the holding water with artificially synthetized pheromone (Wuertz, 1997). Thus, it is important to explore pheromones related to aggressive behaviour, as this behaviour compromises these activities, and improve welfare (Ashley, 2007; Naumowicz et al., 2017; Sloman et al., 2000; Wuertz, 1997).
A major market that has been neglected is the production of ornamental fish. It is estimated that this trade involves up to 1.5 billion individuals per year, with mortality reaching 73% because of stressors that affect their welfare similar to aquaculture (Huntingford et al., 2006). Nonetheless, to improve welfare by expanding the space of animal rearing would constitute an economic impact to producers. In addition, after the fish arrive at peoples' home as pets, aggression continues to be a problem, mainly because the animals are often confined in small aquaria (Oldfield, 2011). Ornamental species often require larger aquaria and complex habitats, which allow more natural behaviour, thereby improving welfare through reduced aggression (Oldfield, 2011). Reduction of stock density is effective in the reduction of aggression in some ornamental species, such as swordtails (Xiphophorus helleri) (Magellan et al., 2012), angelfish (Pterophyllum scalare) and tiger barbs (Puntigrus tetrazona) (Stevens et al., 2017). The use of pheromones could constitute an improvement in this market not only to increase profitability but also, equally importantly, to improve the quality of life for the fish produced.
D. rerio is one of the most used vertebrate species in biological research (Lawrence, 2007), and the welfare of such experimental animals is therefore an important consideration (Toni et al., 2019). Recently, environmental enrichment to maximize the well‐being of D. rerio used for experimental purposes was explored (Woodward et al., 2019). Curiously, this attempt led to increased aggression, as individuals would become territorial over objects placed in the tanks (Woodward et al., 2019). The authors concluded that when considering environmental enrichment of a certain species, its natural behaviour should be taken into consideration (Woodward et al., 2019).
In this context, the discovery of a pheromone released during dominance contests that modulates behaviour by reducing aggression would be a promising tool to promote welfare. Nonetheless, such a pheromone has yet to be unequivocally identified in fishes. Thus, a better understanding of factors, including chemical communication, regulating social interactions is most pertinent from both scientific and practical viewpoints.
2. CHEMICAL COMMUNICATION MOLECULES
2.1. Pheromones and cues
In chemical communication the message is transferred via the release and detection of chemical cues and/or pheromones (Scott‐Phillips, 2008). Chemical cues can be defined as any stimuli capable of triggering a chemo‐sensory response in an animal (Sorensen & Wisenden, 2015), whereas pheromones are chemical cues released by one individual that cause innate behavioural and/or physiological responses in conspecifics (Kekan et al., 2017). Pheromones are categorized as “signals” that evolved to alter the behaviour of other organisms and are effective because the receiver's response has also evolved (Smith & Harper, 2003). Chemical cues transmit information unidirectionally to the receiver and provide no advantage to the sender; conversely, pheromones are used to communicate, which means that there is feedback from the receiver to the sender, and both may benefit (Laidre & Johnstone, 2013; Sorensen & Stacey, 1999). It has been proposed that chemical cues might have been the evolutionary basis for pheromones, by an evolutionary process referred to as ritualization (Smith & Harper, 2003; Wyatt, 2014). Pheromonal systems have evolved specifically for communication and are defined according to the biological response that they induce (Sorensen et al., 2010). Accordingly, pheromones that elicit immediate behavioural responses are classified as “releaser” pheromones, whereas those causing physiological effects, which may later also drive behavioural change, are classified as “primer” pheromones (Sorensen & Wisenden, 2015). As functional definitions, nonetheless, these terms are not mutually exclusive (Sorensen et al., 2010). Hormonal metabolites that drive sexual arousal are classic examples of releaser pheromones, whereas primer pheromones are exemplified by preovulatory compounds produced by females that induce endocrine changes in male conspecifics leading to increases in milt production (Sorensen & Wisenden, 2015). Some pheromones derive from, and can be confused with, hormones – hormonal pheromones (Stacey, 2011); nonetheless, hormones are a class of signalling molecules that are produced in internal glands and transported by the circulatory system to regulate physiology and behaviour of the producer individual itself (Karlson & Lüscher, 1959).
Fish pheromones include: dominance pheromones (important for the establishment and maintenance of social hierarchies); kin recognition and aggregation pheromones, (important to avoid predation and also facilitate migratory orientation); and reproductive pheromones, which are important for mate‐choice and reproductive behaviour (Sorensen & Wisenden, 2015).
Examples of chemical cues in fishes are kairomones, odorants originating from other species such as predators or prey, e.g., post‐ingestion cues released from the predator's diet (Sorensen & Wisenden, 2015).
In fishes, chemical stimuli known to be excreted and/or to induce behavioural and physiological responses in conspecifics include amino acids (and their derivatives), bile acids, gonadal steroids (and their conjugates) and prostaglandins (Hara, 1994; Sorensen et al., 2010). Pheromones may be single compounds or – more often – multicomponent mixtures and thus a distinction of pheromones and pheromonal components/constituents is important (Sorensen et al., 2010). Slight variation in the mix may reflect the individual's physiological state, including status within a hierarchy, and affect the response in conspecifics (Sorensen & Wisenden, 2015).
2.2. Secretion, transmission and reception
2.2.1. Secretion
The study of pheromone production is common in mammals and insects, but scarce in fishes. Pheromones in insects and mammals are often secreted by a variety of specialized glands, which can be internal or external (Wyatt, 2014). Most studies in fishes deal with reproductive pheromones and show that these are mostly produced by the gonads (testes and ovaries) (Hurk & Resink, 1992). In the male peacock blenny (Salaria pavo), pheromones involved in sexual attraction of females are secreted by an anal gland located on the anal fin‐rays (Laumen et al., 1974; Serrano et al., 2008b). The testicular glands, anal glands and/or the blind pouches (paired evaginations of the spermatic ducts) may be sites of pheromone production (Serrano et al., 2008a). In the black goby (Gobius niger), these attractants appear to be synthesized in the mesorchial gland, which is a part of the testis (Colombo et al., 1980; Colombo & Burighel, 1974). Such structural specialization for sex pheromone production also occurs in the seminal vesicles in African catfish (Clarias gariepinus) (Resink et al., 1989). Females also emit sex pheromones to attract males, and the compounds have been found to be produced in the ovaries (Crow & Liley, 1979). Migratory pheromones in salmonids may be produced in the liver and secreted into the bile (Selset & Doving, 1980). In the sea lamprey (Petromyzon marinus), larval bile acid pheromones are synthesized in the liver, stored in the gallbladder and secreted into the intestine via the bile duct (Buchinger et al., 2015), whereas adult males release them through the gills (Siefkes et al., 2003).
2.2.2. Transmission
Transmission is the course taken by pheromones from release until it reaches the receiver. Pheromones released to the water are transported through turbulence and variable flows, and animals may use natural or self‐generated water currents to amplify the effectiveness of pheromone transmission (Chung‐Davidson et al., 2010). During transmission, pheromone action may be compromised by other dissolved chemicals, which can bind pheromones or inhibit receptors, e.g., humic acids (Hubbard et al., 2002), or disrupt the function or cause degeneration of olfactory sensory neurons (OSN), e.g., heavy metals (Tierney et al., 2010). Pheromones may be released to the environment through the gills, or via the urine, skin mucus, faeces, semen and ovarian fluids (Almeida et al., 2005; Bayani et al., 2017; Brown et al., 1995; Døving et al., 1980; Félix et al., 2013; Giaquinto et al., 2015; Giaquinto & Hara, 2008; Hubbard et al., 2003; Lecchini et al., 2018; Marui & Caprio, 1992; Rosenthal et al., 2011; Saraiva et al., 2017; Scott et al., 2019; Stacey & Sorensen, 2009). In O. mossambicus, C. auratus and S. salar, the urine is an important vehicle for pheromone release (Almeida et al., 2005; Appelt & Sorensen, 1999, 2007; Barata et al., 2007, 2008a). The frequency of urination of male O. mossambicus depends on the social context; dominant males store more urine than subordinate males, and the pheromone concentration is higher (Keller‐Costa et al., 2012, 2014). The size of urinary bladder and its musculature thickness is directly proportional to social status (Keller‐Costa et al., 2012).
2.2.3. Reception
Most terrestrial vertebrates possess two distinct chemosensory organs: the main olfactory epithelium (OE) and the vomeronasal organ. Nonetheless, teleosts lack both the vomeronasal organ and accessory olfactory bulb (OB) (Mombaerts, 2004a). Instead, they possess a single pseudostratified OE on each side of the head, generally organized in a multilamellar olfactory rosette (Figure 2), and their odorant receptors are expressed in the OE with information relayed to the central nervous system by cranial nerve 1, the olfactory nerve (Hashiguchi et al., 2008; Sorensen & Wisenden, 2015). The olfactory rosettes are located inside the nasal cavities, which are usually composed of two inlet nostrils, through which the water enters, and two outlet nostrils located posteriorly (Kermen et al., 2013); cichlids are the notable exception in that they only have a single nostril per side (Escobar‐Camacho & Carleton, 2015). The olfactory rosettes are organized into lamellae, the size and number of which increase throughout development and stabilize at maturity (Olivares & Schmachtenberg, 2019). The shape of the rosettes, the number and morphology of lamellae are highly variable from species to species (Hara & Zielinski, 2006). The OE lies between the inlet and outlet nostrils and contains up to five distinct classes of olfactory receptor neurons (ORNs): ciliated, microvillus, crypt cells, pear and kappe neurons (Figure 3), with the last three being thought to be specific to teleosts. Nonetheless, pear and kappe neurons have so far only been identified in D. rerio (Ahuja et al., 2014; Hansen & Eckart, 1998; Hansen & Finger, 2000; Miyasaka et al., 2014). At the basal level of the OE, basal cells are capable of regeneration in case of epithelium damage (Iqbal & Byrd‐Jacobs, 2010). The OE is exposed to mixtures of different substances dissolved in the water, and the capacity to discriminate between different odorants is important for animals to navigate and take decisions (Chung‐Davidson et al., 2010). Detection of odorants requires their binding to odorant receptors which, in vertebrates, are members of the G‐protein‐coupled receptor superfamily, in the membrane of ORNs in the OE (Buck & Axel, 1991; Fleischer et al., 2009; Sorensen & Wisenden, 2015; Spehr & Munger, 2009). Odorant receptors are highly diverse and can be grouped into olfactory receptors (ORs), trace amine‐associated receptors (TAARs) and type I and II vomeronasal receptors (V1Rs and V2Rs) (Alioto & Ngai, 2005; Fleischer et al., 2009; Mombaerts, 2004a). It has been proposed that ciliated cells express ORs, whereas microvillous and crypt cells express V1Rs and V2Rs (Oka et al., 2012; Yoshihara, 2009). TAARs are expressed in sparse ORNs (Hussain et al., 2009).
FIGURE 2.
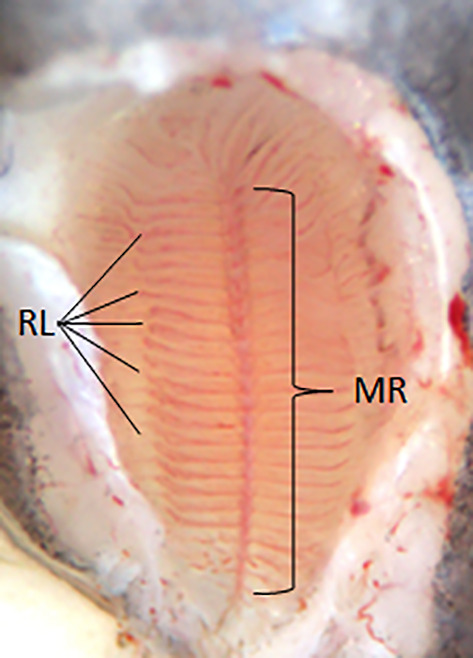
The olfactory rosette of the European eel (Anguilla anguilla L.). The olfactory rosette is composed of a narrow elongated median raphe (MR) in the middle that extends longitudinally from one extremity to another. Numerous radial lamellae (RL) are arranged around the MR and attached to the membrane of the olfactory chamber
FIGURE 3.
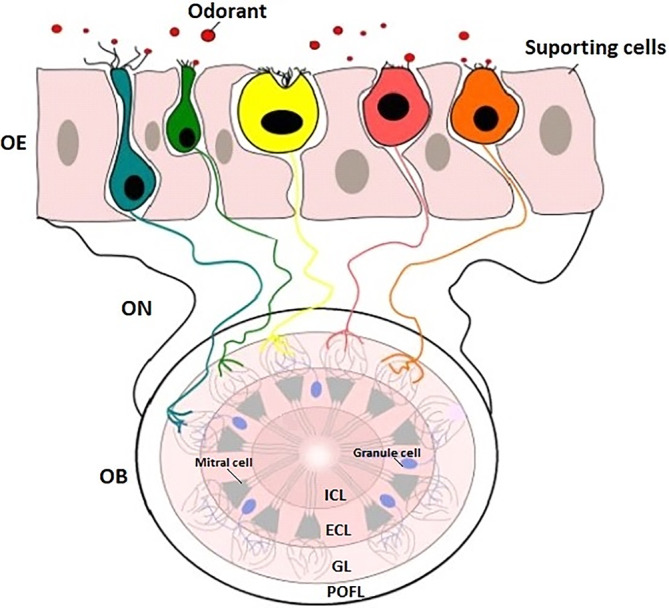
Schematic representation of the organization of the olfactory bulb. The five types of olfactory receptor neurons (ORNs) described in teleosts and their respective laminar position are represented with different colours (blue, green, yellow, pink and orange). The odorant molecules (red circles) are detected in the olfactory epithelium (OE) by the five different types of sensory neurons. The soma of the ORNs are located at different depths in the OE and each has specific characteristics: ciliated neurons (blue) that are the most basal ORNs have round cell bodies and thin dendrites that terminate in clusters of cilia on the epithelial surface; the microvillous neurons (green) that are also known to have round soma, but thicker dendrites with bundles of microvilli on their apical surface; the crypt neurons (yellow) that have globular‐shaped and exhibit both microvilli and cilia on their apical surface and are located more apically than ciliated and microvillous neurons; the kappe neurons (pink) are in pear‐shaped form with an apical appendage resembling a cap (German: Kappe), and these neurons do not have cilia and are the ORNs type located more apically and, finally, the pear neurons (orange) that are also located apically, are pear‐shaped and possess short apical dendrites. The ORNs project their axons, via the olfactory nerve (ON), to different glomeruli located in the olfactory bulb (OB). The OB is composed of four different layers: POFL: primary olfactory fibre layer; GL: glomerular layer; ECL: external cell layer; ICL: internal cell layer. The axons of the olfactory neurons establish contact with the dendrites of the mitral cells in each glomerulus (adapted from Ahuja et al., 2014; Kermen et al., 2013)
Some studies have given insights into the ORNs and their interaction with pheromones. For example, in O. mykiss, ciliated ORN have a generalist response to a wide range of odorants, among them pheromones and amino acids, whereas microvillous ORNs are specific for amino acid detection (Sato & Suzuki, 2001). In the channel catfish (Ictalurus punctatus), amino acids are detected by both ciliated and microvillous ORNs, and bile salts by ciliated ORNs (Hansen et al., 2003). In the crucian carp (Carassius carassius), detection of alarm cues occurs in ciliated ORs (Døving & Lastein, 2009; Hamdani & Døving, 2002) as with some sex pheromones (Lastein et al., 2006). A spatial coding of the olfactory input occurs in the OB, because each ORN expressing a particular OR sends its axon to the same glomeruli (e.g., amino acids induce activity in the glomeruli of the lateral bulb; bile salts induce the medial part and the posterior part is induced by alarm cues (Lastein et al., 2006). This “olfactory coding” that stipulates that the OB is organized into subregions (“glomeruli”) to process certain classes of odorant has been extensively studied in D. rerio, another cyprinid (Ahuja et al., 2013; Friedrich & Korsching, 1998; Sato et al., 2005). Crypt ORNs have been suggested to be involved in sex pheromone detection (Kermen et al., 2013). These ORNs have a seasonal variation in C. carassius; during winter few crypt ORS are dispersed in the OE; throughout spring the majority are located deep in the OE not yet exposed to the environment but during summer, the spawning season, they appear at the surface of the OE (Hamdani et al., 2008). Furthermore, in mature O. mykiss crypt ORS are larger than those of juveniles and locate preferentially the apical surface of the OE (Bazáes & Schmachtenberg, 2012). Nevertheless, crypt ORNs detect odorants, such as amino acids and bile salts, other than sex pheromones (Schmachtenberg, 2006; Vielma et al., 2008). Nonetheless, in mature O. mykiss, a preference exists for gonadal extracts and hormones from the opposite sex, supporting the involvement of these ORNs in reproductive chemical communication in fishes (Bazáes & Schmachtenberg, 2012). The recently identified pear ORNs may be involved in the detection of food‐derived odorants (VanHook, 2017); nonetheless, studies correlating these ORNs and the kappe ORNs with pheromone detection have not yet been carried out.
The processing of odours in vertebrates occurs in the OB, a brain structure organized in four layers that receives input from the ORNs axons via the olfactory nerves (Figure 3) (Kermen et al., 2013). The primary olfactory fibre layer, the most external layer, is composed of axons of the ORNs, the dendrites of which extend into the following inner layer, the glomerular layer, where ORNs form glutamatergic synapses with the dendrites of the mitral cells, which have their somas located in a deeper layer, the external cell layer (Braubach et al., 2012; Hara & Zielinski, 2006; Vassar et al., 1994). The granule cells are located in the external cell layer that, in D. rerio, are GABAergic cells without axons and make dendro‐dendritic synaptic connections with principal cells (Kermen et al., 2013). In the internal cell layer, the deepest layer of the OB, the axons of the mitral cells are located, which connect to telencephalon and diencephalon brain areas as targets to further process olfactory input, where it is integrated with other information to mediate appropriate output responses (Kermen et al., 2013; Miyasaka et al., 2014).
Studies in mammals initially suggested that ORNs express a single OR (Chess et al., 1994; Serizawa et al., 2004). Nonetheless, this “ one receptor–one neuron” theory has been revised (Mombaerts, 2004b; Tan et al., 2015), in that an ORN initially express several ORs, but epigenetic regulation occurs during development leading to expression of a single OR per ORN (Tan et al., 2015). Thus, this specificity of one OR per ORN and its projection to a specific glomerulus are key events for the organization and function of the olfactory system and result in the production of a topographic odour map in the brain (Sato et al., 2007). Nonetheless, it appears that the “one receptor–one neuron” may have an exception in D. rerio (Sato et al., 2007). In this species, multiple ORs exist in an ORNs subpopulation and may represent a specific way to integrate information from multiple odours (Sato et al., 2007).
Studies focusing on whether olfactory processing in higher‐order brain centre is influenced by an animal's physiological condition are scarce. Nonetheless, a recent study in A. burtoni using local field potential recordings from the ventral telencephalon of dominant and subordinate males revealed that the social rank and reproductive state influence the neuronal response properties (Nikonov & Maruska, 2019). More specifically, dominant males had a high percentage of neurons that responded to several odour types, which may indicate that males in reproductive and territory defending conditions exhibit a differential sensitivity (Nikonov & Maruska, 2019).
2.3. Chemical identity of fish aggression pheromones
Table 1 summarizes studies linking pheromones to dominance and aggression. One of the first studies to suggest that aggressive behaviour is modulated by pheromones was conducted in B. splendens, showing that they secrete substances that reduce aggression in conspecifics, and that these substances are secreted by either one or both combatants in response to prolonged fighting stress (Colyer & Jenkins, 1976). In bullhead catfish (lctalurus nebulosus), individuals detect the body odours of conspecifics, and these compounds play a role in signalling dominance and territorial relationships and evoke increased aggression towards chemical “strangers” (Bryant & Atema, 1987).
TABLE 1.
Summary of studies suggesting that fishes use chemical communication in an aggressive context
| Species | Identity | Transmission | Behaviour elicited/function | Reference |
|---|---|---|---|---|
| Etta splendens | n.i. Pheromone | n.i. | Reduces aggressiveness in conspecifics | (Colyer & Jenkins, 1976) |
| Ictalurus nebulosus | n.i. Pheromone | n.i. | Signals dominance and increases aggression towards strangers | (Bryant & Atema, 1987) |
| Gasterosteus aculeatus | n.i. Chemical cue | n.i. | Promotes aggression from males and gravid females | (Waas & Colgan, 1992) |
| Carassius auratus | Androstenedione (pré‐ovulatory pheromone) | n.i. | Courtship and aggressive behaviour | (Poling et al., 2001) |
| Gobius niger | n.i Sexual pheromone. | Sperm | Attracts females and induces aggressive displays in males | (Locatello et al., 2002) |
| Oreochromis mossambicus | n.i. Pheromone | Urine | Signals social status to male rivals | (Almeida et al., 2005) |
| Danio rerio | n.i. Pheromone | n.i | Suppress other females' reproduction according to their social rank | (Gerlach, 2006) |
| Oreochromis mossambicus | n.i. Pheromone | Urine | Signals dominance and modulate aggression in rivals contributing to social stability | (Barata et al., 2007) |
| Oreochromis mossambicus | Pheromone aminosterol‐like | Urine | Signals of dominance, thereby influencing female spawning | (Barata et al., 2008a) |
| Oreochromis niloticus | n.i. Pheromone or chemical cue | n.i. | Water renewal increases aggression and leads to social instability | (Gonçalves‐de‐Freitas et al., 2008) |
| Pimephales promelas | n.i. pheromone (bile acids and/or volatile amines) | Urine | Signals dominance | (Martinovic‐Weigelt et al., 2012) |
| Oreochromis mossambicus | Multicomponent pheromone | Urine | Lowers aggression when added to a tank containing a male fighting its mirror image | (Keller‐Costa et al., 2016) |
| Neolamprologus pulcher | n.i. Pheromone | Urine | Communicate the aggressive propensity | (Bayani et al., 2017) |
| Oreochromis mossambicus | n.i. | Urine | Signals social dominance and fighting ability to avoid energetic costs and/or risk of injury in fights. Exposure to urine leads to 11KT response of subordinate males suggesting chemical eavesdropping | (Saraiva et al., 2017) |
| Porichthys notatus | n.i. Pheromone | Sperm | Dominant males suggest that accessory glands may play a role in parental care and chemical signalling | (Miller et al., 2019) |
| Cichlasoma paranaense | n.i. Pheromone/chemical cue | n.i. | Water renewal reduces aggression | (Gauy et al., 2019) |
Note. n.i., non‐identified.
Female D. rerio release pheromones to suppress reproduction by other females, and the extent of this inhibition depends on social rank, which in turn is related to aggressive interactions (Gerlach, 2006). The identification of these compound(s) was not pursued, but it could be a sex pheromone that influences both reproductive and aggressive behaviour.
C. auratus is the best‐studied fish species for reproductive pheromones (Stacey & Sorensen, 2009). Males respond to pre‐ovulatory female pheromone components, and the responses include courtship and aggressive behaviour (Poling et al., 2001). Androstenedione (AD) is released by females at the end of vitellogenesis, eliciting aggressive behaviour in mature males (Poling et al., 2001). Sexually active males also release considerable amounts of this steroid (50 ng h−1), which suppresses female responsiveness to pheromones (Sorensen et al., 2005). The olfactory system is highly sensitive to AD; because AD is released by both sexes, discrimination of the sex of the donor may be based on the bouquet of C19 and C21 steroids released and detected, which is different between males and females; during sexual arousal in males, the ratio of C19 to C21 steroids was 50:1, markedly different from that (1:7) in females (Sorensen et al., 2005).
Territorial male fathead minnows (Pimephales promelas) use urinary cues to signal dominance Martinovic‐Weigelt et al., 2012). Reproduction in this species depends on the male's ability to acquire and defend a high‐quality nesting territory, and to attract a female to his nest. Dominant males use aggression to visually and physically suppress subordinates to deter them from their territory and females (Unger, 1983). Based on their different concentrations in urine from dominant and subordinate males, bile acids and volatile amines were suggested as the active components (Martinovic‐Weigelt et al., 2012). In N. pulcher, urine is also the vehicle of chemical signals to communicate aggressive propensity and appropriate agonistic responses are dependent on chemical information (Bayani et al., 2017). In the cichlids O. mossambicus and N. pulcher, male aggression levels and urination frequency during fights depend on the social rank of the opponents; dominant males urinate more often, and the urinary emissions act as a signal before the physical contact to inform the opponent of their social status or, perhaps, RHP (Barata et al., 2007; Bayani et al., 2017).
Some males use an alternative reproductive strategy – sneaking – to gain fertilization without investing in territorial defence (Taborsky, 1994). Sneakers are usually smaller and behave like females to access the territory without being confronted by the dominant male, and ultimately fertilize a proportion of the ova (Taborsky, 1994). Sneaking is an interesting strategy from the chemical communication point of view as sneakers must either be “pheromonally silent” (Locatello et al., 2002; Miller et al., 2019) or mimic females in odour as well as looks. One example is the G. niger, wherein parental males release a sex pheromone produced in the mesorchial gland that attracts females and induces aggressive displays in other males (Locatello et al., 2002). Nonetheless, sneakers have undeveloped mesorchial glands; it has been hypothesized that they are “pheromonally silent” so as not to be detected by the other males, thus facilitating fertilization of eggs by avoiding aggressive encounters with other males (Locatello et al., 2002). A similar strategy is found in the plainfin midshipman (Porichthys notatus). In this species, the dominant male accessory glands may play a role not only in parental care but also in pheromonal signalling. Again, sneakers have smaller accessory glands (Miller et al., 2019). In S. pavo, male sneakers lack both the anal gland and the accessory testicular organs, suggesting that this renders them chemically undetectable by conspecifics (Barata et al., 2008b).
Studies in O. niloticus found that aggression of subordinates, but not dominant fish, was higher when their water was renewed compared with not renewed (Gonçalves‐de‐Freitas et al., 2008). Nonetheless, in another cichlid Cichlasoma paranaense, 50% water replacement led to a reduction in aggression, suggesting the existence of species specificity in the aggressive response to chemical stimuli in the social environment (Gauy et al., 2019).
Studies in O. mossambicus have addressed the identity of putative dominance pheromones. Dominant male O. mossambicus release urine pulses to communicate their status during agonistic interactions with rival males and in the presence of pre‐ovulatory females (Almeida et al., 2005; Barata et al., 2007, 2008a). Two steroid glucuronates, 5β‐pregnane‐3α,17,20β‐triol‐3‐glucuronate (20β‐P‐3‐G) and its 20α‐epimer (20α‐P‐3‐G), were identified in male urine (Keller‐Costa et al., 2014, 2016). The urinary concentration of these steroid glucuronates correlates positively with the donor's social rank, and both have high olfactory potency. Although the two steroid glucuronates are sufficient to prime the female endocrine system to accelerate oocyte maturation, alone they had no clear effect on male behaviour (Hubbard et al., 2014; Keller‐Costa et al., 2014, 2016). Although dominant male urine lowers aggression in males fighting their own mirror image, urine fractionation and reconstitution in combination with mirror assays showed that the dominance pheromone is likely to be a mixture (Keller‐Costa et al., 2016). It was hypothesized that male tilapia signalling of social dominance and fighting ability via pheromonal communication may be an adaptive mechanism to avoid energic costs and/or risk of injury in fights (Saraiva et al., 2017).
Some of the studies discussed here suggest a possible overlap between reproductive and dominance pheromones. This is not unlikely considering that aggressive behaviours can be triggered in several contexts, reproduction being one of them. Most likely, the complexity of some behaviours implies the release of a mixture of compounds, different components of which may acquire more or less importance in different contexts. During reproduction, some might affect male–male rivalry to increase the chances of accessing a sexual partner and others might play a direct role in attracting and promoting spawning of the partners. In this study, the authors use “dominance pheromone” to refer to odorants released to show an individual's – usually male – rank within a social hierarchy. One function of a dominance pheromone would be to reduce aggression in rivals, thus reducing the risk of injury and maintaining a stable social order. Clearly, however, many of the components in such a pheromone may also be used by females in their mate‐choice.
Studies on the use of chemicals cues in aggressive contexts in fishes are scarce. Some crustaceans, however, are good models and this topic was already reviewed (Chung‐Davidson et al., 2010). For example, some crustaceans that lose a fight learn to associate unique cues with the winner (Chung‐Davidson et al., 2010). Among the scant extant fish literature, a behavioural study in G. aculeatus showed that aggression is elicited by both visual and chemical stimuli released by conspecifics (Waas & Colgan, 1992). Odorants from displaying males were detected by non‐territorial males and gravid females, and both attempted to bite or bump in the direction of a water source from a displaying male (Waas & Colgan, 1992). The authors proposed that these cues were associated with different rates of physical activity (Waas & Colgan, 1992). Sexual ornaments (odorants that advertise mate quality) are further evidence of chemical cues used in aggressive behaviour; these compounds are bifunctional, both attracting sexual partners and inducing aggressive behaviour from rivals (Sorensen & Wisenden, 2015).
3. METHODOLOGICAL CONSIDERATIONS AND FUTURE DIRECTIONS
The identification of dominance pheromones requires the compound(s) to be isolated from tissues or body fluids and demonstration that is capable of eliciting dominance behaviour. This requires an inter‐disciplinary approach including behavioural, physiological and chemical methods. An important component of the isolation procedure is to have standardized behavioural and physiological assays to test the extracts, fractions and isolates (Barata et al., 2008a; Breithaupt & Thiel, 2011; Sorensen et al., 2010).
The methodologies to measure aggressive behaviour, such as live encounters, video playbacks, mirrors and robots, have been a subject of much discussion (Balzarini et al., 2014; D'Eath, 1998; Huntingford, 1980; Patricelli, 2010; Ramos & Gonçalves, 2019; Romano et al., 2017). This may be because of intrinsic characteristics of the methodology and species‐specific responses. In B. splendens, responses to the mirror assay were similar to responses to a live conspecific and provided less variability (Ramos & Gonçalves, 2019). Nonetheless, another study revealed that the cleaner wrasse (Labroides dimidiatusa) may recognize its mirror image as itself (Kohda et al., 2018). Studies also reported differences between mirror and live assay at the behaviour, endocrine and transcriptomic levels (Balzarini et al., 2014; Desjardins & Fernald, 2010; Oliveira et al., 2005). A study exploring the mirror assay, live conspecific, video assay and model replicas to test for aggression in D. rerio revealed significant differences between the assays, concluding that the specific research goals should be considered when selecting the appropriate stimulus to trigger aggression (Way et al., 2015).
From the few studies that attempted to identify dominance pheromones, these seem at least in some cases to be a mix of compounds (Section 2.3). This makes the identification of the individual components more complex, as each separate component may not have a measurable effect.
Another confounding factor is that communication can be multimodal (see Section 1.3) and to fully understand a behaviour is important to consider the different sensory channels involved in communication of aggressive behaviour. Few studies have been performed combining chemical senses with other sensorial modalities. For instance, the cichlid Neolamprologus pulcher shows similar behaviour when using vision or olfaction (Fischer et al., 2017); and in the G. aculeatus kin recognition, visual and olfactory cues combined are involved (Mehlis et al., 2008).
Another aspect that would benefit standardization is the measurement of aggression. There is currently wide variation in how aggression is measured in fishes, from single behaviours to compound indexes (Budaev, 2010; Félix et al., 2013; Teles & Oliveira, 2016). Ideally, there should be consistency in methodology, too, but difficulties may arise because of variation in aggressive displays across species (Noleto‐Filho et al., 2019). Behaviour is usually recorded on video. Video‐taping allows multiple viewings for precise and detailed measurement of aggressive behaviours; furthermore, they can be analysed “blind” in that the scorer may be unaware of the treatment. Studies using different species have followed this approach (Barata et al., 2007; Dunlap & Larkins‐Ford, 2003; Giaquinto & Volpato, 1998; Oliveira et al., 2011). Numerous displays are used by researchers to evaluate the fish's agonistic behaviour and can be quantified in terms of frequency and duration. Some examples are: time spent with the gill covers extended, proximity to the opponent, tail beats, attempted bites, swimming, flight and nipping (Giaquinto & Volpato, 1998; McGregor et al., 2001). Video‐taping and counting the aggressive displays also enable researchers to identify the winner, the loser and provide rankings. Some behaviours may be species‐ and sex‐specific; in male B. splendens, gill flaring is the initial aggressive display towards both males and females but after prolonged exposure to intruders, there is a switch to fin‐spreading (Forsatkar et al., 2016). Change of colour patterns in cichlids can also be viewed as a species‐specific feature; an example is the oscar (Astronotus ocellatus) that, when defeated, changes from olive‐green to brown/black (Beeching, 1995). It is now possible to extract information on aggressive displays using purpose‐designed software, which improves data analysis compared to unaided human observation (Way et al., 2016). Progress in artificial intelligence for automatic detection of behavioural patterns has emerged as a new tool. Because analysis of animal behaviour is time‐consuming and can be biased by the observer, studies using this technology show progress in this field (Han et al., 2018; Yang et al., 2020).
Usually, studies exploring aggressive behaviour use ethograms and quantify the number of aggressive responses; and there is a separation of “high” and “low” intensity displays, based on the energetic cost (Noleto‐Filho et al., 2017, 2019). Quantifying aggressive behaviour by behavioural units is a common practice; nonetheless, this may generate a bias when pooling variables that are shown in different temporal patterns (Noleto‐Filho et al., 2017). The use of a Bayesian Hierarchical Linear Model (BHLM) was suggested to overcome this; it provided a clear description of the changes even when patterns were tenuous (Noleto‐Filho et al., 2017, 2019).
4. FINAL REMARKS
Understanding the underlying physiological mechanisms of aggression is of great interest at several levels, yet our knowledge is far from complete. Chemical communication is predominant in many fishes but, although some pheromones and other chemical cues associated with reproduction, migration and alarm have been characterized, those associated with modulation of aggression remain to be identified. Nevertheless, the few studies so far provide clear evidence that a modulation of aggression by chemical cues exists in fishes. The combination of behavioural, physiological and analytical chemical approaches together with the right choice of model species is likely to advance our understanding of the role(s) of chemical communication in aggression over the coming years, and potentially use these compounds as tools to improve fish welfare.
ACKNOWLEDGEMENTS
This study was supported by the Portuguese Science and Technology Foundation (FCT) through project UIDB/04326/2020 and fellowship SFRH/BD/143872/2019 to MCS, and by the Macao Science and Technology Development Fund (FDCT) through project 093/2017/A2.
da Silva MC, Canário AVM, Hubbard PC, Gonçalves DMF. Physiology, endocrinology and chemical communication in aggressive behaviour of fishes. J Fish Biol. 2021;98:1217–1233. 10.1111/jfb.14667
Funding information FDCT
Contributor Information
Melina Coelho da Silva, Email: mcsilva@ualg.pt.
Adelino Vicente Mendonça Canário, Email: acanario@ualg.pt.
Peter Colin Hubbard, Email: phubbard@ualg.pt.
David Manuel Flores Gonçalves, Email: david.goncalves@usj.edu.mo.
REFERENCES
- Abbott, J. C. , & Dill, L. M. (1989). The relative growth of dominant and subordinate juvenile steelhead trout (Salmo gairdneri) fed equal rations. Behaviour, 108, 104–113. [Google Scholar]
- Abril‐de‐Abreu, R. , Cruz, J. , & Oliveira, R. F. (2015). Social eavesdropping in zebrafish: Tuning of attention to social interactions. Scientific Reports, 5, 12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja, G. , Ivandić, I. , Saltürk, M. , Oka, Y. , Nadler, W. , & Korsching, S. I. (2013). Zebrafish crypt neurons project to a single, identified mediodorsal glomerulus. Scientific Reports, 3, 2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja, G. , Nia, S. B. , Zapilko, V. , Shiriagin, V. , Kowatschew, D. , Oka, Y. , & Korsching, S. I. (2014). Kappe neurons, a novel population of olfactory sensory neurons. Scientific Reports, 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioto, T. S. , & Ngai, J. (2005). The odorant receptor repertoire of teleost fish. BMC Genomics, 6, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, O. G. , Miranda, A. , Frade, P. , Hubbard, P. C. , Barata, E. N. , & Canário, A. V. M. (2005). Urine as a social signal in the Mozambique tilapia (Oreochromis mossambicus). Chemical Senses, 30(Suppl 1), 309–310. [DOI] [PubMed] [Google Scholar]
- Almeida, O. , Canário, A. V. M. , & Oliveira, R. F. (2014a). Castration affects reproductive but not aggressive behavior in a cichlid fish. General and Comparative Endocrinology, 207, 34–40. [DOI] [PubMed] [Google Scholar]
- Almeida, O. , Gonçalves‐de‐Freitas, E. , Lopes, J. S. , & Oliveira, R. F. (2014b). Social instability promotes hormone–behavior associated patterns in a cichlid fish. Hormones and Behavior, 66, 369–382. [DOI] [PubMed] [Google Scholar]
- Antunes, R. A. , & Oliveira, R. F. (2009). Hormonal anticipation of territorial challenges in cichlid fish. Proceedings of the National Academy of Sciences, 106, 15985–15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelt, C. W. , & Sorensen, P. W. (1999). Freshwater fish release urinary pheromones in a pulsatile manner. In Johnston R. E., Müller‐Schwarze D., & Sorensen P. W. (Eds.), Advances in chemical signals in vertebrates (pp. 247–256). Boston, MA: Springer. [Google Scholar]
- Appelt, C. W. , & Sorensen, P. W. (2007). Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Animal Behaviour, 74, 1329–1338. [Google Scholar]
- Ashley, P. J. (2007). Fish welfare: Current issues in aquaculture. Applied Animal Behaviour Science, 104, 199–235. [Google Scholar]
- Backström, T. , & Winberg, S. (2009). Arginine‐vasotocin influence on aggressive behavior and dominance in rainbow trout. Physiology & Behavior, 96, 470–475. [DOI] [PubMed] [Google Scholar]
- Bakker, T. C. M. , Bruijn, E. F.‐D. , & Sevenster, P. (1989). Asymmetrical effects of prior winning and losing on dominance in sticklebacks (Gasterosteus aculeatus). Ethology, 82, 224–229. [Google Scholar]
- Ball, J. N. , & Baker, B. I. (1969). 1 ‐ The pituitary gland: Anatomy and histophysiology. In Hoar W. S. & Randall D. J. (Eds.), Fish physiology (pp. 1–110). New York: Academic Press. [Google Scholar]
- Balzarini, V. , Taborsky, M. , Wanner, S. , Koch, F. , & Frommen, J. (2014). Mirror, mirror on the wall: The predictive value of mirror tests for measuring aggression in fish. Behavioral Ecology and Sociobiology, 68, 871–878. [Google Scholar]
- Barata, E. N. , Hubbard, P. C. , Almeida, O. G. , Miranda, A. , & Canário, A. V. M. (2007). Male urine signals social rank in the Mozambique tilapia (Oreochromis mossambicus). BMC Biology, 5, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barata, E. N. , Fine, J. M. , Hubbard, P. C. , Almeida, O. G. , Frade, P. , Sorensen, P. W. , & Canário, A. V. M. (2008a). A sterol‐like odorant in the urine of Mozambique tilapia males likely signals social dominance to females. Journal of Chemical Ecology, 34, 438–449. [DOI] [PubMed] [Google Scholar]
- Barata, E. N. , Serrano, R. M. , Miranda, A. , Nogueira, R. , Hubbard, P. C. , & Canário, A. V. M. (2008b). Putative pheromones from the anal glands of male blennies attract females and enhance male reproductive success. Animal Behaviour, 75, 379–389. [Google Scholar]
- Bardach, J. E. , Magnuson, J. J. , May, R. C. , & Reinhart, J. M. (1980). In Bardach J. E., Magnuson J. J., May R. C., & Reinhart J. M. (Eds.), Fish behavior and its use in the capture and culture of fishes. Manila, Philippines: International Center For Living Aquatic Resources Managment ‐ IOLARM. [Google Scholar]
- Barki, A. , Jones, C. , & Karplus, I. (2011). Chemical communication and aquaculture of decapod crustaceans: Needs, problems, and possible solutions. In Breithaupt T. & Thiel M. (Eds.), Chemical communication in crustaceans (pp. 485–506). New York, NY: Springer. [Google Scholar]
- Barlow, G. W. , Rogers, W. , & Fraley, N. (1986). Do midas cichlids win through prowess or daring? It depends. Behavioral Ecology and Sociobiology, 19, 1–8. [Google Scholar]
- Baskaran, G. , & Sathyanesan, A. G. (1992). Tetrapod‐like hypothalamo‐hypophysial portal system in the teleost Megalops cyprinoides (Broussonet). General and Comparative Endocrinology, 86, 211–219. [DOI] [PubMed] [Google Scholar]
- Bayani, D. M. , Taborsky, M. , & Frommen, J. G. (2017). To pee or not to pee: Urine signals mediate aggressive interactions in the cooperatively breeding cichlid Neolamprologus pulcher . Behavioral Ecology and Sociobiology, 71, 37. [Google Scholar]
- Bazáes, A. , & Schmachtenberg, O. (2012). Odorant tuning of olfactory crypt cells from juvenile and adult rainbow trout. The Journal of Experimental Biology, 215, 1740 LP–1748. [DOI] [PubMed] [Google Scholar]
- Beacham, J. L. (1988). The relative importance of body size and aggressive experience as determinants of dominance in pumpkinseed sunfish, Lepomis gibbosus . Animal Behaviour, 36, 621–623. [Google Scholar]
- Beeching, S. C. (1995). Colour pattern and inhibition of aggression in the cichlid fish Astronotus ocellatus . Journal of Fish Biology, 47, 50–58. [Google Scholar]
- Bell, C. C. , Myers, J. P. , & Russell, C. J. (1974). Electric organ discharge patterns during dominance related behavioral displays in Gnathonemus petersii (Mormyridae). Journal of Comparative Physiology, 92, 201–228. [Google Scholar]
- Béné, C. , Barange, M. , Subasinghe, R. , Pinstrup‐Andersen, P. , Merino, G. , Hemre, G. I. , & Williams, M. (2015). Feeding 9 billion by 2050 – Putting fish back on the menu. Food Security, 7, 261–274. [Google Scholar]
- Béné, C. , Arthur, R. , Norbury, H. , Allison, E. H. , Beveridge, M. , Bush, S. , … Williams, M. (2016). Contribution of fisheries and aquaculture to food security and poverty reduction: Assessing the current evidence. World Development, 79, 177–196. [Google Scholar]
- Bertucci, F. , Matos, R. J. , & Dabelsteen, T. (2014). Knowing your audience affects male‐male interactions in Siamese fighting fish (Betta splendens). Animal Cognition, 17, 229–236. [DOI] [PubMed] [Google Scholar]
- Borg, B. (1994). Androgens in teleost fishes. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 109, 219–245. [PubMed] [Google Scholar]
- Bradbury, J. W. , & Vehrencamp, S. L. (2015). In Bradbury J. W. & Vehrencamp S. L. (Eds.), Principles of animal communication (2nd ed.). New York, NY: Oxford University Press Inc. [Google Scholar]
- Bratton, B. O. , & Kramer, B. (1989). Patterns of the electric organ discharge during courtship and spawning in the mormyrid fish, Pollimyrus isidori . Behavioral Ecology and Sociobiology, 24, 349–368. [Google Scholar]
- Braubach, O. R. , Fine, A. , & Croll, R. P. (2012). Distribution and functional organization of glomeruli in the olfactory bulbs of zebrafish (Danio rerio). Journal of Comparative Neurology, 520, 2317–2339. [DOI] [PubMed] [Google Scholar]
- Brazeau, P. , Vale, W. , Burgus, R. , Ling, N. , Butcher, M. , Rivier, J. , & Guillemin, R. (1973). Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science, 179, 77–79. [DOI] [PubMed] [Google Scholar]
- Breithaupt, T. , & Thiel, M. (2011). In Breithaupt T. & Thiel M. (Eds.), Chemical communication in crustaceans (1st ed.). New York, NY: Springer. [Google Scholar]
- Brown, G. E. , Chivers, D. P. , & Smith, R. J. F. (1995). Fathead minnows avoid conspedfic and heterospedfic alarm pheromones in the faeces of northern pike. Journal of Fish Biology, 47, 387–393. [Google Scholar]
- Bruce, K. E. , & White, W. G. (1995). Agonistic relationships and sexual behaviour patterns in male guppies, Poecilia reticulata . Animal Behaviour, 50, 1009–1021. [Google Scholar]
- Bryant, B. P. , & Atema, J. (1987). Diet manipulation affects social behavior of catfish ‐ importance of body odor. Journal of Chemical Ecology, 13, 1645–1661. [DOI] [PubMed] [Google Scholar]
- Buchinger, T. J. , Siefkes, M. J. , Zielinski, B. S. , Brant, C. O. , & Li, W. (2015). Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Frontiers in Zoology, 12, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, L. , & Axel, R. (1991). A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell, 65, 175–187. [DOI] [PubMed] [Google Scholar]
- Budaev, S. (2010). Using principal components and factor analysis in animal behaviour research: Caveats and guidelines. Ethology, 116, 472–480. [Google Scholar]
- Bullock, T. H. , Bodznick, D. A. , & Northcutt, R. G. (1983). The phylogenetic distribution of electroreception: Evidence for convergent evolution of a primitive vertebrate sense modality. Brain Research Reviews, 6, 25–46. [DOI] [PubMed] [Google Scholar]
- Butler, J. M. , & Maruska, K. P. (2015). The mechanosensory lateral line is used to assess opponents and mediate aggressive behaviors during territorial interactions in an African cichlid fish. Journal of Experimental Biology, 218, 3284–3294. [DOI] [PubMed] [Google Scholar]
- Carpenter, R. , Maruska, K. , Becker, L. , & Fernald, R. (2014). Social opportunity rapidly regulates expression of CRF and CRF receptors in the brain during social ascent of a teleost fish, Astatotilapia burtoni . PLoS One, 9, e96632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrolles, L. , Ammar, I. B. , Fernandez, M. S. A. , Boyer, N. , Attia, J. , Fonseca, P. J. , … Beauchaud, M. (2017). Appraisal of unimodal cues during agonistic interactions in Maylandia zebra . PeerJ, 2017, e3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase, I. D. , Bartolomeo, C. , & Dugatkin, L. A. (1994). Aggressive interactions and inter‐contest interval: How long do winners keep winning? Animal Behaviour, 48, 393–400. [Google Scholar]
- Chess, A. , Simon, I. , Cedar, H. , & Axel, R. (1994). Allelic inactivation regulates olfactory receptor gene expression. Cell, 78, 823–834. [DOI] [PubMed] [Google Scholar]
- Chung‐Davidson, Y.‐W. , Huertas, M. , & Li, W. (2010). A review of research in fish pheromones. In Breithaupt T. & Thiel M. (Eds.), Chemical communication in crustaceans (pp. 467–482). New York, NY: Springer. [Google Scholar]
- Collias, N. E. (1944). Aggressive behavior among vertebrate animals. Physiological Zoology, 17, 83–123. [Google Scholar]
- Colombo, L. , & Burighel, P. (1974). Fine structure of the testicular gland of the black goby, Gobius jozo L. Cell and Tissue Research, 154, 39–49. [DOI] [PubMed] [Google Scholar]
- Colombo, L. , Marconato, A. , Belvedere, P. C. , & Friso, C. (1980). Endocrinology of teleost reproduction: A testicular steroid pheromone in the black goby, Gobius jozo L. Italian Journal of Zoology, 47, 355–364. [Google Scholar]
- Colyer, S. W. , & Jenkins, C. (1976). Pheromonal control of aggressive display in Siamese fighting fish (Betta splendens). Perceptual and Motor Skills, 42, 47–54. [DOI] [PubMed] [Google Scholar]
- Crow, R. T. , & Liley, N. R. (1979). A sexual pheromone in the guppy, Poecilia reticulata (Peters). Canadian Journal of Zoology, 57, 184–188. [Google Scholar]
- D'Eath, R. (1998). Can video images imitate real stimuli in animal behavior experiments? Biological Reviews of the Cambridge Philosophical Society, 73, 267–292. [Google Scholar]
- Dawkins, M. S. , & Guilford, T. (1993). Colour and pattern in relation to sexual and aggressive behaviour in the bluehead wrasse Thalassoma bifasciatum . Behavioural Processes, 30, 245–251. [DOI] [PubMed] [Google Scholar]
- Desjardins, J. K. , Hazelden, M. R. , Van der Kraak, G. J. , & Balshine, S. (2005). Male and female cooperatively breeding fish provide support for the “Challenge Hypothesis”. Behavioral Ecology, 17, 149–154. [Google Scholar]
- Desjardins, J. K. , & Fernald, R. D. (2010). What do fish make of mirror images? Biology Letters, 6, 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doutrelant, C. , McGregor, P. K. , & Oliveira, R. F. (2001). The effect of an audience on intrasexual communication in male Siamese fighting fish, Betta splendens . Behavioral Ecology, 12, 283–286. [Google Scholar]
- Døving, K. , & Lastein, S. (2009). The alarm reaction in fishes—odorants, modulations of responses, neural pathways. Annals of the New York Academy of Sciences, 1170, 413–423. [DOI] [PubMed] [Google Scholar]
- Døving, K. , Selset, R. , & Thommesen, G. (1980). Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108, 123–131. [DOI] [PubMed] [Google Scholar]
- Doyon, C. , Gilmour, K. M. , Trudeau, V. L. , & Moon, T. W. (2003). Corticotropin‐releasing factor and neuropeptide Y mRNA levels are elevated in the preoptic area of socially subordinate rainbow trout. General and Comparative Endocrinology, 133, 260–271. [DOI] [PubMed] [Google Scholar]
- Dunlap, K. D. , & Larkins‐Ford, J. (2003). Production of aggressive electrocommunication signals to progressively realistic social stimuli in male Apteronotus leptorhynchus . Ethology, 109, 243–258. [Google Scholar]
- Ejike, C. , & Schreck, C. B. (1980). Stress and social hierarchy rank in coho salmon. Transactions of the American Fisheries Society, 109, 423–426. [Google Scholar]
- Escobar‐Camacho, D. , & Carleton, K. L. (2015). Sensory modalities in cichlid fish behavior. Current Opinion in Behavioral Sciences, 6, 115–124. 10.1016/j.cobeha.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix, A. S. , Faustino, A. I. , Cabral, E. M. , & Oliveira, R. F. (2013). Noninvasive measurement of steroid hormones in zebrafish holding‐water. Zebrafish, 10, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald, R. (1976). The effect of testosterone on the behavior and coloration of adult male cichlid fish (Haplochromis burtoni, Günther). Hormone Research, 7, 172–178. [DOI] [PubMed] [Google Scholar]
- Filby, A. L. , Paull, G. C. , Hickmore, T. F. A. , & Tyler, C. R. (2010). Unravelling the neurophysiological basis of aggression in a fish model. BMC Genomics, 11, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, S. , Oberhummer, E. , Cunha‐Saraiva, F. , Gerber, N. , & Taborsky, B. (2017). Smell or vision? The use of different sensory modalities in predator discrimination. Behavioral Ecology and Sociobiology, 71(10), 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer, J. , Breer, H. , & Strotmann, J. (2009). Mammalian olfactory receptors. Frontiers in Cellular Neuroscience, 3, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsatkar, M. N. , Nematollahi, M. A. , & Brown, C. (2016). Male Siamese fighting fish use gill flaring as the first display towards territorial intruders. Journal of Ethology, 35, 51–59. [Google Scholar]
- Francis, R. C. (1983). Experiential effects on agonistic behavior in the paradise fish, Macropodus opercularis . Behaviour, 85, 292–313. [Google Scholar]
- Frey, D. F. , & Miller, R. J. (1972). The establishment of dominance relationships in the blue gourami, Trichogaster trichopterus (Pallas). Behaviour, 42, 8–62. [Google Scholar]
- Friedrich, R. W. , & Korsching, S. I. (1998). Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage‐sensitive axon tracer. Journal of Neuroscience, 18, 9977–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommen, J. G. (2020). Aggressive communication in aquatic environments. Functional Ecology, 34, 364–380. [Google Scholar]
- Ganga, R. , Tort, L. , Acerete, L. , Montero, D. , & Izquierdo, M. (2006). Modulation of ACTH‐induced cortisol release by polyunsaturated fatty acids in interrenal cells from gilthead seabream, Sparus aurata . The Journal of Endocrinology, 190, 39–45. [DOI] [PubMed] [Google Scholar]
- Gauy, A. C. D. S. , Bolognesi, M. C. , & Gonçalves‐de‐Freitas, E. (2019). Unusual effect of chemical communication on social aggression in juvenile cichlid fish Cichlasoma paranaense (Cichliformes: Cichlidae). Neotropical Ichthyology, 17, e180159[1]–e180159[7]. [Google Scholar]
- Gentry, R. R. , Froehlich, H. E. , Grimm, D. , Kareiva, P. , Parke, M. , Rust, M. , … Halpern, B. S. (2017). Mapping the global potential for marine aquaculture. Nature Ecology and Evolution, 1, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Gerlach, G. (2006). Pheromonal regulation of reproductive success in female zebrafish: Female suppression and male enhancement. Animal Behaviour, 72, 1119–1124. [Google Scholar]
- Giaquinto, P. C. , Barreto, R. , Volpato, G. , Fernandes‐de‐Castilho, M. , & Gonçalves‐de‐Freitas, E. (2015). Bile acids as potential pheromones in pintado catfish Pseudoplatystoma corruscans (Spix & Agassiz, 1829): Eletrophysiological and behavioral studies. Neotropical Ichthyology, 13, 237–244. [Google Scholar]
- Giaquinto, P. C. , & Volpato, G. (1998). Chemical communication, aggression, and conspecific recognition in the fish Nile tilapia. Physiology & Behavior, 62, 1333–1338. [DOI] [PubMed] [Google Scholar]
- Giaquinto, P. , & Hara, T. (2008). Discrimination of bile acids by the rainbow trout olfactory system: Evidence as potential pheromone. Biological Research, 41, 33–42. [PubMed] [Google Scholar]
- Godin, J.‐G. , A. Dill, P. , & E. Drury, D. (2011). Effects of thyroid hormones on behavior of yearling Atlantic salmon (Salmo salar). Journal of the Fisheries Research Board of Canada, 31, 1787–1790. [Google Scholar]
- Godwin, J. , & Thompson, R. (2012). Nonapeptides and social behavior in fishes. Hormones and Behavior, 61, 230–238. [DOI] [PubMed] [Google Scholar]
- Gonçalves‐de‐Freitas, E. , Teresa, F. B. , Gomes, F. S. , & Giaquinto, P. C. (2008). Effect of water renewal on dominance hierarchy of juvenile Nile tilapia. Applied Animal Behaviour Science, 112, 187–195. [Google Scholar]
- Grossman, G. (1980). Food, fights, and burrows: The adaptive significance of intraspecific aggression in the bay goby (Pisces: Gobiidae). Oecologia, 45, 261–266. [DOI] [PubMed] [Google Scholar]
- Gubbels, M. R. B. , & Jorgensen, T. N. (2018). Androgen‐induced immunosuppression. Frontiers in Immunology, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller, J. (1995). Biochemical background for an analysis of cost‐benefit interrelations in aggression. Neuroscience & Biobehavioral Reviews, 19, 599–604. [DOI] [PubMed] [Google Scholar]
- Hamdani, E. H. , & Døving, K. B. (2002). The alarm reaction in crucian carp is mediated by olfactory neurons with long dendrites. Chemical Senses, 27, 395–398. [DOI] [PubMed] [Google Scholar]
- Hamdani, E. H. , Lastein, S. , Gregersen, F. , & Døving, K. B. (2008). Seasonal variations in olfactory sensory neurons–fish sensitivity to sex pheromones explained? Chemical Senses, 33, 119–123. [DOI] [PubMed] [Google Scholar]
- Han, S. , Taralova, E. , Dupre, C. , & Yuste, R. (2018). Comprehensive machine learning analysis of Hydra behavior reveals a stable basal behavioral repertoire. eLife, 7, e32605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, A. , & Eckart, Z. (1998). The peripheral olfactory organ of the zebrafish, Danio rerio: An ultrastructural study. Chemical Senses, 23, 39–48. [DOI] [PubMed] [Google Scholar]
- Hansen, A. , & Finger, T. E. (2000). Phyletic distribution of crypt‐type olfactory receptor neurons in fishes. Brain, Behavior and Evolution, 55, 100–110. [DOI] [PubMed] [Google Scholar]
- Hansen, A. , Rolen, S. H. , Anderson, K. , Morita, Y. , Caprio, J. , & Finger, T. E. (2003). Correlation between olfactory receptor cell type and function in the channel catfish. Journal of Neuroscience, 23, 9328–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, T. J. (1994). The diversity of chemical stimulation in fish olfaction and gustation. Reviews in Fish Biology and Fisheries, 4, 1–35. [Google Scholar]
- Hara, T. , & Zielinski, B. (2006). Olfaction. In Fish physiology: Sensory systems neuroscience (pp. 1–43). London: Academic Press. [Google Scholar]
- Hardy, I. C. W. , & Briffa, M. (2013). In Hardy I. C. W. & Briffa M. (Eds.), Animal contests. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Hashiguchi, Y. , Futura, Y. , & Nishida, M. (2008). Evolutionary patterns and selective pressures of odorant/pheromone receptor gene families in teleost fishes. PLoS One, 3(12), e4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschenhauser, K. , Taborsky, M. , Oliveira, T. , Canario, A. V. M. , Oliveira, R. F. F. , Canàrio, A. V. M. , & Oliveira, R. F. F. (2004). A test of the ‘challenge hypothesis’ in cichlid fish: Simulated partner and territory intruder experiments. Animal Behaviour, 68, 741–750. [Google Scholar]
- Hofmann, H. A. , & Fernald, R. D. (2000). Social status controls somatostatin neuron size and growth. The Journal of Neuroscience, 20, 4740–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, H. A. , & Trainor, B. C. (2006). Somatostatin regulates aggressive behavior in an African cichlid fish. Endocrinology, 147, 5119–5125. [DOI] [PubMed] [Google Scholar]
- Hopkins, C. D. (1974). Electric communication: Functions in the social behavior of Eigenmannia virescens. In Behaviour, 50, (3‐4), (pp. 270–305). [Google Scholar]
- Hsu, Y. , & Wolf, L. L. (1999). The winner and loser effect: Integrating multiple experiences. Animal Behaviour, 57, 903–910. [DOI] [PubMed] [Google Scholar]
- Hsu, Y. , Earley, R. L. , & Wolf, L. L. (2006). Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biological Reviews of the Cambridge Philosophical Society, 81, 33–74. [DOI] [PubMed] [Google Scholar]
- Hsu, Y. , Huang, Y. Y. , & Wu, Y. T. (2014). Multiple contest experiences interact to influence each other's effect on subsequent contest decisions in a mangrove killifish. Animal Cognition, 17, 165–175. [DOI] [PubMed] [Google Scholar]
- Hubbard, P. C. , Mota, V. C. , Keller‐Costa, T. , da Silva, J. P. , Canário, A. V. M. , & Canario, A. (2014). Chemical communication in tilapia: A comparison of Oreochromis mossambicus with O. niloticus . General and Comparative Endocrinology, 207, 13–20. [DOI] [PubMed] [Google Scholar]
- Hubbard, P. C. , Barata, E. N. , & Canario, A. V. M. (2002). Possible disruption of pheromonal communication by humic acid in the goldfish, Carassius auratus . Aquatic Toxicology, 60, 169–183. [DOI] [PubMed] [Google Scholar]
- Hubbard, P. C. , Barata, E. N. , Canario, A. , & Canário, A. V. M. (2003). Olfactory sensitivity of the gilthead seabream (Sparus auratus L) to conspecific body fluids. Journal of Chemical Ecology, 29, 2481–2498. [DOI] [PubMed] [Google Scholar]
- Huffman, L. S. , Hinz, F. I. , Wojcik, S. , Aubin‐Horth, N. , & Hofmann, H. A. (2015). Arginine vasotocin regulates social ascent in the African cichlid fish Astatotilapia burtoni . General and Comparative Endocrinology, 212, 106–113. [DOI] [PubMed] [Google Scholar]
- Huntingford, F. A. (1980). A review of the methods used to describe and measure aggressive behavior in physiological studies. Aggressive Behavior, 6, 205–215. [Google Scholar]
- Huntingford, F. A. A. , Adams, C. , Braithwaite, V. , Kadri, S. , Pottinger, T. , Sandøe, P. , & Turnbull, J. F. (2006). Issues in fish welfare. Journal of Fish Biology, 68, 332–372. [Google Scholar]
- Huntingford, F. A. , Taylor, A. C. , Sneddon, L. , & Neat, F. (2001). Prowess and the resolution of animal fights. In Esprnark Y., Amundsen T., & Rosenqvist G. (Eds.), Animal signals: Signalling and signal design in animal communication (pp. 259–276). Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- Huntingford, F. A. , & Turner, A. K. (1987). Animal conflict (1st ed.). London, UK: Chapman and Hall. [Google Scholar]
- Hupé, G. J. , & Lewis, J. E. (2008). Electrocommunication signals in free swimming brown ghost knifefish, (Apteronotus leptorhynchus). Journal of Experimental Biology, 211, 1657–1667. [DOI] [PubMed] [Google Scholar]
- Hurk, R. , & Resink, J. (1992). Male reproductive system as sex pheromone producers in teleost fish. Journal of Experimental Zoology, 261, 204–213. [Google Scholar]
- Hussain, A. , Saraiva, L. R. , & Korsching, S. I. (2009). Positive Darwinian selection and the birth of an olfactory receptor clade in teleosts. Proceedings of the National Academy of Sciences of the United States of America, 106, 4313–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, M. J. , & Iwata, M. (1998). Effect of thyroxine on decrease of aggressive behavior of four salmonids during the parr‐smolt transformation. Aquaculture, 168, 169–175. [Google Scholar]
- Iqbal, T. , & Byrd‐Jacobs, C. (2010). Rapid degeneration and regeneration of the zebrafish olfactory epithelium after triton X‐100 application. Chemical Senses, 35, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson, E. , Johnsson, J. I. , & Björnsson, B. T. (1998). Growth hormone increases aggressive behavior in juvenile rainbow trout. Hormones and Behavior, 33, 9–15. [DOI] [PubMed] [Google Scholar]
- Karlson, P. , & Lüscher, M. (1959). ‘Pheromones’: A new term for a class of biologically active substances. Nature, 183, 55–56. [DOI] [PubMed] [Google Scholar]
- Kekan, P. , Ingole, S. , Sirsat, S. , Bharucha, S. , Kharde, S. , & Nagvekar, A. (2017). The role of pheromones in farm animals ‐ A review. Agricultural Reviews, 38, 83–93. [Google Scholar]
- Keller‐Costa, T. , Hubbard, P. C. , Paetz, C. , Nakamura, Y. , Da Silva, J. P. , Rato, A. , … Canario, A. V. (2014). Identity of a tilapia pheromone released by dominant males that primes females for reproduction. Current Biology, 24, 2130–2135. [DOI] [PubMed] [Google Scholar]
- Keller‐Costa, T. , Lopes, O. S. , Almeida, O. , Hubbard, P. C. , Iacovella, A. , Lima, M. , … Canário, A. V. M. (2012). Muscular hypertrophy of urinary bladders in dominant tilapia facilitates the control of aggression through urinary signals. Behaviour, 149, 953–975. [Google Scholar]
- Keller‐Costa, T. , Saraiva, J. L. , Hubbard, P. C. , Barata, E. N. , & Canário, A. V. (2016). A multi‐component pheromone in the urine of dominant male tilapia (Oreochromis mossambicus) reduces aggression in rivals. Journal of Chemical Ecology, 42, 173–182. [DOI] [PubMed] [Google Scholar]
- Kermen, F. , Franco, L. M. , Wyatt, C. , & Yaksi, E. (2013). Neural circuits mediating olfactory‐driven behavior in fish. Frontiers in neural circuits, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszczyńska, A. , Sokołowska, E. , & Kulczykowska, E. (2012). Variation in brain arginine vasotocin (AVT) and isotocin (IT) levels with reproductive stage and social status in males of three‐spined stickleback (Gasterosteus aculeatus). General and Comparative Endocrinology, 175, 290–296. [DOI] [PubMed] [Google Scholar]
- Koebele, B. P. (1985). Growth and the size hierarchy effect: An experimental assessment of three proposed mechanisms; activity differences, disproportional food acquisition, physiological stress. Environmental Biology of Fishes, 12, 181–188. [Google Scholar]
- Kohda, M. , Hotta, T. , Takeyama, T. , Awata, S. , Tanaka, H. , Asai, J. Y. , & Jordan, A. L. (2018). Cleaner wrasse pass the mark test. What are the implications for consciousness and self‐awareness testing in animals? PLoS Biology, 17, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, B. (1990). In Bradshaw S. D., Burggren W., Heller H. C., Ishii S., Langer H., Neuweiler G., & Randal D. J. (Eds.), Electrocommunication in teleost fishes: Behavior and experiments. Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Kupsala, S. , Jokinen, P. , & Vinnari, M. (2013). Who cares about farmed fish? Citizen perceptions of the welfare and the mental abilities of fish. Journal of Agricultural and Environmental Ethics, 26, 119–135. [Google Scholar]
- Ladich, F. (1997). Agonistic behavior and significance of sounds in vocalizing fish. Marine and Freshwater Behaviour and Physiology, 29, 87–108. [Google Scholar]
- Ladich, F. , & Myrberg, A. A. (2006). Agonistic behaviour and acoustic communication. In Ladich F., Colin S., Moller P., & Kapoor B. G. (Eds.), Communication in fishes (pp. 121–148). New Hampshire: Science Publishers. [Google Scholar]
- Laidre, M. E. , & Johnstone, R. A. (2013). Animal signals. Current Biology, 23, R829–R833. [DOI] [PubMed] [Google Scholar]
- Larson, E. T. , O'Malley, D. M. , Melloni, R. H. , O'Malley, D. M. , & Melloni, R. H. (2006). Aggression and vasotocin are associated with dominant–subordinate relationships in zebrafish. Behavioural Brain Research, 167, 94–102. [DOI] [PubMed] [Google Scholar]
- Lastein, S. , Hamdani, E. H. , & Døving, K. B. (2006). Gender distinction in neural discrimination of sex pheromones in the olfactory bulb of crucian carp, Carassius carassius . Chemical Senses, 31, 69–77. [DOI] [PubMed] [Google Scholar]
- Laumen, J. , Pern, U. , & Blüm, V. (1974). Investigations on the function and hormonal regulation of the anal appendices in Blennius pavo (Risso). Journal of Experimental Zoology, 190, 47–56. [DOI] [PubMed] [Google Scholar]
- Lawrence, C. (2007). The husbandry of zebrafish (Danio rerio): A review. Aquaculture, 269, 1–20. [Google Scholar]
- Lecchini, D. , Tapissier‐Bontemps, N. , Banaigs, B. , Sasal, P. , & Reverter, M. (2018). Biological and ecological roles of external fish mucus: A review. Fishes, 3, 41. [Google Scholar]
- Locatello, L. , Mazzoldi, C. , & Rasotto, M. B. (2002). Ejaculate of sneaker males is pheromonally inconspicuous in the black goby, Gobius niger (Teleostei, Gobiidae). Journal of Experimental Zoology, 293, 601–605. [DOI] [PubMed] [Google Scholar]
- Longo, S. B. , Clark, B. , York, R. , & Jorgenson, A. K. (2019). Aquaculture and the displacement of fisheries captures. Conservation Biology, 33, 832–841. [DOI] [PubMed] [Google Scholar]
- Magellan, K. , Johnson, A. , Willaimson, L. , Richardson, M. , Watt, W. , & Kaiser, H. (2012). Alteration of tank dimensions reduces aggression in the swordtail. Journal of Applied Ichthyology, 28, 91–94. [Google Scholar]
- Magnhagen, C. , Braithwaite, V. , Forsgren, E. , Kapoor, B. G. , & Magurran, A. E. (2008). In Magnhagen C., Braithwaite V., Forsgren E., & Kapoor B. G. (Eds.), Fish behaviour. Boca Raton, FL: Taylor & Francis. [Google Scholar]
- Martinovic‐Weigelt, D. , Ekman, D. R. , Villeneuve, D. L. , James, C. M. , Teng, Q. , Collette, T. W. , & Ankley, G. T. (2012). Fishy aroma of social status: Urinary chemo‐signalling of territoriality in male fathead minnows (Pimephales promelas). PLoS One, 7, e46579–e46579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui, T. , & Caprio, J. (1992). Fish chemoreception (1st ed.). Malta: Springer Science+Business Media Dordrecht. [Google Scholar]
- Maruska, K. P. , & Fernald, R. D. (2012). Contextual chemosensory urine signaling in an African cichlid fish. The Journal of Experimental Biology, 215, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos, R. J. , & McGregor, P. K. (2002). The effect of the sex of an audience on male‐male displays of Siamese fighting fish (Betta splendens). Behaviour, 139, 1211–1221. [DOI] [PubMed] [Google Scholar]
- McDonald, A. L. , Heimstra, N. W. , & Damkot, D. K. (1968). Social modification of agonistic behaviour in fish. Animal Behaviour, 16, 437–441. [DOI] [PubMed] [Google Scholar]
- McGregor, P. K. , Peake, T. M. , & Lampe, H. M. (2001). Fighting fish Betta splendens extract relative information from apparent interactions: What happens when what you see is not what you get. Animal Behaviour, 62, 1059–1065. [Google Scholar]
- Mehlis, M. , Bakker, T. C. M. , & Frommen, J. G. (2008). Smells like sib spirit: Kin recognition in three‐spined sticklebacks (Gasterosteus aculeatus) is mediated by olfactory cues. Animal Cognition, 11, 643–650. [DOI] [PubMed] [Google Scholar]
- Miller, J. S. , Mazzoldi, C. , Rasotto, M. B. , & Balshine, S. (2019). Differential investment in male accessory glands: Lessons from a marine fish with alternative reproductive tactics. Marine Biology, 166, 37. [Google Scholar]
- Miyasaka, N. , Arganda‐Carreras, I. , Wakisaka, N. , Masuda, M. , Sümbül, U. , Seung, H. S. , & Yoshihara, Y. (2014). Olfactory projectome in the zebrafish forebrain revealed by genetic single‐neuron labelling. Nature Communications, 9, 1–14. [DOI] [PubMed] [Google Scholar]
- Mombaerts, P. (2004a). Genes and ligands for odorant, vomeronasal and taste receptors. Nature Reviews Neuroscience, 5, 263. [DOI] [PubMed] [Google Scholar]
- Mombaerts, P. (2004b). Odorant receptor gene choice in olfactory sensory neurons: The one receptor‐one neuron hypothesis revisited. Current Opinion in Neurobiology, 14, 31–36. [DOI] [PubMed] [Google Scholar]
- Naumowicz, K. , Pajdak, J. , Terech‐Majewska, E. , & Szarek, J. (2017). Intracohort cannibalism and methods for its mitigation in cultured freshwater fish. Reviews in Fish Biology and Fisheries, 27, 193–208. [Google Scholar]
- Neat, F. C. , Huntingford, F. A. , & Beveridge, M. M. C. C. (1998). Fighting and assessment in male cichlid fish: The effects of asymmetries in gonadal state and body size. Animal Behaviour, 55, 883–891. [DOI] [PubMed] [Google Scholar]
- Nelson, R. J. (2005). Biology of aggression. New York, NY: Oxford University Press. [Google Scholar]
- Nicieza, A. G. , & Metcalfe, N. B. (1999). Costs of rapid growth: The risk of aggression is higher for fast‐growing salmon. Functional Ecology, 13, 793–800. [Google Scholar]
- Nikonov, A. A. , & Maruska, K. P. (2019). Male dominance status regulates odor‐evoked processing in the forebrain of a cichlid fish. Scientific Reports, 9, 5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noleto‐Filho, E. M. , Gauy, A. C. , Pennino, M. G. , Gonçalves de Freitas, E. , & Gonçalves‐de‐Freitas, E. (2017). Bayesian analysis improves experimental studies about temporal patterning of aggression in fish. Behavioural Processes, 145, 18–26. [DOI] [PubMed] [Google Scholar]
- Noleto‐Filho, E. M. , Pennino, M. G. , Gauy, A. C. D. S. , Bolognesi, M. C. , & Gonçalves‐de‐Freitas, E. (2019). The bias of combining variables on fish's aggressive behavior studies. Behavioural Processes, 164, 65–77. [DOI] [PubMed] [Google Scholar]
- Ogino, Y. , Miyagawa, S. , & Iguchi, T. (2016). 11‐Ketotestosterone. In Takei Y., Ando H., & Tsutsui K. (Eds.), Handbook of hormones (pp. 518–519). San Diego, CA: Academic Press. [Google Scholar]
- Oka, Y. , Saraiva, L. R. , Korsching, S. I. , Oka, Y. , & Saraiva, L. R. (2012). Crypt neurons express a single v1r‐related ora gene. Chemical Senses, 37, 219–227. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. G. (2011). Aggression and welfare in a common aquarium fish, the midas cichlid. Journal of Applied Animal Welfare Science, 14, 340–360. [DOI] [PubMed] [Google Scholar]
- Olivares, J. , & Schmachtenberg, O. (2019). An update on anatomy and function of the teleost olfactory system. PeerJ, 7, e7808–e7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, R. F. , Hirschenhauser, K. , Carneiro, L. A. , & Canario, A. V. M. (2002). Social modulation of androgen levels in male teleost fish. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 132, 203–215. [DOI] [PubMed] [Google Scholar]
- Oliveira, R. F. , Lopes, M. , A. Carneiro, L. , Canario, A. , Carneiro, L. A. , & Canário, A. V. M. (2001). Watching fights raises fish hormone levels. Nature, 409, 475. [DOI] [PubMed] [Google Scholar]
- Oliveira, R. F. , Carneiro, L. A. , & Canário, A. V. M. (2005). No hormonal response in tied fights. Nature, 437, 207–208. [DOI] [PubMed] [Google Scholar]
- Oliveira, R. F. , Silva, A. , & Canário, A. V. M. (2009). Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proceedings of the Royal Society of London B: Biological Sciences, 276, 2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, R. F. , Silva, J. F. , & Simões, J. M. (2011). Fighting zebrafish: Characterization of aggressive behavior and winner–loser effects. Zebrafish, 8, 73–81. [DOI] [PubMed] [Google Scholar]
- Oliveira, R. , & Gonçalves, D. (2008). Hormones and social behaviour of teleost fish. In Magnhagen C., Braithwaite V. A., & Forsgren E. (Eds.), Fish behaviour (pp. 61–125). New York, NY: CRC Press. [Google Scholar]
- Øverli, Ø. , Kotzian, S. , & Winberg, S. (2002). Effects of cortisol on aggression and locomotor activity in rainbow trout. Hormones and Behavior, 42, 53–61. [DOI] [PubMed] [Google Scholar]
- Parikh, V. N. , Clement, T. , & Fernald, R. D. (2006). Physiological consequences of social descent: Studies in Astatotilapia burtoni . Journal of Endocrinology, 190, 183–190. [DOI] [PubMed] [Google Scholar]
- Parker, G. A. (1974). Assessment strategy and the evolution of fighting behaviour. Journal of Theoretical Biology, 47, 223–243. [DOI] [PubMed] [Google Scholar]
- Patrão, M. T. , Silva, E. J. , & Avellar, M. C. (2009). Androgens and the male reproductive tract: An overview of classical roles and current perspectives. Arquivos Brasileiros de Endocrinologia & Metabologia, 53(8), 934–945. [DOI] [PubMed] [Google Scholar]
- Patricelli, G. (2010). Robotics in the study of animal behavior. In Choe J. (Ed.), Encyclopedia of animal behavior (pp. 91–99). London: Elsevier Academic Press. [Google Scholar]
- Peake, T. M. , & McGregor, P. K. (2004). Information and aggression in fishes. Learning & Behavior, 32, 114–121. [DOI] [PubMed] [Google Scholar]
- Peter, R. E. , Yu, K. ‐L. , Marchant, T. A. , & Rosenblum, P. M. (1990). Direct neural regulation of the teleost adenohypophysis. Journal of Experimental Zoology, 256, 84–89. [Google Scholar]
- Pitcher, T. J. (1993). In Pitcher T. J. (Ed.), Behaviour of teleost fishes (2nd ed.). London, UK: Chapman and Hall. [Google Scholar]
- Poling, K. R. , Fraser, E. J. , & Sorensen, P. W. (2001). The three steroidal components of the goldfish preovulatory pheromone signal evoke different behaviors in males. Comparative Biochemistry and Physiology ‐ B Biochemistry and Molecular Biology, 129, 645–651. [DOI] [PubMed] [Google Scholar]
- Ramos, A. , & Gonçalves, D. (2019). Artificial selection for male winners in the Siamese fighting fish Betta splendens correlates with high female aggression. Frontiers in Zoology, 16, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reebs, S. (2001). In Reebs S. (Ed.), Fish Behavior in the Aquarium and in the Wild. London, UK: Comstock Publishing Associates. [Google Scholar]
- Resink, J. W. , Schoonen, W. G. E. J. , Albers, P. C. H. , Filé, D. M. , Notenboom, C. D. , Hurk, R. V. D. , & Van Oordt, P. G. W. J. (1989). The chemical nature of sex attracting pheromones from the seminal vesicle of the African catfish, Clarias gariepinus . Aquaculture, 83, 137–151. [Google Scholar]
- Romano, D. , Benelli, G. , Donati, E. , Remorini, D. , Canale, A. , & Stefanini, C. (2017). Multiple cues produced by a robotic fish modulate aggressive behaviour in Siamese fighting fishes. Scientific Reports, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal, G. G. , Fitzsimmons, J. N. , Woods, K. U. , Gerlach, G. , Fisher, H. S. , U Woods, K. , … Fisher, H. S. (2011). Tactical release of a sexually‐selected pheromone in a swordtail fish. PLoS One, 6, e16994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo, N. , & Bass, A. H. (2006). New insights into neuropeptide modulation of aggression: Field studies of arginine vasotocin in a territorial tropical damselfish. Proceedings of the Royal Society B: Biological Sciences, 273, 3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva, J. L. , Keller‐Costa, T. , Hubbard, P. C. , Rato, A. , & Canário, A. V. M. M. (2017). Chemical diplomacy in male tilapia: Urinary signal increases sex hormone and decreases aggression. Scientific Reports, 7, 7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K. , & Suzuki, N. (2001). Whole‐cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urine in rainbow trout. Chemical Senses, 26, 1145–1156. [DOI] [PubMed] [Google Scholar]
- Sato, Y. , Miyasaka, N. , & Yoshihara, Y. (2005). Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. Journal of Neuroscience, 25, 4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y. , Miyasaka, N. , & Yoshihara, Y. (2007). Hierarchical regulation of odorant receptor gene choice and subsequent axonal projection of olfactory sensory neurons in zebrafish. The Journal of Neuroscience, 27, 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffel, A. , & Kramer, B. (2000). Electric signals in the social behavior of sympatric elephantfish (Mormyridae, Teleostei) from the upper Zambezi River. Die Naturwissenschaften, 87, 142–147. [DOI] [PubMed] [Google Scholar]
- Schmachtenberg, O. (2006). Histological and electrophysiological properties of crypt cells from the olfactory epithelium of the marine teleost Trachurus symmetricus . Journal of Comparative Neurology, 495, 113–121. [DOI] [PubMed] [Google Scholar]
- Scott‐Phillips, T. C. (2008). Defining biological communication. Journal of Evolutionary Biology, 21, 387–395. [DOI] [PubMed] [Google Scholar]
- Scott, A. M. , Zhang, Z. , Jia, L. , Li, K. , Zhang, Q. , Dexheimer, T. , … Li, W. (2019). Spermine in semen of male sea lamprey acts as a sex pheromone. PLoS Biology, 17, e3000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selset, R. , & Doving, K. B. (1980). Behaviour of mature anadromous char (Salmo alpinus L.) towards odorants produced by smolts of their own population. Acta Physiologica Scandinavica, 108, 113–122. [DOI] [PubMed] [Google Scholar]
- Semsar, K. , Kandel, F. L. , & Godwin, J. (2001). Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Hormones and Behavior, 40, 21–31. [DOI] [PubMed] [Google Scholar]
- Serizawa, S. , Miyamichi, K. , & Sakano, H. (2004). One neuron‐one receptor rule in the mouse olfactory system. TRENDS in Genetics, 20, 648–653. [DOI] [PubMed] [Google Scholar]
- Serrano, R. M. , Lopes, O. , Hubbard, P. C. , Araújo, J. , Canário, A. V. M. , & Barata, E. N. (2008a). 11‐ketotestosterone stimulates putative sex pheromone production in the male peacock blenny, Salaria pavo (Risso 1810). Biology of Reproduction, 79, 861–868. [DOI] [PubMed] [Google Scholar]
- Serrano, R. M. , Lopes, O. , Hubbard, P. C. , Araújo, J. , Canário, A. V. M. , & Barata, E. N. (2008b). Seasonal cell differentiation and olfactory potency of secretions by the anal glands of male peacock blenny Salaria pavo . Journal of Fish Biology, 73, 1790–1798. [Google Scholar]
- Siefkes, M. (2017). Use of physiological knowledge to control the invasive sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes. Conservation Physiology, 5, cox031[1]–cox031[18]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefkes, M. J. , Scott, A. P. , Zielinski, B. , Yun, S.‐S. , & Li, W. (2003). Male sea lampreys, Petromyzon marinus L., excrete a sex pheromone from gill epithelia. Biology of Reproduction, 69, 125–132. [DOI] [PubMed] [Google Scholar]
- Siegel, A. , Bhatt, S. , & Zalcman, S. (2009). Aggression: Neurochemical and molecular mechanisms. In Squire L. (Ed.), Encyclopedia of neuroscience (pp. 159–165). Oxford, UK: Academic Press. [Google Scholar]
- Simpson, M. J. A. (1968). The display of the Siamese fighting fish, Betta splendens . Animal Behaviour Monographs, 1, 1–73. [Google Scholar]
- Sloman, K. A. , Motherwell, G. , O'connor, K. I. , & Taylor, A. C. (2000). The effect of social stress on the standard metabolic rate (SMR) of brown trout, Salmo trutta . Fish Physiology and Biochemistry, 23, 49–53. [Google Scholar]
- Smith, B. R. , & Blumstein, D. T. (2008). Fitness consequences of personality: A meta‐analysis. Behavioral Ecology, 19, 448–455. [Google Scholar]
- Smith, J. , & Harper, D. (2003). Animal signals. Oxford, UK: Oxford University Press. [Google Scholar]
- Smith, R. J. F. (1970). Control of prespawning behaviour of sunfish (Lepomis gibbosus and Lepomis megalotis) II. Environmental factors. Animal Behaviour, 18, 575–587. [DOI] [PubMed] [Google Scholar]
- Sorensen, P. W. , Hoye, T. R. , & R. Hoye, T. (2010). Pheromones in vertebrates. In Liu H.‐W. & Lew M. (Eds.), Comprehensive natural products II: Chemistry and biology (pp. 225–262). Lodon: Elsevier Science. [Google Scholar]
- Sorensen, P. W. , Pinillos, M. , & Scott, A. P. (2005). Sexually mature male goldfish release large quantities of androstenedione into the water where it functions as a pheromone. General and Comparative Endocrinology, 140, 164–175. [DOI] [PubMed] [Google Scholar]
- Sorensen, P. W. , & Stacey, N. E. (1999). Advances in Chemical Signals in Vertebrates (pp. 15–47). London: Springer. [Google Scholar]
- Sorensen, P. W. , & Wisenden, B. D. (2015). Fish pheromones and related cues (1st ed.). Ames, IA: John Wiley & Sons, Inc. [Google Scholar]
- Spehr, M. , & Munger, S. D. (2009). Olfactory receptors: G protein‐coupled receptors and beyond. Journal of Neurochemistry, 109, 1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey, N. (2011). Hormonally derived sex pheromones in fishes. In Hormones and reproduction of vertebrates: Fishes (pp. 169–192). London: Elsevier. [Google Scholar]
- Stacey, N. , & Sorensen, P. (2009). 18‐ hormonal pheromones in fish. In Pfaff D. W., Arnold A. P., Etgen A. M., Fahrbach S. E., & Rubin R. T. (Eds.), Hormones, brain and behavior (pp. 639–682). San Diego, CA: Elsevier. [Google Scholar]
- Stevens, C. H. , Croft, D. P. , Paull, G. C. , & Tyler, C. R. (2017). Stress and welfare in ornamental fishes: What can be learned from aquaculture? Journal of Fish Biology, 91, 409–428. [DOI] [PubMed] [Google Scholar]
- Taborsky, M. (1994). Sneakers, satellites, and helpers ‐ parasitic and cooperative behavior cooperative in fish reproduction. In Slater M., Rosenblatt P. J. B., Snowdon J. S., & Milinski C. T. (Eds.), Advances in the study of behavior (pp. 1–100). San Diego, CA: Elsevier Academic Press Inc. [Google Scholar]
- Tan, L. , Li, Q. , & Xie, X. S. (2015). Olfactory sensory neurons transiently express multiple olfactory receptors during development. Molecular Systems Biology, 11, 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles, M. C. , Gozdowska, M. , Kalamarz‐Kubiak, H. , Kulczykowska, E. , & Oliveira, R. F. (2016). Agonistic interactions elicit rapid changes in brain nonapeptide levels in zebrafish. Hormones and Behavior, 84, 57–63. [DOI] [PubMed] [Google Scholar]
- Teles, M. , & Oliveira, R. F. (2016). Quantifying aggressive behavior in zebrafish. In Kawakami K., Patton E. E., & Orger M. (Eds.), Methods in molecular biology (pp. 293–305). New York, NY: Springer New York. [DOI] [PubMed] [Google Scholar]
- Thompson, R. R. , & Walton, J. C. (2004). Peptide effects on social behavior: Effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behavioral Neuroscience, 118, 620–626. [DOI] [PubMed] [Google Scholar]
- Tierney, K. B. , Baldwin, D. H. , Hara, T. J. , Ross, P. S. , Scholz, N. L. , & Kennedy, C. J. (2010). Olfactory toxicity in fishes. Aquatic Toxicology, 96, 2–26. [DOI] [PubMed] [Google Scholar]
- Toni, M. , Manciocco, A. , Angiulli, E. , Alleva, E. , Cioni, C. , & Malavasi, S. (2019). Review: Assessing fish welfare in research and aquaculture, with a focus on European directives. Animal, 13, 161–170. [DOI] [PubMed] [Google Scholar]
- Torrezani, C. S. , Ferreira Pinho‐Neto, C. , Miyai, C. , Sanches, F. , & Barreto, R. (2013). Structural enrichment reduces aggression in Tilapia rendalli . Marine and Freshwater Behaviour and Physiology, 46, 183–190. [Google Scholar]
- Tricas, T. C. , & Carlson, B. A. (2012). Electroreceptors and magnetoreceptors. In Sperelakis N. (Ed.), Cell physiology source book (pp. 705–725). San Diego, CA: Academic Press. [Google Scholar]
- Unger, L. M. (1983). Nest defense by deceit in the fathead minnow, Pimephales promelas . Behavioral Ecology and Sociobiology, 13, 125–130. [Google Scholar]
- VanHook, A. M. (2017). An adenosine receptor for olfaction. Science Signaling, 10, 1437, –1447.e4. [DOI] [PubMed] [Google Scholar]
- Vassar, R. , Chao, S. K. , Sitcheran, R. , Nuñez, J. M. , Vosshall, L. B. , & Axel, R. (1994). Topographic organization of sensory projections to the olfactory bulb. Cell, 79, 981–991. [DOI] [PubMed] [Google Scholar]
- Vielma, A. , Ardiles, A. , Delgado, L. , & Schmachtenberg, O. (2008). The elusive crypt olfactory receptor neuron: Evidence for its stimulation by amino acids and cAMP pathway agonists. Journal of Experimental Biology, 211, 2417–2422. [DOI] [PubMed] [Google Scholar]
- Villars, T. A. (1983). Hormones and aggressive behavior in teleost fishes. In Svare B. B. (Ed.), Hormones and aggressive behavior (pp. 407–433). Boston, MA: Springer. [Google Scholar]
- Vu, T. D. , Iwasaki, Y. , Shigenobu, S. , Maruko, A. , Oshima, K. , Iioka, E. , … Okada, N. (2020). Behavioral and brain‐transcriptomic synchronization between the two opponents of a fighting pair of the fish Betta splendens . PLoS Genetics, 16, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waas, J. R. , & Colgan, P. W. (1992). Chemical cues associated with visually elaborate aggressive displays of 3‐spine sticklebacks. Journal of Chemical Ecology, 18, 2277–2284. [DOI] [PubMed] [Google Scholar]
- Wallen, K. , & Wojciechowski‐Metzlar, C. I. (1985). Social conditioning and dominance in male Betta splendens . Behavioural Processes, 11, 181–188. [DOI] [PubMed] [Google Scholar]
- Way, G. , Ruhl, N. , Snekser, J. , Kiesel, A. , & Mcrobert, S. (2015). A comparison of methodologies to test aggression in zebrafish. Zebrafish, 12, 144–151. [DOI] [PubMed] [Google Scholar]
- Way, G. P. , Southwell, M. , & McRobert, S. P. (2016). Boldness, aggression, and shoaling assays for zebrafish behavioral syndromes. Journal of Visualized Experiments, 2016, 54049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer, G. (1997). Effects of rearing conditions on the health and physiological quality of fish in intensive culture. In Iwama G., Pickering A., Sumpter J., & Schreck C. (Eds.), Fish stress and health in aquaculture (pp. 35–71). Cambridge, MA: Cambridge University Press. [Google Scholar]
- Weiss, C. S. , & Coughlin, J. P. (1979). Maintained aggressive behavior in gonadectomized male Siamese fighting fish (Betta splendens). Physiology & Behavior, 23, 173–177. [DOI] [PubMed] [Google Scholar]
- Westby, G. W. M. (1975). Further analysis of the individual discharge characteristics predicting social dominance in the electric fish, Gymnotus carapo . Animal Behaviour, 23, 249–260. [DOI] [PubMed] [Google Scholar]
- Wingfield, J. C. , Hegner, R. E. , Dufty, A. M. , & Ball, G. F. (1990). The ‘challenge hypothesis’: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist, 136, 829–846. [Google Scholar]
- Woodward, M. A. , Winder, L. A. , & Watt, P. J. (2019). Enrichment increases aggression in zebrafish. Fishes, 4.1–13. [Google Scholar]
- Wuertz, S. (1997). The significance of pheromones for fish and shellfish farming. Aquaculture Ireland, 77, 19–21. [Google Scholar]
- Wyatt, T. (2014). Pheromones and animal behavior: Chemical signals and signatures (2nd ed.). Cambridge, MA: Cambridge University Press. [Google Scholar]
- Yang, L. , Liu, Y. , Yu, H. , Fang, X. , Song, L. , Li, D. , & Chen, Y. (2020). Computer vision models in intelligent aquaculture with emphasis on fish detection and behavior analysis: A review. Archives of Computational Methods in Engineering, 1, 3. [Google Scholar]
- Yoshihara, Y. (2009). Molecular genetic dissection of the zebrafish olfactory system. In Korsching S. & Meyerhof W. (Eds.), Chemosensory systems in mammals, fishes, and insects (pp. 1–19). Berlin, Germany: Springer Berlin Heidelberg. [Google Scholar]
- Zakon, H. , Oestreich, J. , Tallarovic, S. , & Triefenbach, F. (2002). EOD modulations of brown ghost electric fish: JARs, chirps, rises, and dips. Journal of Physiology Paris, 96, 451–458. [DOI] [PubMed] [Google Scholar]
- Zupanc, G. K. H. (2002). From oscillators to modulators: Behavioral and neural control of modulations of the electric organ discharge in the gymnotiform fish, Apteronotus leptorhynchus . Journal of Physiology‐Paris, 96, 459–472. [DOI] [PubMed] [Google Scholar]


