Abstract
Objective
To assess the efficacy of medication review as an isolated intervention and with several co‐interventions for preventing hospital readmissions in older adults.
Methods
Ovid MEDLINE, Embase, The Cochrane Central Register of Controlled Trials and CINAHL were searched for randomized controlled trials evaluating the effectiveness of medication review interventions with or without co‐interventions to prevent hospital readmissions in hospitalized or recently discharged adults aged ≥65, until September 13, 2019. Included outcomes were “at least one all‐cause hospital readmission within 30 days and at any time after discharge from the index admission.”
Results
Twenty‐five studies met the inclusion criteria. Of these, 11 studies (7,318 participants) contributed to the network meta‐analysis (NMA) on all‐cause hospital readmission within 30 days. Medication review in combination with (a) medication reconciliation and patient education (risk ratio (RR) 0.45; 95% confidence interval (CI) 0.26–0.80) and (b) medication reconciliation, patient education, professional education and transitional care (RR 0.64; 95% CI 0.49–0.84) were associated with a lower risk of all‐cause hospital readmission compared to usual care. Medication review in isolation did not significantly influence hospital readmissions (RR 1.06; 95% CI 0.45–2.51). The NMA on all‐cause hospital readmission at any time included 24 studies (11,677 participants). Medication review combined with medication reconciliation, patient education, professional education and transitional care resulted in a reduction of hospital readmissions (RR 0.82; 95% CI 0.74–0.91) compared to usual care. The quality of the studies included in this systematic review raised some concerns, mainly regarding allocation concealment, blinding and contamination.
Conclusion
Medication review in combination with medication reconciliation, patient education, professional education and transitional care, was associated with a lower risk of hospital readmissions compared to usual care. An effect of medication review without co‐interventions was not demonstrated. Trials of higher quality are needed in this field.
Keywords: hospital readmission, medication review, older adults
Key Points
A medication review in combination with medication reconciliation, patient education, professional education and transitional care is associated with a decreased risk of hospital readmissions.
An effect of medication review as an isolated intervention was not demonstrated.
Why Does this Paper Matter?
A medication review can be implemented in isolation or in combination with co‐interventions, such as medication reconciliation or training of healthcare professionals. It is unclear whether a medication review alone or in combination with co‐interventions, effectively prevents hospital readmissions.
1. INTRODUCTION
Hospitalizations can have detrimental effects on older patient outcomes. 1 , 2 Following hospitalization, older adults are at risk for complications like delirium, falls, functional decline and subsequent institutionalization or readmission. 1 , 2 Medication related readmissions occur frequently, particularly in older adults. 3
Improving medication appropriateness may reduce medication related problems and the number of hospital readmissions. Medication appropriateness is present when therapeutic objectives are being achieved or there is a reasonable chance they will be achieved and the benefits of the medication outweigh the risks for an individual patient. 4 Relevant systematic reviews often recommend a medication review to improve the quality of prescriptions in older patients. 5 , 6 , 7 , 8 Christensen et al conducted a Cochrane review assessing the effect of medication review in hospitalized patients. 9 A medication review is an intervention which can be implemented in isolation or in combination with one or more co‐interventions. Co‐interventions, i.e., medication reconciliation, education of patients/healthcare professionals, use of Computerized Decision Support tool, prescribing criteria like START/STOPP criteria 10 or the Beers' criteria, 11 complement or structure the basic critical evaluation of a patient's medication, and may all have a different effects on hospital readmissions.
Frequently the terms for medication review and co‐interventions as listed previously are erroneously used interchangeably. Thus, leading to substantial heterogeneity. Medication related problems frequently occur on transition from one health care setting to another. 12 , 13 However, Christensen et al excluded studies where medication review recommendations were implemented after discharge.
An internationally accepted standardized approach for implementing medication reviews in research and clinical settings is lacking. It is unclear whether a medication review alone or in combination with co‐interventions, effectively prevents hospital readmissions. Published data are conflicting, possibly due to the heterogeneity of the interventions evaluated and the timing of execution. 5 , 6 , 14 , 15 , 16 , 17 To address this, we categorized all medication review interventions by the presence of associated co‐interventions. A network meta‐analysis (NMA) permitted the synthesis of relative effects from studies comparing competing interventions, even if these interventions were not directly compared to each other in the literature. 18 , 19 We included studies where the intervention was implemented during admission or within 2 weeks of discharge.
The aim of this systematic review and NMA was to determine and compare the impact of medication review in isolation or with co‐interventions, during hospitalization or within 2 weeks of discharge, on hospital readmissions.
2. METHODS
2.1. Protocol
The study protocol was registered online (PROSPERO, registration number CRD42020150799).
2.2. Study identification
Replicating the search strategy of Christensen et al 9 we searched online repositories Ovid MEDLINE, Embase, The Cochrane Central Register of Controlled Trials and CINAHL from January 1 2014 to September 13 2019, without language restriction (Tables S11–S14). Original studies of Christensen et al were identified via reference lists and rescreened. Bibliographical hand searches of relevant systematic reviews were also conducted. 7 , 8 , 9 , 15 , 17 , 21 , 22 , 23 , 24 , 25 , 26 , 27
2.3. Eligibility criteria
Controlled trials (randomized, quasi and cluster) evaluating the effectiveness of medication review interventions with or without co‐interventions to prevent hospital readmissions in adults aged 65 years and older were included. Participants were hospitalized or recently discharged (the medication review was conducted within 2 weeks of discharge) to the community, nursing home or rehabilitation center. Comparison treatments were usual care, a sham intervention or another version of a medication review intervention.
Included outcomes were (i) at least one all‐cause hospital readmission, (ii) at least one medication‐related readmission at any time, and (iii) all‐cause hospital readmission rate. Details of the study population, interventions, comparators and outcomes are described in Table S10.
2.4. Study selection
Study selection was performed by two researchers (LD and LB). A supervised test screening (RJPMS) of application of inclusion criteria was conducted prior to the start of the title/abstract screening phase and the full text screening phase to ensure consistency, i.e., 50 studies with 98% agreement between researchers. Each researcher independently screened half of the titles and abstracts identified by the systematic search. Each researcher subsequently independently screened half of the included full texts for inclusion. Uncertainties were resolved by discussion or by involvement of a third author (WK, RJPMS, HLK). Several publications from the same patient cohort were considered as one study with one or more companion reports.
2.5. Data extraction
One researcher (LD) extracted all descriptive and outcome data. Outcome data were verified by a second researcher (LB). Conflicts were discussed and resolved by the two researchers. For every included study data was extracted by means of a bespoke report‐form capturing study design, population characteristics, intervention characteristics and reported outcomes (Table S15).
Reported interventions were categorized into nine intervention components: medication review, medication reconciliation, shared decision making, patient education/ medication counseling, health professional education, use of validated methods, use of Computerized Decision Support, compliance aid and transitional care (Tables 1 and S1). The list of the nine intervention components was developed during the preparation of the study protocol. The list is based on medication review interventions in previously published RCTs and clinical experience of several pharmacologists among the co‐authors. One researcher (LD) categorized the interventions of the included studies by this list. A second researcher (HLK) blindly evaluated this categorization for a random sample of included studies (n = 10, 40%), and there was a 90% overlap. Inconsistencies were solved during a consensus meeting. Information about the co‐interventions found in the included studies is presented in Table S2.
TABLE 1.
Intervention components to prevent hospital readmissions
| Intervention component | Abbreviation |
|---|---|
| Medication review | Mdrev |
| Medication reconciliation | Mdrec |
| Shared decision making | Sdm |
| Patient education/medication counseling | Pedu |
| Health professional education | Hpedu |
| Use of validated methods | Vm |
| Use of Computerized Decision Support | Cds |
| Compliance aid | Ca |
| Transitional care | Tc |
2.6. Risk of bias assessment
The risk of bias assessment was performed using the Effective Practice and Organization of Care (EPOC) version of Cochrane's Risk of Bias tool. 20 The risk of bias assessment was performed by one researcher (LD) and verified by a second researcher (LB). Discrepancies were discussed and resolved.
2.7. Data analysis
When the number of included studies was sufficient (i.e., less interventions than studies providing data), we performed random‐effects NMA for each of the aforementioned outcomes using the netmeta command in R Statistical Software (Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013). 18 , 19 , 21 , 22 After categorization of the interventions of included studies (Table 1), many studies turned out to consist of multi‐component interventions (e.g., medrev + medrec + pedu). Therefore, as per protocol, we additionally analyzed the effect of the (combination of) components, also known as component NMA (CNMA) (for details: see Supplement, additional information regarding NMA). 23
We calculated risk ratios (RRs) along with their 95% confidence intervals (CIs) for each intervention versus usual care. For each outcome we used P‐scores to rank intervention effects. 23 P‐scores measure the certainty that an intervention is better than the competing interventions of the network, and take values between 0 and 1; the higher the P‐score, the more beneficial the intervention.
We did not check for inconsistency, i.e., the occurrence of conflicting direct and indirect evidence, as for none of the interventions versus usual care there was both direct and indirect evidence available. We assessed transitivity clinically. 18
Further subgroup analyses (participants aged ≥75 years, multi‐morbid participants and nursing home residents) were not feasible due to the low number of studies identified.
2.8. Confidence in the NMA results
We evaluated the credibility of the NMA results using the CINeMA approach. 24 CINeMA is an online web application, considering six domains (within‐study bias, across‐study bias, indirectness, imprecision, heterogeneity, and incoherence) to judge the confidence on NMA results. For each treatment comparison we rated the corresponding treatment effect on each of the aforementioned six domains as either “no concerns”, “minor concerns,” or “major concerns” (for details: see online Supplement, additional information regarding the CINeMA approach).
3. RESULTS
3.1. Study selection
We identified 4,045 studies through database search. Figure S1. illustrates study identification and selection. Five additional RCTs were identified, two 25 , 26 from the list of excluded studies of Christensen et al and three 27 , 28 , 29 from screening reference lists of 12 relevant systematic reviews. After screening, 25 studies 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 and one companion report 50 were included in the final analysis.
3.2. Study and participant characteristics
An executive summary of included study and participant characteristics is presented in Table 2. (detailed individual study description is available in Tables S2 and S3). In 12 studies (48%) 26 , 28 , 31 , 33 , 36 , 37 , 38 , 43 , 44 , 45 , 48 , 49 , the mean/median age was 75–84 years and in four studies (16%) ≥85 years. 25 , 29 , 32 , 34 In the majority of the studies, at least half of the study population was female (n = 19, 76%). 25 , 26 , 27 , 28 , 29 , 31 , 32 , 33 , 35 , 36 , 37 , 38 , 39 , 40 , 43 , 44 , 45 , 48 , 49
TABLE 2.
Summary of participant and study characteristics of the 25 included randomized controlled trials
| Participant or study characteristic | Number of studies (%) | Citation number for each study in each row |
|---|---|---|
| Mean/median age (years) | ||
| 65–74 | 9 (36%) | 27, 30, 35, 39, 40, 41, 42, 46, 47 |
| 75–84 | 12 (48%) | 26, 28, 31, 33, 36, 37, 38, 43, 44, 45, 48, 49 |
| ≥85 | 4 (16%) | 25, 29, 32, 34 |
| Female (%) | ||
| 25–49 | 5 (20%) | 30, 34, 42, 46, 47 |
| 50–74 | 19 (76%) | 25, 26, 27, 28, 29, 31, 32, 33, 35, 36, 37, 38, 39, 40, 43, 44, 45, 48, 49 |
| Not reported | 1 (4%) | 41 |
| Year of publication | ||
| 2000–2004 | 1a (4%) | 28 |
| 2005–2009 | 5a (20%) | 26, 27, 29, 32, 41 |
| 2010–2014 | 3a (12%) | 25, 38, 45 |
| 2015–2019 | 16 (64%) | 30, 31, 33, 34, 35, 36, 37, 39, 40, 42, 43, 44, 46, 47, 48, 49 |
| Continent | ||
| Europe | 16 (64%) | 25, 26, 27, 29, 30, 32, 37, 38, 41, 42, 43, 44, 45, 46, 48, 49 |
| North America | 6 (24%) | 30, 31, 34, 35, 42, 49 |
| Australia/New Zealand | 1 (4%) | 28 |
| South America | 1 (4%) | 46 |
| Asia | 1(4%) | 48 |
| Study design | ||
| Parallel | 19 (76%) | 25, 27, 28, 29, 31, 32, 33, 34, 36, 37, 38, 39, 40, 41, 42, 45, 46, 47, 49 |
| Quasi randomized | 4 (16%) | 26, 35, 44, 48 |
| Cluster | 2 (8%) | 30, 43 |
| Site | ||
| Single center | 17 (68%) | 26, 27, 28, 30, 31, 32, 34, 36, 37, 38, 42, 44, 45, 46, 47, 48, 49 |
| Multicenter | 8 (32%) | 25, 29, 33, 35, 39, 40, 41, 43 |
| Setting | ||
| Hospital | 21 (84%) | 25, 26, 27, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 44, 45, 46, 47, 48, 49 |
| Community | 3 (12%) | 28, 29, 42 |
| Community pharmacy | 1 (4%) | 43 |
| Duration of follow‐up (weeks) | ||
| 0–4 | 7 (28%) | 30, 34, 35, 44, 46, 47, 49 |
| 5–12 | 6 (24%) | 28, 31, 37, 38, 42, 48 |
| 13–26 | 7 (28%) | 25, 27, 29, 33, 40, 43, 45 |
| 27–52 | 5 (20%) | 26, 32, 36, 39, 41 |
| Sample size | ||
| <100 | 4 (16%) | 34, 36, 37, 38 |
| 100–499 | 16 (64%) | 26, 27, 28, 30, 31, 32, 33, 39, 42, 43, 44, 45, 46, 47, 48, 49 |
| 500–999 | 3 (12%) | 25, 29, 41 |
| ≥1,000 | 2 (8%) | 35, 40 |
| Regular used medication, mean/median number | ||
| 0–5 | 0 | |
| 6–10 | 18 (72%) | 25, 26, 27, 28, 29, 30, 32, 33, 35, 37, 38, 39, 40, 43, 44, 46, 47, 48 |
| 11–15 | 1 (4%) | 42 |
| >15 | 2 (8%) | 34, 36 |
| Not reported | 4 (16%) | 31, 41, 45, 49 |
| Chronic conditions, mean/median number | ||
| 0–5 | 4 (16%) | 25, 28, 43, 46 |
| Not reported | 21 (84%) | 26, 27, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 44, 45, 47, 48, 49 |
These studies were identified from screening the reference list of relevant systematic reviews and the list of included and excluded studies by Christensen et al. The studies identified through database search were all published after 2014.
In four studies (16%), 28 , 29 , 42 , 43 the intervention was community based within 2 weeks of discharge. The duration of follow‐up of all studies varied from 4 weeks to 1 year. Study size ranged from 22 to 4,049, over half (n = 21, 84%) of included studies had a study population ≥ 100. 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 35 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 The mean/median number of regularly used medication was at least 6 and in most studies ranged between 6 and 10 (n = 18, 72%). 25 , 26 , 27 , 28 , 29 , 30 , 32 , 33 , 35 , 37 , 38 , 39 , 40 , 43 , 44 , 46 , 47 , 48
A summary of the characteristics of the medication review interventions is provided in Table S5.
3.3. Risk of bias of included studies
Individual risk of bias assessments of included studies are presented in Table S4 and the aggregate risk of bias assessment per domain in Figure S2.
Most studies had a low risk of bias for the domains “random sequence generation” (n = 19, 76%) 25 , 27 , 28 , 29 , 31 , 32 , 33 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 45 , 46 , 47 , 49 , “similarity of baseline characteristics” (n = 24, 96%) 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 and “other bias” (n = 20, 80%). 25 , 26 , 27 , 29 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 42 , 44 , 45 , 46 , 47 , 48 Allocation concealment was adequately performed in one third, inadequately in one third and unclear in the remaining third of the trials. In 76% of the studies, no information was reported at baseline on hospital (re)admissions in the preceding months (n = 19). 27 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 42 , 43 , 44 , 45 , 46 , 47 , 48 Blinding of participants or personnel was not performed in the majority of studies. Blinded outcome assessment was performed in 17 studies (68%). 25 , 26 , 28 , 29 , 30 , 32 , 33 , 34 , 35 , 36 , 37 , 39 , 40 , 41 , 45 , 46 , 47 In 19 studies (76%) there was a high risk of bias for contamination. 25 , 26 , 27 , 28 , 29 , 31 , 32 , 33 , 34 , 36 , 37 , 38 , 39 , 40 , 42 , 45 , 47 , 48 , 49 For the domains “incomplete outcome data” (n = 16, 64%) 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 40 , 42 , 47 and “selective outcome reporting” (n = 13, 52%), 26 , 30 , 31 , 32 , 33 , 36 , 37 , 39 , 40 , 42 , 44 , 45 , 49 more than half of the studies scored a low risk of bias.
3.4. Network meta‐analysis
NMA was performed for the outcomes “all‐cause hospital readmissions within 30 days” and “all‐cause hospital readmissions at any time.” There was insufficient reported data to perform a NMA for other outcomes as the number of interventions evaluated was higher than the number of studies providing data.
3.4.1. All‐cause hospital readmissions within 30 days
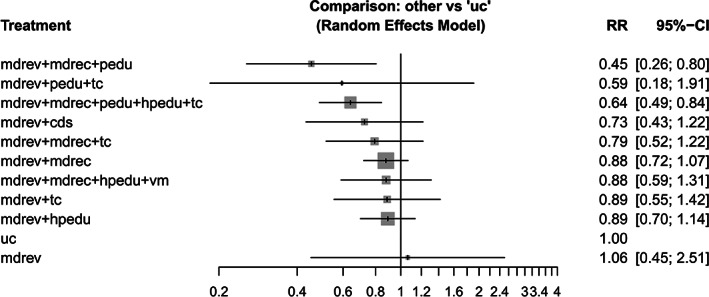
For the outcome “at least one all‐cause hospital readmission within 30 days after discharge,” the NMA included 11 studies (7,318 participants) 30 , 31 , 33 , 34 , 35 , 40 , 44 , 46 , 47 , 48 , 49 and 10 interventions that were all compared with usual care (Figure S3). Each intervention was directly compared to usual care, except for the medication review intervention without any co‐interventions, for which only indirect evidence was present. The RRs and 95% CIs for every intervention versus usual care, resulting from the primary analysis in which each existing combination of components was analyzed as a distinct intervention, are presented in Table 3 and Figure 1.
TABLE 3.
Risk ratios with 95% confidence intervals (95% CIs), P‐scores and CINeMA confidence ratings for the interventions versus usual care for the outcomes all‐cause hospital readmissions within 30 days and all‐cause hospital readmissions at any time
| Intervention | Studies (N) | Participants (N) | Risk ratio (95% CI) | P‐score | Confidence rating, all domains a | Confidence rating, four remaining domains a |
|---|---|---|---|---|---|---|
| All‐cause hospital readmissions within 30 days | ||||||
| mdrev + mdrec + pedu | 1 | 207 | 0.45 (0.26–0.80) | 0.92 | Low b , c | Moderate d , e |
| mdrev + pedu + tc | 1 | 104 | 0.59 (0.18–1.91) | 0.67 | Low b , c | Moderate e , f |
| mdrev + mdrec + pedu + hpedu + tc | 1 | 1,467 | 0.64 (0.49–0.84) | 0.76 | Low b , c | Moderate d , e |
| mdrev + cds | 1 | 254 | 0.73 (0.43–1.22) | 0.61 | Low b , c | Moderate d , e , f |
| mdrev + mdrec + tc | 1 | 429 | 0.79 (0.52–1.22) | 0.52 | Low b , c | Low d , e , f , g |
| mdrev + mdrec | 2 | 4,201 | 0.88 (0.72–1.07) | 0.40 | Low b , c | Moderate d , e , f |
| mdrev + mdrec + hpedu + vm | 1 | 166 | 0.88 (0.59–1.31) | 0.40 | Low b , c | Moderate e , f |
| mdrev + tc | 2 | 380 | 0.89 (0.55–1.42) | 0.39 | Low b , c | Moderate e , f |
| mdrev + hpedu | 1 | 1,467 | 0.89 (0.70–1.14) | 0.37 | Low b , c | Moderate d , e , f |
| mdrev | 0 | NA | 1.06 (0.45–2.51) | 0.26 | Low b , c | Moderate d , f , g |
| All‐cause hospital readmissions at any time | ||||||
| mdrev + pedu + tc | 1 | 104 | 0.59 (0.18–1.91) | 0.78 | Low b , c | Moderate f |
| mdrev + pedu + mdt + tc | 1 | 121 | 0.62 (0.38–1.02) | 0.90 | Low b , c | High |
| mdrev + mdrec + pedu | 1 | 207 | 0.76 (0.55–1.04) | 0.80 | Low b , c | High |
| mdrev + mdrec + pedu + hpedu + tc | 2 | 2,229 | 0.82 (0.74–0.91) | 0.77 | Low b , c | High |
| mdrev + mdrec + hpedu + vm | 1 | 166 | 0.88 (0.59–1.31) | 0.61 | Low b , c | Moderate f |
| mdrev + tc | 2 | 380 | 0.89 (0.55–1.42) | 0.59 | Low b , c | Moderate f |
| mdrev + mdrec + pedu + tc | 3 | 1,205 | 0.91 (0.79–1.04) | 0.60 | Low b , c | High |
| mdrev + mdrec | 5 | 4,708 | 0.92 (0.82–1.05) | 0.56 | Low b , c | High |
| mdrev + mdrec + tc | 1 | 429 | 0.94 (0.74–1.19) | 0.52 | Low b , c | Moderate f , g |
| mdrev + hpedu | 1 | 1,467 | 0.97 (0.86–1.10) | 0.46 | Low b , c | High |
| mdrev + mdrec + pedu + hpedu | 1 | 141 | 1.01 (0.58–1.76) | 0.44 | Low b , c | Moderate f , g |
| mdrev + pedu + cds + tc | 1 | 345 | 1.02 (0.82–1.26) | 0.39 | Low b , c | Moderate d , f |
| mdrev + cds | 2 | 554 | 1.02 (0.79–1.31) | 0.40 | Low b , c | Moderate f |
| mdrev + pedu + hpedu + ca + tc | 1 | 855 | 1.22 (1.01–1.46) | 0.17 | Low b , c | High |
| mdrev | 0 | NA | 1.50 (0.84–2.69) | 0.13 | Low b , c | Moderate d , f , g |
| mdrev + mdrec + pedu + hpedu + vm + tc | 1 | 123 | 2.22 (1.29–3.83) | 0.02 | Low b , c | High |
Abbreviations: mdrev, medication review; mdrec, medication reconciliation; pedu, patient education/medication counseling; hpedu, health professional education; vm, use of validated methods; cds, use of Computerized Decision Support; ca, compliance aid; tc, transitional care.
The result of the assessment for the domains “within‐study bias” and “reporting bias” was the same for every comparison, i.e., major concerns for “within‐study bias” and “reporting bias” was suspected (first column). To maintain a distinctive character, the remaining four of the six planned domains were taken into account, i.e. “indirectness,” “imprecision,” “heterogeneity,” and “incoherence” (second column).
Within‐study bias.
Reporting bias.
Heterogeneity.
Incoherence.
Imprecision.
Indirectness.
FIGURE 1.
Summary risk ratios (RRs) with 95% confidence intervals (95% CIs) resulting from the primary network meta‐analysis for every intervention consisting of one or more components versus usual care for the outcome all‐cause hospital readmissions within 30 days, including 11 studies. Abbreviations: mdrev, medication review; mdrec, medication reconciliation; pedu, patient education/medication counseling; hpedu, health professional education; vm, use of validated methods; cds, use of Computerized Decision Support; ca, compliance aid; tc, transitional care
Two interventions were associated with a statistically significant decrease in hospital readmissions: (a) medication review in combination with medication reconciliation and patient education (RR 0.45; 95% CI 0.26–0.80; P‐score 0.92) and (b) medication review in combination with medication reconciliation, patient education, professional education and transitional care (RR 0.64; 95% CI 0.49–0.84; P‐score 0.76).
Analysis of effects of single components (Table S7) showed that patient education significantly reduced hospital readmissions (RR 0.64; 95% CI 0.41–0.99).
Analysis of effects of the compound interventions (interventions rebuilt by adding up the separate effects of the components) (Table S6) demonstrated a statistically significant effect for (a) medication review in combination with medication reconciliation and patient education (RR 0.54; 95% CI 0.35–0.85) and (b) medication review in combination with medication reconciliation, patient education, professional education and transitional care (RR 0.63; 95% CI 0.48–0.82).
3.4.2. All‐cause hospital readmissions at any time
For the outcome “at least one all‐cause hospital readmission at any time,” the NMA included 24 studies (11,677 participants) 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 and 17 interventions (Figure S4). All interventions consisting of multiple components were compared with usual care. There was no direct evidence for medication review without any co‐interventions versus usual care. Table 3 and Figure 2 present the RRs for each intervention versus usual care from the primary analysis. The combination of medication review, medication reconciliation, patient education, professional education and transitional care, was associated with a statistically significant reduction of hospital readmission at any time (RR 0.82; 95% CI 0.74–0.91; P‐score 0.77).
FIGURE 2.
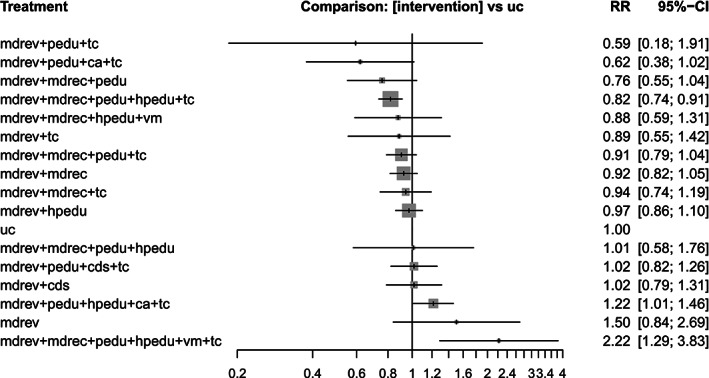
Summary risk ratios (RRs) with 95% confidence intervals (95% CIs) resulting from the primary network meta‐analysis for every intervention consisting of one or more components versus usual care for the outcome all‐cause hospital readmissions at any time, including 24 studies. Abbreviations: mdrev, medication review; mdrec, medication reconciliation; pedu, patient education/medication counseling; hpedu, health professional education; vm, use of validated methods; cds, use of Computerized Decision Support; ca, compliance aid; tc, transitional care
Two interventions were associated with a statistically significant increase of hospital readmissions: (a) medication review in combination with patient education, professional education, compliance aid and transitional care (RR 1.22; 95% CI 1.01–1.46; P‐score 0.17) and (b) medication review in combination with medication reconciliation, patient education, professional education, use of validated methods and transitional care (RR 2.22; 95% CI 1.29–3.83; P‐score 0.02).
For the separate components and for the compound interventions, there were no statistically significant effects on hospital readmissions (Tables S8 and S9).
We performed an additional pairwise meta‐analysis for those studies in which medication review was performed after discharge, to compare the effect of a medication review in general with usual care. NMA was not possible due to the limited number of studies (n = 4). Medication review had no statistically significant effect on hospital readmissions at any time, when compared to usual care (RR 1.14; 95% CI 0.75–1.74).
3.5. Confidence in the NMA results
For all comparisons, major concerns for “within‐study bias” and “reporting bias” were present, mainly due to lack of blinding of personnel and participants (which is the result of the nature of the intervention) and due to the fact that there are no established statistical methods to explore reporting bias, respectively, resulting in low overall confidence in all effects. To allow for discrimination based on the other domains, we also assessed the confidence rating for every comparison by only taking into account the ratings for the remaining four domains. Table 3 presents these confidence ratings for every intervention versus usual care, along with the reason(s) for downgrading. Based on these four domains only, the confidence in the NMA results was moderate to high for the majority of the comparisons.
4. DISCUSSION
This systematic review and NMA updated current literature on the effect of different medication review interventions during hospital admission and transition of care, on prevention of hospital readmissions in participants aged 65 years and older. 9 Medication review in combination with medication reconciliation and patient education was associated with a significant reduction of all‐cause hospital readmissions within 30 days. This also applied for medication review in combination with medication reconciliation, patient education, professional education and transitional care. Medication review as an isolated intervention had no significant effect on hospital readmissions. This comparison was based on the NMA with indirect evidence. In this review, most studies compared active interventions to usual care, resulting in most effect estimates being informed either by direct or indirect evidence. Hence, most effect estimates are imprecise. This was evidenced both by the wide 95% CI and the CINeMA analysis where non‐significant effect estimates extended to clinically relevant regions (RR < 0.8 or RR > 1.25). In the CNMA, the RR for medication review as an isolated intervention was also not statistically significant. For the outcome “at least one all‐cause hospital readmission at any time,” medication review in combination with medication reconciliation, patient education, professional education and transitional care was associated with a statistically significant reduction, although the risk reduction was less pronounced than for hospital readmissions 30 days after discharge. An effect of medication review as an isolated intervention or performed after discharge was not demonstrated.
A number of previous studies have highlighted the importance of co‐interventions. 7 , 12 , 15 , 40 Multifaceted programs including a medication review, medication reconciliation, patient counseling and follow‐up by primary care physician, pharmacists, and nursing home physicians, reduced the risk of hospital readmissions. 12 , 40 A previous meta‐analysis on the effectiveness of medication review as an isolated short‐term intervention also found no effect on hospital admissions. 5
The two combinations of intervention components that were associated with a statistically significant increase of hospital readmissions at any time, were both investigated by one study each, with a high summary risk of bias and were directly compared to usual care. 29 , 43 In these two studies, possible explanations for this unexpected finding were (a) an increase of help seeking behavior after disease‐specific education from the pharmacist leading to better recognition of warning signs, (b) more adverse events as a result of improved compliance, (c) study‐related involvement in medication management may have increased the complexity of care causing anxiety, confusion or dependence on health services, or (d) chance (type I error). 29 , 43
A strength of this study is the application of standard NMA as well as CNMA, in which we determined the effect of both the individual intervention components and the combinations of these components. Medication review is a very heterogeneous strategy and we investigated which particular combination of components of a medication review was most effective. An additional advantage of NMA is the ranking of interventions according to their effectiveness using P‐scores.
Another strength is that we focused both on studies in which the intervention was implemented during admission and studies that applied the intervention within 2 weeks of discharge. By including the latter studies, the medication review was performed at a time period in which the risk of medication related harm or medication errors is expected to be the highest (i.e., during transition of care). 12 , 13
This study has some limitations relating to the studies we included. The quality of the included studies raised some concerns. In two third of the studies, the risk of bias for allocation concealment was high or unclear. However, baseline characteristics were similar between study arms in all but one study, 33 indicating that randomization worked well. Although blinding of participants or personnel was not possible due to the nature of the interventions, blinded outcome assessment was performed in 68% of the studies. In addition, the outcome of hospital readmissions is a fairly objective outcome as often data on hospital readmissions was extracted from national registers. We also noted a high risk of bias for contamination in 76% of the studies: the pharmacist's recommendations in the intervention group probably have led to a learning effect for the prescriber, and this may have also influenced the way the prescriber managed the medication in the control group. This type of bias, however, may have resulted in an underestimation of the intervention effect in those studies.
Also, we found that the majority of the studies did not report on mean number of chronic conditions, medication appropriateness and number of recommendations following the medication review, while this information may give an indication of the potential effect of the medication review. The use of different inclusion criteria regarding polypharmacy, use of a specific drug class and comorbidities, might also impact the effect of the intervention.
There were some limitations to the review process. The outcome all‐cause hospital readmissions at any time is inherently heterogeneous. We accepted any time point for hospital readmissions which ranged from 1 month to 1 year after intervention. This heterogeneity may explain why the effect of medication review interventions was more pronounced for the outcome hospital readmissions within 30 days than readmissions at any time.
The evaluation of many (combinations of) intervention components permitted identification of the most effective combination. However, this also may have decreased the power of the analyses, due to the large number of components relatively to the low number of studies. A second reason for a reduced power of the analyses is the previously mentioned fact that most effect estimates were informed either by direct or indirect evidence.
The results of this study showed that it is not the medication review in itself, medication reconciliation, patient education, healthcare professional education and transitional care are essential elements that need to be implemented in clinical practice to reach effect on hospital readmissions.
For future studies, we advise adequate allocation concealment and we suggest to report on hospital (re)admissions in the preceding months at baseline, as multiple previous hospital (re)admissions are associated with an increased risk for readmissions. 51
In the included studies, follow‐up duration was heterogeneous and often short. To determine the effect of a medication review in both the short and long term, we recommend that future studies pursue longer follow‐up duration. Outcome definition, including "hospital readmissions,” was very heterogeneous. 52 Use of a recently published core outcome set for clinical trials of medication review could overcome this challenge and enable NMA. 53 Furthermore, we recommend future studies that perform a medication review after hospital discharge to confirm the current finding of no effect of this intervention on hospital readmissions. Finally, we propose to focus on participants with multi‐morbidity, polypharmacy and increasing age (>75 years), who are at higher risk of medication related problems and medication errors, but have been poorly represented in studies.
5. CONCLUSION
This systematic review and NMA demonstrates that medication review in combination with medication reconciliation, patient education, professional education and transitional care is associated with a decreased risk of hospital readmissions within 30 days, compared to usual care. Therefore it is important to combine it with these co‐interventions when implementing a medication review. An effect of medication review as an isolated intervention or performed after discharge could not be demonstrated. The effect of a medication review with co‐interventions on hospital readmissions during a longer period of time after discharge was less pronounced.
CONFLICT OF INTEREST
The authors have no financial or personal conflicts.
FINANCIAL DISCLOSURE
This work is part of the project “OPERAM: OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly” supported by the European Union's Horizon 2020 research and innovation programme under the grant agreement No 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137. The opinions expressed and arguments employed herein are those of the authors and do not necessarily reflect the official views of the European Union and the Swiss government.
AUTHOR CONTRIBUTIONS
The study protocol was created by RCMAR, LD, LB, HLK, ST, SZ, NR, RJPMS, AWR, MDN, MEV, ELMJ, OD, DM and WK. LD executed the literature search. Study selection was performed by LD and LB under supervision of RJPMS, WK and HLK. Data extraction was performed by LD and LB. Data analysis was performed by ST, SZ and DM. Interpretation of the results was performed by LD, RJPMS, HLK and WK. The manuscript was drafted by LD, RJPMS, WK and HLK. The manuscript was revised by LB, ST, SZ, NR, AWR, MDN, RCMAR, MEV, ELMJ, OD and DM. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
SPONSOR'S ROLE
The sponsor had no input on the study design, methods, data collection, data analysis, interpretation of the results, report writing, approval of the manuscript or the decision to submit the paper for publication.
Supporting information
Table S1: Medication review interventions categorized into nine components.
Figure S1: Flow diagram of study selection.
Table S2. Individual study characteristics of the 25 randomized controlled studies included in the analysis.
Table S3: Individual participant characteristics of the 25 randomized controlled studies included in the analysis.
Table S4: Individual Cochrane EPOC risk of bias assessment of the included studies.
Figure S2: Aggregate risk of bias assessment per domain.
Table S5: Description of how the medication review was conducted.
Figure S3: Network plot for the outcome all‐cause hospital readmissions within 30 days.
Table S6: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from network meta‐analysis (left) and component network meta‐analysis (right) for every intervention versus usual care for the outcome all‐cause hospital readmissions within 30 days.
Table S7: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from the component network meta‐analysis for every intervention component versus usual care for the outcome all‐cause hospital readmissions within 30 days.
Figure S4: Network plot for the outcome all‐cause hospital readmissions at any time.
Table S8: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from network meta‐analysis (left) and component network meta‐analysis (right) for every intervention versus usual care for the outcome all‐cause hospital readmissions at any time.
Table S9: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from the component network meta‐analysis for every intervention component versus usual care for the outcome all‐cause hospital readmissions at any time.
Table S10: Study population, interventions, comparators, and outcomes.
Table S11: Electronic search strategy MEDLINE.
Table S12: Electronic search strategy Embase.
Table S13: Electronic search strategy The Cochrane Library.
Table S14: Electronic search strategy CINAHL.
Table S15: List and definition of all variable data collected.
ACKNOWLEDGMENTS
Dautzenberg L, Bretagne L, Koek HL, et al. Medication review interventions to reduce hospital readmissions in older people. J Am Geriatr Soc. 2021;69:1646–1658. 10.1111/jgs.17041
The abstract of this paper has been presented at the European Geriatric Medicine Society (EUGMS) E‐Congress 2020.
Funding information European Union's Horizon 2020 research and innovation programme, Grant/Award Number: 6342388; Swiss State Secretariat for Education, Research and Innovation (SERI), Grant/Award Number: 15.0137
REFERENCES
- 1. Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the women's health and aging study I. J Am Geriatr Soc. 2009;57(10):1757‐1766. 10.1111/j.1532-5,415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Szlejf C, Farfel JM, Curiati JA, Couto Juniorde E d B, Jacob‐Filho W, Azevedo RS. Medical adverse events in elderly hospitalized patients: a prospective study. Clinics. 2012;67(11):1247‐1,252. 10.6061/clinics/2012(11)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002‐2012. 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 4. Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045‐1,051. [DOI] [PubMed] [Google Scholar]
- 5. Huiskes VJB, Burger DM, Van Den Ende CHM, Van Den Bemt BJF. Effectiveness of medication review: a systematic review and meta‐analysis of randomized controlled trials. BMC Fam Pract. 2017;18(1):5. 10.1186/s12875-016-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. Br J Clin Pharmacol. 2008;65(3):303‐316. 10.1111/j.1365-2,125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graabæk T, Kjeldsen L. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: a systematic review. Basic Clin Pharmacol Toxicol. 2013;112(6):359‐373. 10.1111/bcpt.12062. [DOI] [PubMed] [Google Scholar]
- 8. Blenkinsopp A, Bond C, Raynor DK. Medication reviews. Br J Clin Pharmacol. 2012;74(4):573‐580. 10.1111/j.1365-2,125.2012.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2016(2):CD008986. 10.1002/14651858.CD008986.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (screening tool of older Person's prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72‐83. 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 11. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157(14):1531‐1,536. [PubMed] [Google Scholar]
- 12. Ensing HT, Stuijt CCM, Van Den Bemt BJF, et al. Identifying the optimal role for pharmacists in care transitions: a systematic review. J Manag Care Spec Pharm. 2015;21(8):614‐638. 10.18553/jmcp.2015.21.8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wheeler AJ, Scahill S, Hopcroft D, Stapleton H. Reducing medication errors at transitions of care is everyone's business. Aust Prescr. 2018;41(3):73‐77. 10.18773/austprescr.2018.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings: a systematic review and meta‐analysis. JAMA Intern Med. 2015;175(1):76‐87. 10.1001/jamainternmed.2014.5841. [DOI] [PubMed] [Google Scholar]
- 15. Hatah E, Braund R, Tordoff J, Duffull S. A systematic review and meta‐analysis of pharmacist‐led fee‐for‐services medication review. Br J Clin Pharmacol. 2014;77(1):102‐115. 10.1111/bcp.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wallerstedt SM, Kindblom JM, Nylén K, Samuelsson O, Strandell A. Medication reviews for nursing home residents to reduce mortality and hospitalization: systematic review and meta‐analysis. Br J Clin Pharmacol. 2014;78(3):488‐497. 10.1111/bcp.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas R, Huntley AL, Mann M, et al. Pharmacist‐led interventions to reduce unplanned admissions for older people: a systematic review and meta‐analysis of randomised controlled trials. Age Ageing. 2014;43(2):174‐187. 10.1093/ageing/aft169. [DOI] [PubMed] [Google Scholar]
- 18. Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80‐97. 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 19. Mavridis D, Giannatsi M, Cipriani A, Salanti G. A primer on network meta‐analysis with emphasis on mental health. Evid Based Ment Health. 2015;18(2):40‐46. 10.1136/eb-2015-102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cochrane Effective Practice and Organisation of Care (EPOC) . Suggested Risk of bias criteria for EPOC reviews. EPOC resources for review authors, 2017. http://Epoc.Cochrane.Org/Resources/Epoc-Resources-Review-Authors.
- 21. Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi‐arm studies in network meta‐analysis. Stat Med. 2014;33(25):4353‐4369. 10.1002/sim.6236. [DOI] [PubMed] [Google Scholar]
- 22. Rücker G, Schwarzer G, Krahn U, König J, Schwarzer MG. Package 'netmeta'. Network meta‐analysis using frequentist methods (Version 0.7‐0). Published online 2015.
- 23. Rücker G, Petropoulou M, Schwarzer G. Network meta‐analysis of multicomponent interventions. Biometrical J. 2020;62:808‐821. 10.1002/bimj.201800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. Cinema: an approach for assessing confidence in the results of a network meta‐analysis. PLoS Med. 2020;17(4):e1003082. 10.1371/JOURNAL.PMED.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legrain S, Tubach F, Bonnet‐Zamponi D, et al. A new multimodal geriatric discharge‐planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017‐2028. 10.1111/j.1532-5415.2011.03628.x. [DOI] [PubMed] [Google Scholar]
- 26. Spinewine A, Swine C, Dhillon S, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc. 2007;55(5):658‐665. 10.1111/j.1532-5,415.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- 27. Mannheimer B, Ulfvarson J, Eklöf S, et al. Drug‐related problems and pharmacotherapeutic advisory intervention at a medicine clinic. Eur J Clin Pharmacol. 2006;62(12):1075‐1,081. 10.1007/s00228-006-0214-z. [DOI] [PubMed] [Google Scholar]
- 28. Naunton M, Peterson GM. Evaluation of home‐based follow‐up of high‐risk elderly patients discharged from hospital. J Pharm Pract Res. 2003;33(3):176‐182. 10.1002/jppr2003333176. [DOI] [Google Scholar]
- 29. Holland R, Lenaghan E, Harvey I, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. Br Med J. 2005;330(7486):293. 10.1136/bmj.38338.674583.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edey R, Edwards N, Von Sychowski J, Bains A, Spence J, Martinusen D. Impact of deprescribing rounds on discharge prescriptions: an interventional trial. Int J Clin Pharm. 2019;41(1):159‐166. 10.1007/s11096-018-0753-2. [DOI] [PubMed] [Google Scholar]
- 31. Elliott LS, Henderson JC, Neradilek MB, Moyer NA, Ashcraft KC, Thirumaran RK. Clinical impact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: a prospective pilot randomized controlled trial. PLoS One. 2017;12(2):e0170905. 10.1371/journal.pone.0170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894‐900. 10.1001/archinternmed.2009.71. [DOI] [PubMed] [Google Scholar]
- 33. Gustafsson M, Sjölander M, Pfister B, Jonsson J, Schneede J, Lövheim H. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: a randomized controlled trial. Eur J Clin Pharmacol. 2017;73(7):827‐835. 10.1007/s00228-017-2249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haag JD, Davis AZ, Hoel RW, et al. Impact of pharmacist‐provided medication therapy management on healthcare quality and utilization in recently discharged elderly patients. Am Heal Drug Benefits. 2016;9(5):259‐268. [PMC free article] [PubMed] [Google Scholar]
- 35. Hohl CM, Partovi N, Ghement I, et al. Impact of early in‐hospital medication review by clinical pharmacists on health services utilization. PLoS One. 2017;12(2):e0170495. 10.1371/journal.pone.0170495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lenssen R, Schmitz K, Griesel C, et al. Comprehensive pharmaceutical care to prevent drug‐related readmissions of dependent‐living elderly patients: a randomized controlled trial. BMC Geriatr. 2018;18(1):135. 10.1186/s12877-018-0814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lisby M, Bonnerup DK, Brock B, et al. Medication review and patient outcomes in an orthopedic department: a randomized controlled study. J Patient Saf. 2018;14(2):74‐81. 10.1097/PTS.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 38. Lisby M, Thomsen A, Nielsen LP, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol. 2010;106(5):422‐427. 10.1111/j.1742-7,843.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 39. Nielsen TRH, Honoré PH, Rasmussen M, Andersen SE. Clinical effects of a pharmacist intervention in acute wards – a randomized controlled trial. Basic Clin Pharmacol Toxicol. 2017;121(4):325‐333. 10.1111/bcpt.12802. [DOI] [PubMed] [Google Scholar]
- 40. Ravn‐Nielsen LV, Duckert M‐L, Lund ML, et al. Effect of an in‐hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med. 2018;178(3):375‐382. 10.1001/jamainternmed.2017.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. J Eval Clin Pract. 2007;13(5):781‐788. 10.1111/j.1365-2,753.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 42. Tuttle KR, Alicic RZ, Short RA, et al. Medication therapy management after hospitalization in CKD: a randomized clinical trial. Clin J Am Soc Nephrol. 2018;13(2):231‐241. 10.2215/CJN.06790617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van der Heijden AAWA, de Bruijne MC, Nijpels G, Hugtenburg JG. Cost‐effectiveness of a clinical medication review in vulnerable older patients at hospital discharge, a randomized controlled trial. Int J Clin Pharm. 2019;41(4):963‐971. 10.1007/s11096-019-00825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van der Linden L, Decoutere L, Walgraeve K, et al. Combined use of the rationalization of home medication by an adjusted STOPP in older patients (RASP) list and a pharmacist‐led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs Aging. 2017;34(2):123‐133. 10.1007/s40266-016-0424-8. [DOI] [PubMed] [Google Scholar]
- 45. Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health‐related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf. 2011;20(9):738‐746. 10.1136/bmjqs.2009.039693. [DOI] [PubMed] [Google Scholar]
- 46. Bonetti AF, Bagatim BQ, Mendes AM, et al. Impact of discharge medication counseling in the cardiology unit of a tertiary hospital in Brazil: a randomized controlled trial. Clinics. 2018;73(8):e325. 10.6061/clinics/2018/e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brühwiler LD, Beeler PE, Böni F, et al. A RCT evaluating a pragmatic in‐hospital service to increase the quality of discharge prescriptions. Int J Qual Heal Care. 2019;31(8):1‐7. 10.1093/intqhc/mzz043. [DOI] [PubMed] [Google Scholar]
- 48. Chiu PKC, Lee AWK, See TYW, Chan FHW. Outcomes of a pharmacist‐led medication review programme for hospitalised elderly patients. Hong Kong Med J. 2018;24(2):98‐106. 10.12809/hkmj176871. [DOI] [PubMed] [Google Scholar]
- 49. Cossette B, Éthier J‐F, Joly‐Mischlich T, et al. Reduction in targeted potentially inappropriate medication use in elderly inpatients: a pragmatic randomized controlled trial. Eur J Clin Pharmacol. 2017;73(10):1237‐1,245. 10.1007/s00228-017-2,293-4. [DOI] [PubMed] [Google Scholar]
- 50. Wallerstedt SM, Bladh L, Ramsberg J. A cost‐effectiveness analysis of an in‐hospital clinical pharmacist service. BMJ Open. 2012;2(1):e000329. 10.1136/bmjopen-2011-000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hallgren J, Aslan AKD. Risk factors for hospital readmission among Swedish older adults. Eur Geriatr Med. 2018;9(5):603‐611. 10.1007/s41999-018-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beuscart JB, Pont LG, Thevelin S, et al. A systematic review of the outcomes reported in trials of medication review in older patients: the need for a core outcome set. Br J Clin Pharmacol. 2017;83(5):942‐952. 10.1111/bcp.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beuscart JB, Knol W, Cullinan S, et al. International core outcome set for clinical trials of medication review in multi‐morbid older patients with polypharmacy. BMC Med. 2018;16(1):21. 10.1186/s12916-018-1,007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Medication review interventions categorized into nine components.
Figure S1: Flow diagram of study selection.
Table S2. Individual study characteristics of the 25 randomized controlled studies included in the analysis.
Table S3: Individual participant characteristics of the 25 randomized controlled studies included in the analysis.
Table S4: Individual Cochrane EPOC risk of bias assessment of the included studies.
Figure S2: Aggregate risk of bias assessment per domain.
Table S5: Description of how the medication review was conducted.
Figure S3: Network plot for the outcome all‐cause hospital readmissions within 30 days.
Table S6: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from network meta‐analysis (left) and component network meta‐analysis (right) for every intervention versus usual care for the outcome all‐cause hospital readmissions within 30 days.
Table S7: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from the component network meta‐analysis for every intervention component versus usual care for the outcome all‐cause hospital readmissions within 30 days.
Figure S4: Network plot for the outcome all‐cause hospital readmissions at any time.
Table S8: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from network meta‐analysis (left) and component network meta‐analysis (right) for every intervention versus usual care for the outcome all‐cause hospital readmissions at any time.
Table S9: Risk ratios (RRs) with 95% confidence intervals (95% CI) resulting from the component network meta‐analysis for every intervention component versus usual care for the outcome all‐cause hospital readmissions at any time.
Table S10: Study population, interventions, comparators, and outcomes.
Table S11: Electronic search strategy MEDLINE.
Table S12: Electronic search strategy Embase.
Table S13: Electronic search strategy The Cochrane Library.
Table S14: Electronic search strategy CINAHL.
Table S15: List and definition of all variable data collected.


