Abstract
Aims
There are limited data examining whether body mass index (BMI) influences the association between cardiovascular biomarkers and incident heart failure (HF).
Methods and results
Thirteen biomarkers representing key HF domains were measured: N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), mid‐regional pro‐A‐type natriuretic peptide (MR‐proANP), cardiac troponin T (cTnT), C‐reactive protein, procalcitonin, galectin‐3, C‐terminal pro‐endothelin‐1 (CT‐proET‐1), mid‐regional pro‐adrenomedullin, plasminogen activator inhibitor‐1, copeptin, renin, aldosterone, and cystatin‐C. Associations of biomarkers with BMI were examined using linear regression models, and with incident HF using Cox regression models. We selected biomarkers significantly associated with incident HF, and evaluated whether BMI modified these associations. Among 8202 individuals, 41% were overweight (BMI 25–30 kg/m2), and 16% were obese (BMI ≥30 kg/m2). Mean age of the cohort was 49 years (range 28–75), and 50% were women. All biomarkers except renin were associated with BMI: inverse associations were observed with NT‐proBNP, MR‐proANP, CT‐proET‐1 and aldosterone whereas positive associations were observed with the remaining biomarkers (all P ≤ 0.001). During 11.3 ± 3.1 years of follow‐up, 357 HF events were recorded. Only NT‐proBNP, MR‐proANP and cTnT remained associated with incident HF (P < 0.001), and a significant biomarker*BMI interaction was not observed (interaction P > 0.1). Combined NT‐proBNP and cTnT measurements modestly improved performance metrics of the clinical HF model in overweight (ΔC‐statistic = 0.024; likelihood ratio χ2 = 38; P < 0.001) and obese (ΔC‐statistic = 0.020; likelihood ratio χ2 = 32; P < 0.001) individuals.
Conclusions
Plasma concentrations of several cardiovascular biomarkers are influenced by obesity. Only NT‐proBNP, MR‐proANP and cTnT were associated with incident HF, and BMI did not modify these associations. A combination of NT‐proBNP and cTnT improves HF risk prediction in overweight and obese individuals.
Keywords: Body mass index, Cardiovascular biomarkers, Heart failure, Associations, Predictive value, General population
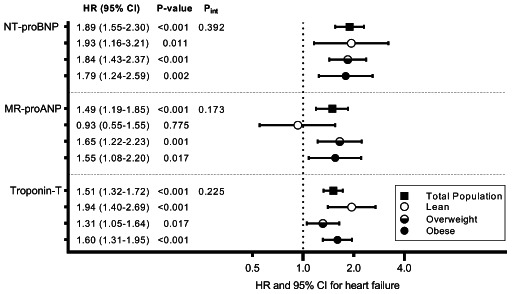
Associations of selected biomarkers with incident heart failure across body mass index categories. Models are adjusted for age, sex, smoking, diabetes mellitus, hypertension, cholesterol, body mass index, myocardial infarction, stroke, atrial fibrillation, and renal dysfunction. In analyses performed in the total population, models were also adjusted for body mass index. Hazard ratio (HR) are presented per standard deviation increase in natural log transformed biomarker. P int represents the P‐value for biomarker*continuous body mass index interaction for heart failure outcome in the total population. CI, confidence interval; MR‐proANP, mid‐regional pro‐A‐type natriuretic peptide; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Introduction
Cardiovascular biomarkers provide information on pathophysiological processes associated with heart failure (HF), may improve HF risk prediction, and could potentially be used for preventative therapies or selection of testing. 1 , 2 While interpreting biomarker values, various factors such as age, sex and renal function should be taken into consideration. Obesity is also an important factor affecting biomarker concentrations. 3
Cardiac natriuretic peptides (NPs) are secreted by cardiomyocytes as a response to myocardial stretch due to volume overload, and circulating NPs are inversely associated with body mass index (BMI). 4 , 5 By contrast, markers of myocardial injury (cardiac troponins), systemic inflammation [C‐reactive protein (CRP), procalcitonin], tissue fibrosis (galectin‐3) and thrombosis [plasminogen activator inhibitor‐1 (PAI‐1)] are known to be elevated in obese individuals. 3 , 6 , 7 , 8 Few data are available examining whether BMI affects plasma concentrations of biomarkers representing other domains pivotal to the pathophysiology of HF syndrome such as endothelial dysfunction, volume status, neurohormonal response and renal impairment. Whether BMI influences the predictive value of cardiovascular biomarkers with incident HF also remains unclear.
We postulated that BMI would influence plasma concentrations of multiple cardiovascular biomarkers, as well as their association with incident HF. Accordingly, we evaluated cross‐sectional associations of 13 cardiovascular biomarkers with BMI, and longitudinal associations of selected biomarkers with incident HF across pre‐specified BMI categories.
Methods
The Prevention of Renal and Vascular End‐stage Disease (PREVEND) study (1997–1998) is an observational cohort study enrolling 8592 participants, and has been described elsewhere. 9 , 10 , 11 From the baseline cohort, we excluded 390 participants (4.5%) for the following reasons: (i) missing data on BMI (n = 93), (ii) BMI <18.5 kg/m2 (n = 74), (iii) estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 (n = 11), and (iv) missing data on clinical covariates (n = 212). This resulted in 8202 participants eligible for the present investigation (online supplementary Figure S1 ), of which none had prevalent HF. The current study conformed to the principles drafted in the Helsinki Declaration. Local medical ethics committee approval was obtained and informed consent was provided by all participants.
Baseline measurements
Body mass index was calculated as the ratio of body weight (kg) and height2 (m2). BMI was categorized into <25 kg/m2 (lean), ≥25 to <30 kg/m2 (overweight), and ≥30 kg/m2 (obese). Details on clinical covariates are provided in the online supplementary material. The following biomarkers were measured in plasma samples obtained during the baseline visit: N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), mid‐regional pro‐A‐type natriuretic peptide (MR‐proANP), high‐sensitivity cardiac troponin T (cTnT), high‐sensitivity CRP, procalcitonin, galectin‐3, C‐terminal pro‐endothelin‐1 (CT‐proET‐1), mid‐regional pro‐adrenomedullin (MR‐proADM), PAI‐1, copeptin, renin, aldosterone, and cystatin‐C. NT‐proBNP was measured using a commercially available electrochemiluminescent sandwich immunoassay (Roche Modular E170, Roche Diagnostics, Mannheim, Germany). 5 This assay had an analytical range from 5 to 35 000 ng/L with an intra‐ and inter‐assay imprecision of 1.2–1.5% and 4.4–5.0%, respectively. MR‐proANP was measured with a sandwich immunoassay (MR‐proANP LIA; B.R.A.H.M.S, Hennigsdorf, Germany). The intra‐assay coefficient of variation was <10% for samples containing 23–3000 pmol/L (1 pmol/L = 10.62 ng/L) and 20% for samples containing 18–22.8 pmol/L. The inter‐assay coefficient of variation was 8.0% at 100 pmol/L and 6.5% at 400 pmol/L. cTnT was measured using a fifth‐generation high‐sensitivity assay (Roche Modular E170, Roche Diagnostics). 11 The coefficient of variation at the 99th percentile of the reference range (14 ng/L) was <10%. Above 30 ng/L, cTnT inter‐assay coefficients of variation were between 1% and 5% for all test applications. Limit of blank and limit of detection have been determined to be 3 and 5 ng/L, respectively. Details on other biomarker assays relevant to this study are provided in the online supplementary material.
Incident heart failure
Follow‐up duration was calculated as the period between the baseline screening visit and the last contact date, death, or 31st December 2010, whichever came first. Patient files were checked in two main hospitals covering the region of Groningen for prevalent and incident HF. Individuals suspected of having HF were identified according to guidelines issued by the European Society of Cardiology. 12 An endpoint adjudication committee of seven independent HF experts further evaluated these selected individuals, and two different experts validated each case. A joint decision was made within the committee in the case of disagreement. Aetiology of HF and the date of HF onset were retrieved from clinical charts. Further details can be found elsewhere. 9 , 10
Statistical analyses
Normally distributed data are presented as means ± standard deviation, non‐normally distributed data as medians Q1–Q3 (50th percentile, 25th–75th percentile), and categorical data as percentages. For group comparisons, one‐way analysis of variance (ANOVA) or Kruskal–Wallis test or Pearson's χ2 test were used as appropriate. For subsequent analyses, all biomarkers were natural log‐transformed and standardized. We examined cross‐sectional associations of biomarkers with BMI using linear regression models adjusting for age, sex and renal dysfunction (eGFR <60 mL/min/1.73 m2). Results were displayed as standardized beta coefficients with 95% confidence intervals (CI) based on 1000 bootstrapped estimates. We then identified biomarkers significantly associated with incident HF in the total population using multivariable Cox regression models adjusting for age, sex, smoking, type 2 diabetes mellitus, hypertension, cholesterol, BMI, 10 and also for prevalent myocardial infarction, stroke, atrial fibrillation and renal dysfunction. A Bonferroni‐corrected P‐value of ≤0.004 (i.e. 0.05/13 biomarkers) denoted statistical significance. Next, we examined associations of selected biomarkers with incident HF across pre‐specified BMI categories using multivariable Cox regression models. We tested for biomarker*continuous BMI interaction. For these analyses, a P‐value <0.05 and an interaction P‐value <0.1 denoted statistical significance. 10 To assess the best fitting functional form for biomarker levels and their association with incident HF across BMI categories, we also performed fractional polynomial regression analyses. As sensitivity analyses, we used Fine–Gray models adjusting for the competing risk of death. To account for over‐representation of individuals with increased urinary albumin excretion (> 10 mg/L), a design‐based analysis was performed using statistical weights, which allows conclusions to be generalized to the general population. 9 , 10 Results were expressed as hazard ratios (HR) or sub‐distribution HRs (sHR) with 95% CI based on robust standard error estimates.
Additionally, we constructed a multi‐marker HF model including NT‐proBNP, MR‐proANP and cTnT. We identified biomarkers displaying a statistically significant association with incident HF after adjusting for clinical covariates, and examined whether addition of these biomarkers to the clinical HF model improved discrimination (Harrel's C‐statistic) and model fit [likelihood ratio (LHR) test chi‐squared statistic] in lean, overweight, and obese individuals separately. All statistical analyses were performed using STATA version 14 (Stata Corp., College Station, TX, USA).
Results
Among 8202 participants from the PREVEND study, 3361 (41%) were overweight, and 1303 (16%) were obese. All cardiovascular risk factors (except smoking) were significantly higher across BMI categories (Table 1 ). In linear regression models, cTnT, CRP, procalcitonin, galectin‐3, PAI‐1, MR‐proADM, copeptin and cystatin‐C were positively associated with BMI (P ≤ 0.001) whereas NT‐proBNP, MR‐proANP, CT‐proET‐1 and aldosterone displayed negative associations (P < 0.001). Renin was not associated with BMI (P = 0.72) (Figure 1 and online supplementary Table S1 ).
Table 1.
Baseline characteristics and biomarker levels across body mass index categories
| Total population (n = 8202) | Lean (n = 3538) | Overweight (n = 3361) | Obese (n = 1303) | P‐value | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, years | 49.2 ± 12.6 | 45.0 ± 11.6 | 52.0 ± 12.6 | 53.4 ± 11.8 | <0.001 |
| Female sex | 4099 (50.0) | 1983 (56.0) | 1410 (42.0) | 706 (54.2) | <0.001 |
| Smoking | 3111 (37.9) | 1579 (45) | 1133 (34) | 399 (31) | <0.001 |
| Diabetes mellitus | 317 (3.9) | 51 (1.4) | 154 (4.6) | 112 (8.6) | <0.001 |
| Hypertension | 2789 (34.0) | 636 (18.0) | 1397 (41.6) | 756 (58.0) | <0.001 |
| BMI, kg/m2 | 26.2 ± 4.2 | 22.6 ± 1.6 | 27.1 ± 1.4 | 33.3 ± 3.3 | <0.001 |
| Cholesterol, mmol/L | 5.6 (4.9, 6.3) | 5.2 (4.6, 6.0) | 5.8 (5.1, 6.5) | 5.9 (5.3, 6.6) | <0.001 |
| Atrial fibrillation | 73 (0.9) | 12 (0.3) | 43 (1.3) | 18 (1.4) | <0.001 |
| Myocardial infarction | 508 (6.2) | 162 (4.6) | 245 (7.3) | 101 (7.8) | <0.001 |
| Stroke | 92 (1.1) | 27 (0.8) | 45 (1.3) | 20 (1.5) | 0.023 |
| Renal dysfunction | 279 (3.4) | 53 (1.5) | 147 (4.4) | 70 (6.1) | <0.001 |
| Circulating biomarkers | |||||
| NT‐proBNP, ng/L | 37.4 (16.6, 73.3) | 38.0 (18.1, 70.8) | 35.9 (15.2, 75.2) | 37.9 (15.9, 74.3) | 0.60 |
| MR‐proANP, ng/L | 503.7 (365.8, 689.6) | 506.5 (368.2, 678.3) | 505.5 (366.6, 714.5) | 490.9 (352.7, 693.2) | 0.11 |
| cTnT, ng/L | 2.5 (2.5, 5.0) | 2.5 (2.5, 4.0) | 2.5 (2.5, 5.0) | 3.0 (2.5, 6.0) | <0.001 |
| hs‐CRP, mg/L | 1.3 (0.6, 3.0) | 0.8 (0.3, 1.9) | 1.5 (0.7, 3.2) | 2.7 (1.4, 5.6) | <0.001 |
| Procalcitonin, ng/L | 1.6 (1.3, 2.0) | 1.5 (1.2, 1.8) | 1.7 (1.4, 2.1) | 1.8 (1.5, 2.2) | <0.001 |
| Galectin‐3, mg/L | 10.8 (9.0, 13.0) | 10. 2 (8.6, 12.3) | 11.1 (9.4, 13.3) | 11.7 (9.8, 14.0) | <0.001 |
| CT‐proET‐1, pmol/L | 34.7 (24.5, 44.3) | 34.1 (23.8, 43.0) | 35.4 (25.3, 44.6) | 35.2 (24.4, 46.2) | <0.001 |
| MR‐proADM, nmol/L | 0.38 (0.29, 0.46) | 0.35 (0.27, 0.42) | 0.39 (0.31, 0.48) | 0.44 (0.34, 0.53) | <0.001 |
| PAI‐1, mg/L | 72.3 (41.9, 124.3) | 50.3 (31.4, 84.3) | 87.1 (52.3, 139.7) | 123.8 (75.9, 187.9) | <0.001 |
| Copeptin, pmol/L | 4.7 (2.9, 7.5) | 4.3 (2.7, 7.0) | 4.9 (3.1, 7.8) | 5.2 (3.1, 8.3) | <0.001 |
| Renin, IU/L | 18.0 (11.1, 28.5) | 18.6 (11.6, 29.0) | 17.5 (10.6, 28.0) | 17.6 (10.8, 28.8) | 0.012 |
| Aldosterone, ng/L | 118.2 (93.2, 152.6) | 120.7 (95.1, 156.8) | 117.7 (92.5, 151.4) | 113.5 (90.0, 145.7) | <0.001 |
| Cystatin‐C, mg/L | 0.77 (0.69, 0.88) | 0.75 (0.67, 0.83) | 0.79 (0.71, 0.90) | 0.81 (0.72, 0.91) | <0.001 |
| Outcome during follow‐up | |||||
| Heart failure | 357 (4.4) | 71 (2.0) | 178 (5.3) | 108 (8.3) | <0.001 |
| Overall mortality | 791 (9.6) | 224 (6.3) | 393 (11.7) | 174 (13.4) | <0.001 |
Biomarker concentrations are given as mean ± SD, median (25th, 75th percentile), or n (%).
BMI, body mass index; CT‐proET‐1, C‐terminal pro‐endothelin‐1; cTnT, high‐sensitivity cardiac troponin T; hs‐CRP, high‐sensitivity C‐reactive protein; MR‐proADM, mid‐regional pro‐adrenomedullin; MR‐proANP, mid‐regional pro‐A‐type natriuretic peptide; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAI‐1, plasminogen activator inhibitor‐1.
Figure 1.
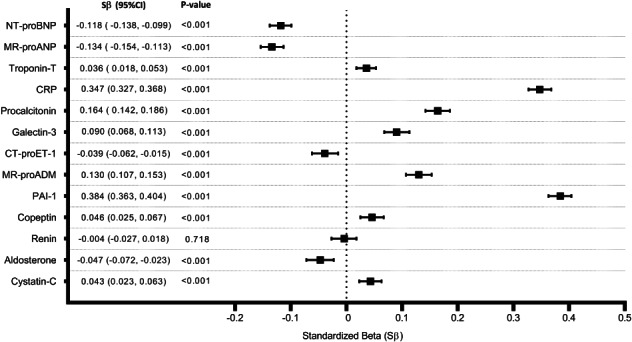
Associations of cardiovascular biomarkers with body mass index. Models are adjusted for age, sex and renal dysfunction. These are bootstrapped (1000x) estimates. Standardized betas represent a unit change in standardized natural log transformed biomarker concentrations per standard deviation increase in body mass index. CI, confidence interval; CRP, C‐reactive protein; CT‐proET‐1, C‐terminal pro‐endothelin‐1; MR‐proADM, mid‐regional pro‐adrenomedullin; MR‐proANP, mid‐regional pro‐A‐type natriuretic peptide; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAI‐1, plasminogen activator inhibitor‐1.
During a mean follow‐up of 11.3 ± 3.1 years, a total of 357 incident HF events were recorded in the total population, with 71 HF events in lean individuals, 178 HF events in overweight individuals, and 108 HF events in obese individuals. This corresponded to an incidence rate of 1.77 per 1000 person‐years (95% CI 1.40–2.23) in lean individuals, 4.69 per 1000 person‐years (95% CI 4.05–5.44) in overweight individuals, and 7.46 per 1000 person‐years (95% CI 6.18–9.01) in obese individuals.
In prospective analyses, only three biomarkers were significantly associated with incident HF in the total population: NT‐proBNP (HR 1.89, 95% CI 1.55–2.30), MR‐proANP (HR 1.49, 95% CI 1.19–1.85), and cTnT (HR 1.51, 95% CI 1.32–1.72) (online supplementary Table S2 ). These associations were not significantly modified by BMI (interaction P > 0.1). NT‐proBNP was strongly associated with incident HF in lean (HR 1.93, 95% CI 1.16–3.21), overweight (HR 1.84, 95% CI 1.43–2.37) and obese (HR 1.79, 95% CI 1.24–2.59) individuals. Subtle differences were, however, observed in associations of MR‐proANP and cTnT with incident HF across BMI categories (Graphical Abstract, online supplementary Figure S2 ). Results did not materially change when we used multivariable Fine–Gray models accounting for death as a competing risk (online supplementary Tables S3 and S4 ).
In a multi‐marker model including clinical risk factors, NT‐proBNP (HR 1.82, 95% CI 1.41–2.36) and cTnT (HR 1.31, 95% CI 1.13–1.15) remained significantly associated with incident HF whereas MR‐proANP was not (HR 1.01, 95% CI 0.78–1.30) (online supplementary Table S5 ). Addition of NT‐proBNP and cTnT individually to the clinical HF risk equation improved model fit in all three BMI categories (P < 0.01). While NT‐proBNP improved discrimination modestly in lean, overweight and obese individuals (ΔC‐statistic = 0.018, 0.021 and 0.015, respectively), addition of cTnT improved discrimination modestly only in overweight and obese individuals (ΔC‐statistic = 0.010 and 0.012, respectively). A combination of NT‐proBNP and cTnT improved discrimination as well as fit of the HF risk prediction model in overweight (ΔC‐statistic = 0.024; LHRχ2 = 38; P < 0.001) and in obese (ΔC‐statistic = 0.020; LHRχ2 = 32; P < 0.001) individuals (Table 2 ).
Table 2.
Predictive value of selected biomarkers across body mass index categories
| C‐statistic | ΔC‐statistic | LHRχ2 | Δχ2 | P‐value | |
|---|---|---|---|---|---|
| Total population (n = 7787) | |||||
| Base model | 0.860 (0.843, 0.878) | – | −4155 | – | – |
| + NT‐proBNP | 0.875 (0.859, 0.892) | 0.015 (0.008, 0.022) | −4087 | 68 | <0.001 |
| + cTnT | 0.869 (0.852, 0.886) | 0.009 (0.003, 0.014) | −4107 | 48 | <0.001 |
| + NT‐proBNP + cTnT | 0.878 (0.861, 0.895) | 0.018 (0.010, 0.025) | −4059 | 96 | <0.001 |
| Lean (n = 3369) | |||||
| Base model | 0.892 (0.855, 0.929) | – | −722 | – | – |
| + NT‐proBNP | 0.910 (0.874, 0.945) | 0.018 (0.006, 0.029) | −708 | 13 | 0.002 |
| + cTnT | 0.891 (0.849, 0.934) | −0.001 (−0.014, 0.013) | −698 | 24 | <0.001 |
| + NT‐proBNP + cTnT | 0.903 (0.863, 0.942) | 0.011 (−0.003, 0.024) | −691 | 31 | <0.001 |
| Overweight (n = 3182) | |||||
| Base model | 0.823 (0.793, 0.854) | – | −1961 | – | – |
| + NT‐proBNP | 0.845 (0.816, 0.873) | 0.021 (0.010, 0.033) | −1928 | 33 | <0.001 |
| + cTnT | 0.833 (0.804, 0.862) | 0.010 (0.001, 0.019) | −1951 | 10 | 0.007 |
| + NT‐proBNP + cTnT | 0.848 (0.819, 0.876) | 0.024 (0.011, 0.037) | −1923 | 38 | <0.001 |
| Obese (n = 1236) | |||||
| Base model | 0.812 (0.775, 0.849) | – | −1039 | – | – |
| + NT‐proBNP | 0.827 (0.788, 0.866) | 0.015 (0.000, 0.030) | −1022 | 17 | <0.001 |
| + cTnT | 0.824 (0.786, 0.862) | 0.012 (0.001, 0.024) | −1019 | 20 | <0.001 |
| + NT‐proBNP + cTnT | 0.832 (0.793, 0.870) | 0.020 (0.005, 0.035) | −1007 | 32 | <0.001 |
cTnT, high‐sensitivity cardiac troponin T; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
For these analyses, 7787 individuals with no missing biomarker measurements were included. Base heart failure model consists of age, sex, smoking, diabetes mellitus, hypertension, cholesterol, myocardial infarction, stroke, atrial fibrillation, and renal dysfunction. Base heart failure model in the total population also included body mass index.
Discussion
Associations of cardiovascular biomarkers with body mass index
We report that the majority of cardiovascular biomarkers were negatively or positively associated with BMI. Specifically, NT‐proBNP and MR‐proANP negatively correlated with BMI after accounting for potential confounders. Indeed, obesity is known to be inversely related to NP concentrations, both in HF patients 13 , 14 , 15 as well as in the general population, 5 and it has been hypothesized that obesity‐associated lowering of NPs may primarily be due to suppression of NP production/release rather than increased degradation. 4 This is because NT‐proBNP, unlike BNP, is not cleared via NP receptor‐C or through neprilysin‐mediated mechanisms.
By contrast, BMI positively correlated with cTnT, and such a trend has also been observed in a few previous studies. 16 , 17 , 18 The exact mechanism underlying obesity‐related myocardial injury is not known, although myocardial damage through paracrine mechanisms and myocardial steatosis due to adipose tissue infiltration may be potential explanations. Furthermore, it also remains unclear whether these associations are due to higher fat mass 18 or higher lean mass 16 or both.
As expected, CRP, procalcitonin, and PAI‐1 were strongly associated with obesity, and our results highlight that adrenomedullin, galectin‐3 and copeptin could also be considered as markers of obesity. A graded association between elevated cystatin‐C and BMI has been previously reported, 19 and we confirm this observation. Although obesity is known to contribute to excess aldosterone in patients with resistant hypertension, 20 we found that aldosterone levels were lower in individuals with a higher BMI. A paradoxical lack of increase in endothelin‐1 levels in obese mice has been previously observed 21 ; we now show that a higher BMI was associated with lower CT‐proET‐1 levels in community‐dwelling adults.
Associations of selected biomarkers with incident heart failure across body mass index categories
Nadruz and colleagues observed that in patients with chronic HF and reduced ejection fraction, NPs had a diminished prognostic value for cardiovascular death/HF admission in individuals with severe obesity. 13 However, in two other studies enrolling patients with acutely decompensated HF, BMI did not modify associations of NPs with 180‐day death. 14 , 15
In a meta‐analysis of multiple community‐based studies with a total of 1938 HF events, NT‐proBNP (tertile 3 vs. tertile 1) had a lower risk ratio for incident HF among individuals belonging to the highest BMI tertile compared with those from other two BMI tertiles. 22 However, in a more recent study enrolling 22 756 individuals with 2095 HF events, BMI did not modify associations of NT‐proBNP with incident HF in both men and women. 23 In the current study, NT‐proBNP levels were lower in individuals with a higher BMI, but this did not translate to differential associations of NT‐proBNP with incident HF across the BMI spectrum (Graphical Abstract). Similarly, despite inverse associations of MR‐proANP with BMI, associations of MR‐proANP with incident HF were not modified by BMI. We did, however, observe that MR‐proANP levels were associated with incident HF in overweight and obese individuals, but not in lean individuals. Collectively, these data suggest that negative cross‐sectional associations of NPs with BMI need not translate to weaker associations of these peptides with incident HF in overweight/obese individuals.
In a multi‐marker model, only NT‐proBNP and cTnT remained associated with incident HF. It is well‐established that adding NPs improves HF risk estimation in the general population, 22 , 24 and we now show that NT‐proBNP measurements have a similar predictive value for incident HF across BMI categories. There are also high‐quality data demonstrating the independent predictive value of cardiac troponins (beyond NPs) for incident HF. 11 , 18 , 23 , 25 Our study adds granularity to these findings, and specifically highlights the potential value of combined NT‐proBNP and cTnT measurements to improve HF risk prediction in overweight and obese individuals. Future studies should examine the value of including both NPs and cardiac troponins in HF prevention programmes across classes of overweight and obesity.
Study limitations
First, despite long‐term follow‐up and a large population, PREVEND is a relatively young cohort with low number of events. Second, the PREVEND study by design included a higher proportion of individuals with urinary albumin excretion >10 mg/mL. We accounted for this by conducting a design‐based analysis. Finally, the current study was conducted on a predominantly white population limiting generalizability to other ethnicities and population groups.
Conclusion
In community‐dwelling adults, plasma concentrations of the majority of cardiovascular biomarkers are negatively or positively influenced by obesity. This, however, does not translate into differential predictive value of a biomarker for incident HF across the BMI spectrum. A combination of NT‐proBNP and cTnT improves prediction of HF in overweight and obese individuals.
Funding
This work was supported by the Netherlands Heart Foundation (CVON SHE‐PREDICTS‐HF, grant 2017‐21, CVON DOSIS, grant 2014‐40, and CVON RED‐CVD, grant 2017‐11), the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VIDI, grant 917.13.350), and the European Research Council (ERC CoG 818715, SECRETE‐HF). The PREVEND study was financially supported by grant E.013 of the Dutch Kidney Foundation. The sponsors/funders did not have any role in the design and conduct of the study, in the collection, analysis, and interpretation of data, and in the preparation, review, or approval of the manuscript.
Conflict of interest: The UMCG, which employs all authors, has received research grants and/or fees from AstraZeneca, Abbott, Bristol‐Myers Squibb, Novartis, Novo Nordisk, and Roche. R.A.d.B. received personal fees from Abbott, AstraZeneca, Novartis, and Roche. All other authors have nothing to disclose.
Supporting information
Figure S1. PREVEND flow diagram: participant selection.
Figure S2. Associations of selected biomarkers with incident heart failure across body mass index categories.
Table S1. Associations of cardiovascular biomarkers with body mass index.
Table S2. Associations of cardiovascular biomarkers with incident heart failure in the total population.
Table S3. Associations of cardiovascular biomarkers with incident heart failure after accounting for death as a competing risk.
Table S4. Associations of selected biomarkers with incident heart failure across body mass index categories after accounting for death as a competing risk.
References
- 1. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 2017;135:e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 2. Ibrahim NE, Januzzi JL. Established and emerging roles of biomarkers in heart failure. Circ Res 2018;123:614–629. [DOI] [PubMed] [Google Scholar]
- 3. Suthahar N, Meems LM, Ho JE, de Boer RA. Sex‐related differences in contemporary biomarkers for heart failure: a review. Eur J Heart Fail 2020;22:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev 2012;17:81–96. [DOI] [PubMed] [Google Scholar]
- 5. Suthahar N, Meijers WC, Ho JE, Gansevoort RT, Voors AA, van der Meer P, Bakker SJL, Heymans S, van Empel V, Schroen B, van der Harst P, van Veldhuisen DJ, de Boer RA. Sex‐specific associations of obesity and N‐terminal pro‐B‐type natriuretic peptide levels in the general population. Eur J Heart Fail 2018;20:1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C‐reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–2135. [DOI] [PubMed] [Google Scholar]
- 7. Abbasi A, Corpeleijn E, Postmus D, Gansevoort RT, de Jong PE, Gans RO, Struck J, Hillege HL, Stolk RP, Navis G, Bakker SJL. Plasma procalcitonin is associated with obesity, insulin resistance, and the metabolic syndrome. J Clin Endocrinol Metab 2010;95:E26–E31. [DOI] [PubMed] [Google Scholar]
- 8. Margaglione M, Cappucci G, D'Addedda M, Colaizzo D, Giuliani N, Vecchione G, Mascolo G, Grandone E, Di Minno G. PAI‐1 plasma levels in a general population without clinical evidence of atherosclerosis. Arterioscler Thromb Vasc Biol 1998;18:562–567. [DOI] [PubMed] [Google Scholar]
- 9. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J 2013;34:1424–1431. [DOI] [PubMed] [Google Scholar]
- 10. Brouwers FP, van Gilst WH, Damman K, van den Berg MP, Gansevoort RT, Bakker SJL, Hillege HL, van Veldhuisen DJ, van der Harst P, de Boer RA. Clinical risk stratification optimizes value of biomarkers to predict new‐onset heart failure in a community‐based cohort. Circ Heart Fail 2014;7:723–731. [DOI] [PubMed] [Google Scholar]
- 11. Suthahar N, Meems LM, van Veldhuisen DJ, Walter JE, Gansevoort RT, Heymans S, Schroen B, van der Harst P, Kootstra‐Ros JE, van Empel V, Mueller C, Bakker SJL, de Boer RA. High‐sensitivity troponin‐T and cardiovascular outcomes in the community: differences between women and men. Mayo Clin Proc 2020;95:1158–1168. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 13. Nadruz W, Claggett BL, McMurray JJ, Packer M, Zile MR, Rouleau JL, Desai AS, Swedberg K, Lefkowitz M, Shi VC, Prescott MF, Solomon SD. Impact of body mass index on the accuracy of N‐terminal pro‐brain natriuretic peptide and brain natriuretic peptide for predicting outcomes in patients with chronic heart failure and reduced ejection fraction: insights from the PARADIGM‐HF Study (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial). Circulation 2016;134:1785–1787. [DOI] [PubMed] [Google Scholar]
- 14. Streng KW, ter Maaten JM, Cleland JG, O'Connor CM, Davison BA, Metra M, Givertz MM, Teerlink JR, Ponikowski P, Bloomfield DM, Dittrich HC, Hillege HL, van Veldhuisen DJ, Voors AA, van der Meer P. Associations of body mass index with laboratory and biomarkers in patients with acute heart failure. Circ Heart Fail 2017;10:e003350. [DOI] [PubMed] [Google Scholar]
- 15. Bhatt AS, Cooper LB, Ambrosy AP, Clare RM, Coles A, Joyce E, Krishnamoorthy A, Butler J, Felker GM, Ezekowitz JA, Armstrong PW, Hernandez AF, O'Connor CM, Mentz RJ. Interaction of body mass index on the association between N‐terminal‐pro‐B‐type natriuretic peptide and morbidity and mortality in patients with acute heart failure: findings from ASCEND‐HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure). J Am Heart Assoc 2018;7:e006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin I levels measured with a high‐sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Coll Cardiol 2013;61:1906–1913. [DOI] [PubMed] [Google Scholar]
- 18. Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, Selvin E, Ballantyne CM, Nambi V. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail 2014;2:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muntner P, Winston J, Uribarri J, Mann D, Fox CS. Overweight, obesity, and elevated serum cystatin C levels in adults in the United States. Am J Med 2008;121:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dudenbostel T, Ghazi L, Liu M, Li P, Oparil S, Calhoun DA. Body mass index predicts 24‐hour urinary aldosterone levels in patients with resistant hypertension. Hypertension 2016;68:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baretella O, Chung SK, Xu A, Vanhoutte PM. Paradoxical lack of increase in endothelin‐1 levels in obese mice – possible role of endothelin‐B receptors. Acta Pharmacol Sin 2017;38:1699–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willeit P, Kaptoge S, Welsh P, Butterworth AS, Chowdhury R, Spackman SA, Pennells L, Gao P, Burgess S, Freitag DF, Sweeting M, Wood AM, Cook NR, Judd S, Trompet S, Nambi V, Olsen MH, Everett BM, Kee F, Ärnlöv J, Salomaa V, Levy D, Kauhanen J, Laukkanen JA, Kavousi M, Ninomiya T, Casas JP, Daniels LB, Lind L, Kistorp CN, Rosenberg J, Mueller T, Rubattu S, Panagiotakos DB, Franco OH, de Lemos JA, Luchner A, Kizer JR, Kiechl S, Salonen JT, Goya Wannamethee S, de Boer RA, Nordestgaard BG, Andersson J, Jørgensen T, Melander O, Ballantyne C, DeFilippi C, Ridker PM, Cushman M, Rosamond WD, Thompson SG, Gudnason V, Sattar N, Danesh J, Di Angelantonio E. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual‐participant‐data meta‐analysis. Lancet Diabetes Endocrinol 2016;4:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suthahar N, Lau ES, Blaha MJ, Paniagua SM, Larson MG, Psaty BM, Benjamin EJ, Allison MA, Bartz TM, Januzzi JL, Levy D, Meems LM, Bakker SJL, Lima JAC, Cushman M, Lee DS, Wang TJ, DeFilippi CR, Herrington DM, Nayor M, Vasan RS, Gardin JM, Kizer JR, Bertoni AG, Allen NB, Gansevoort RT, Shah SJ, Gottdiener JS, Ho JE, de Boer RA. Sex‐specific associations of cardiovascular risk factors and biomarkers with incident heart failure. J Am Coll Cardiol 2020;76:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clerico A, Zaninotto M, Passino C, Aspromonte N, Piepoli MF, Migliardi M, Perrone M, Fortunato A, Padoan A, Testa A, Dellarole F, Trenti T, Bernardini S, Sciacovelli L, Colivicchi F, Gabrielli D, Plebani M. Evidence on clinical relevance of cardiovascular risk evaluation in the general population using cardio‐specific biomarkers. Clin Chem Lab Med 2020;59:79–90. [DOI] [PubMed] [Google Scholar]
- 25. Yan I, Börschel CS, Neumann JT, Sprünker NA, Makarova N, Kontto J, Kuulasmaa K, Salomaa V, Magnussen C, Iacoviello L, Di Castelnuovo A, Costanzo S, Linneberg A, Söderberg S, Zeller T, Ojeda‐Echevarria FM, Blankenberg S, Westermann D. High‐sensitivity cardiac troponin I levels and prediction of heart failure: results from the BiomarCaRE Consortium. JACC Heart Fail 2020;8:401–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PREVEND flow diagram: participant selection.
Figure S2. Associations of selected biomarkers with incident heart failure across body mass index categories.
Table S1. Associations of cardiovascular biomarkers with body mass index.
Table S2. Associations of cardiovascular biomarkers with incident heart failure in the total population.
Table S3. Associations of cardiovascular biomarkers with incident heart failure after accounting for death as a competing risk.
Table S4. Associations of selected biomarkers with incident heart failure across body mass index categories after accounting for death as a competing risk.


