Abstract
Introduction
Fornix deep brain stimulation (fx‐DBS) is under investigation for treatment of Alzheimer's disease (AD). We investigated the anatomic correlates of flashback phenomena that were reported previously during acute diencephalic stimulation.
Methods
Thirty‐nine patients with mild AD who took part in a prior fx‐DBS trial (NCT01608061) were studied. After localizing patients’ implanted electrodes and modeling the volume of tissue activated (VTA) by DBS during systematic stimulation testing, we performed (1) voxel‐wise VTA mapping to identify flashback‐associated zones; (2) machine learning–based prediction of flashback occurrence given VTA overlap with specific structures; (3) normative functional connectomics to define flashback‐associated brain‐wide networks.
Results
A distinct diencephalic region was associated with greater flashback likelihood. Fornix, bed nucleus of stria terminalis, and anterior commissure involvement predicted memory events with 72% accuracy. Flashback‐inducing stimulation exhibited greater functional connectivity to a network of memory‐evoking and autobiographical memory‐related sites.
Discussion
These results clarify the neuroanatomical substrates of stimulation‐evoked flashbacks.
Keywords: Alzheimer's disease, brain connectivity, deep brain stimulation, fornix, magnetic resonance imaging, memory
1. INTRODUCTION
Deep brain stimulation targeting the fornix region (fx‐DBS) is currently under investigation for the treatment of Alzheimer's disease (AD). 1 , 2 , 3 , 4 A recent paper reported on acute flashback‐like phenomena—the involuntary recall of autobiographical memories described by earlier authors as “reminiscences” 5 , 6 —that were experienced by a subset of AD patients during initial postoperative fx‐DBS programming; these were associated with specific stimulation settings and appeared to be unrelated to intrinsic whole‐brain or hippocampal volume. 7 Pioneering 19th and 20th century experiments involving intraoperative direct electrical stimulation of the exposed cortex 8 , 9 , 10 , 11 , 12 provided tremendous insight into the causal relationships between cortical areas and reminiscences, and these insights still stand. 5 , 6 However, the relationship between these memory phenomena and deep brain structures has not been systematically investigated and it remains unknown which specific structures give rise to the flashbacks reported in the aforementioned fx‐DBS population. Although the fornix is the designated target for therapeutic neuromodulation and is well‐known to be a critical component of the brain's memory circuits, 13 , 14 , 15 the stimulated region encompasses a number of other white matter tracts and nuclei that could also conceivably play a role. These include the anterior commissure, 14 , 16 septal nuclei, 17 and bed nucleus of stria terminalis, 18 each of which has been implicated in memory function and—like the fornix—is intimately connected to medial temporal lobe structures like the hippocampus and entorhinal/perirhinal cortex. 19 , 20 , 21
To address this question and further elucidate the architecture of human memory experience, we investigated the neuroanatomical substrate of stimulation‐induced flashbacks in patients with mild AD who were undergoing bilateral fx‐DBS. We hypothesized that the fornix itself would be a key contributor to this phenomenon. However, given that the fornix is presumably engaged in most cases of fx‐DBS and yet not all patients experience flashbacks, we expected that nearby structures might also be necessary substrates. To facilitate this investigation, we performed (1) volume of tissue activated modeling and voxel‐wise linear modeling of stimulation resulting in memory events as compared with stimulation without events; (2) machine learning–based prediction of flashback occurrence given involvement of specific brain structures; (3) normative resting state functional magnetic resonance imaging (rsfMRI) connectomics involving the aforementioned stimulation volumes; and (4) validation of the normative connectivity results by comparison with both brain areas previously shown to elicit flashbacks when electrically stimulated and also with regions and networks heavily implicated in autobiographical memory and memory retrieval.
2. MATERIALS AND METHODS
The analysis involved behavioral observations and pre‐ and postoperative structural MRI data from 39 patients with mild AD (Table 1 ) who were treated with bilateral fx‐DBS as part of a previously described, 42‐patient, multicenter clinical trial (ClinicalTrials.gov number: NCT01608061). 3 ***Each patient who was enrolled in this trial, which was approved by independent research ethics boards at every participating site, provided written informed consent. As specified previously, patients were diagnosed by standardized criteria and expert examination, with the criteria for mild probable AD being scores of 0.5 or 1 on the Clinical Dementia Rating scale and scores of 12‐24 on the Alzheimer's Disease Assessment Scale‐11. 22 Additional inclusion and exclusion criteria for enrollment are outlined in Table S1. All patients were implanted with bilateral quadripolar (four contacts each) DBS electrodes (model 3387, Medtronic, Minneapolis, MN) and connected to an implantable pulse generator 23 (Table S2). Of the 42 patients enrolled in the clinical trial, 3 were excluded due to inadequate image quality, which precluded precise electrode localization.
TABLE 1.
Patient demographics and baseline clinical characteristics
| All patients (n = 39) | Patients without flashbacks (n = 21) | Patients with at least one flashback (n = 18) | |
|---|---|---|---|
| Age at surgery, mean (SD), years | 67.7 (8.0) | 67.7 (7.0) | 67.7 (9.2) |
| Sex | 19f, 20 m | 11f, 10 m | 8f, 10 m |
| Baseline Alzheimer's Disease Assessment Scale‐Cognitive Subscale score, mean (SD) | 19.5 (5.6) | 19.2, (5.8) | 19.9, (5.6) |
| Disease duration at surgery, mean (SD), years | 2.1 (1.7) | 2.0 (1.7) | 2.3 (1.8) |
| Age at diagnosis, mean (SD), years | 65.6 (8.1) | 65.7 (7.1) | 65.5 (9.4) |
| Number of patients diagnosed at age < 65 | 14 | 8 | 6 |
During initial postoperative programming of the device, each electrode contact (four per lead) was tested with high‐frequency (130 Hz, 90 μs) stimulation beginning at a low voltage (∼1 volt) and increasing in 1 volt increments up to the maximal voltage tolerated (max = 10 volts). For each contact (eight per patient), if any setting induced a flashback, the lowest flashback‐inducing voltage setting was sampled, along with the voltage setting(s) immediately below and—if present—above that did not induce flashbacks. For all contacts without induced memory events, the largest voltage setting tested was utilized. This conservative selection method was designed to avoid false‐positive results. Classification of memory events was determined using the TEMPau (Test Episodique de Mémoire du Passé autobiographique) scale. 24 Although the quality of reminiscences elicited by electrical stimulation varies, 5 this paper's primary aim was to elucidate the neural correlates of flashback phenomena in general rather than those underlying subtle variation in memory quality; as such, only presence (TEMPau score 1‐4; “memory‐yes”) or absence of memory events (TEMPau score 0; “memory‐no”) were considered for analysis.
RESEARCH IN CONTEXT
Systematic Review: Pioneering 19th and 20th century experiments involving intraoperative direct electrical brain stimulation provided tremendous insight into the causal relationships between cortical areas and memory. As evidenced by recent reviews (Curot et al., 2017, 2020) and our own inspection of the published literature, few modern studies have directly built on this research legacy.
Interpretation: Our analysis of a serendipitous phenomenon observed during deep brain stimulation (DBS) of the fornix region allowed us to contribute to this classical literature, describing specific diencephalic structures–namely the fornix, anterior commissure, and bed nucleus of stria terminalis—that predict induction of memory flashbacks and implicating a network of areas previously shown to evoke memories when stimulated.
Future Directions: These findings help clarify the neuroanatomical underpinnings of stimulation‐induced flashbacks. Given that the fornix DBS is under investigation for its potential to alleviate memory impairment in Alzheimer's disease, this insight might inform therapeutic approaches.
2.1. Volume of tissue activated modeling
First, we used patient‐specific anatomic MRI, stimulation settings, and volume of tissue activated (VTA) modeling techniques to estimate the extent of tissue directly modulated by DBS during each observation. Following non‐uniformity correction of all MR images, VTA modeling was conducted using a well‐described pipeline (lead‐DBS v2.0; https://www.lead-dbs.org/). This involved localization of electrode contacts on postoperative MRI acquisitions by two experienced users (AH and GJBE), non‐linear normalization to MNI152 standard space (using “low variance” ANTS (http://stnava.github.io/ANTs/) with an additional subcortical affine transformation when necessary) via coregistered preoperative images, and estimation of the shape/extent of the electrical field using finite element method modeling with 0.2 V/mm gradient thresholding (FieldTrip‐SimBio pipeline; http://fieldtriptoolbox.org). 25 , 26 A VTA was estimated for each of the sampled “memory‐yes” and “memory‐no” observations using the corresponding stimulation setting (contact and voltage) and peri‐electrode conductivity estimates (gray matter: 0.33 S/m; white matter: 0.14 S/m; cerebrospinal fluid: 1.79 S/m; electrode contact: 108 S/m; insulated electrode components: 10−16 S/m) derived from standard space tissue priors. Left‐sided VTAs were flipped in the sagittal plane to facilitate group‐level analysis. Figure 1 provides a visual summary of the major neuroimaging processing steps used in this paper.
FIGURE 1.
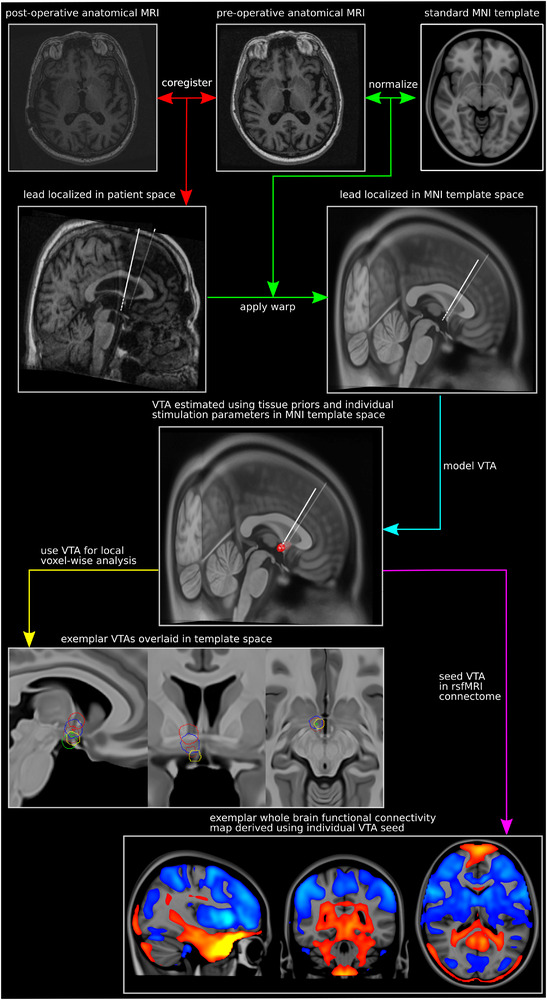
Visual summary of neuroimaging methods. The major methodological steps (colored arrows) and their corresponding descriptions are shown alongside exemplar brain images. First, patient‐acquired pre‐ and postoperative anatomic MRI scans were coregistered together, and each patient's leads were precisely localized in patient space based on the post‐operative acquisition (red arrows). Next, the coregistered patient scans were normalized to a standard MNI152 template, and the resultant transforms were used to warp the lead models to MNI space (green arrows). VTAs were then modeled for each “memory‐yes/memory‐no” observation in MNI space using the corresponding stimulation settings and conductivity estimates from standard space tissue priors (turquoise arrow). Finally, the created VTAs (n = 386) were employed as (1) inputs for local voxel‐wise mapping analysis (yellow arrow) and (2) seeds for rsfMRI functional connectivity mapping (magenta arrow). MNI = Montreal Neurological Institute; rsfMRI = resting state functional magnetic resonance imaging; VTA = volume of tissue activated
2.2. Whole‐brain voxel‐wise analysis of flashback‐inducing VTAs
Next, “memory‐yes” and “memory‐no” VTAs were stratified by contact and stimulation voltage in order to examine the possible effects of these factors independent of VTA location. Simple linear models were estimated to assess the relationship between contact and voltage, and between voltage and memory events. Subsequently, whole‐brain voxel‐wise logistic regression comparing “memory‐yes” and “memory‐no” VTAs was conducted to identify brain areas associated with flashbacks while controlling for stimulation voltage.
2.3. Support‐vector machine classification
Support‐vector machine (SVM) learning was then used to further interrogate the brain structures driving flashbacks and determine the extent to which their involvement could predict memory events. Specifically, the presence and extent (in mm3) of overlap between VTAs and structures (as defined using a manually segmented high‐fidelity diencephalic atlas) 27 within memory‐associated regions were calculated and used to classify VTAs as “memory‐yes” or “memory‐no”. Modeling was performed with balanced data sets of 343 observations for both “memory‐yes” and “memory‐no” groups; additional observations for the “memory‐yes” cohort were created by random sampling with replacement. The most parsimonious model that best classified these observations was identified and validated using a 10‐fold (random split in 10 balanced (“memory‐yes” vs “memory‐no”) groups, 3 with 35 members per group and 7 with 34) cross‐validation approach. In addition, an alternative model classifying memory events on the basis of voltage and electrode contact alone was created for comparative purposes.
2.4. Connectomic mapping of flashback‐inducing VTAs
To investigate wider brain networks associated with flashback‐inducing stimulation, whole‐brain connectivity maps were derived for each VTA using a high‐quality normative 3 Tesla rsfMRI data set (http://neuroinformatics.harvard.edu/gsp/) as described previously. 25 , 28 , 29 , 30 , 31 Normative data were used for the primary analysis instead of patient‐derived rsfMRI images because the latter were not acquired in the majority of patients and were of low fidelity (eg, 1.5 Tesla) when present. Per this connectomic method, correlations with the seed region (ie, the VTA) were obtained for each voxel in the brain based on the time course of low‐frequency blood oxygen level–dependent (BOLD) signal fluctuations across 1000 healthy subjects (age range: 18‐35 years; 57% female) (in‐house MATLAB script, The MathWorks, Inc., Version R2018a. Natick, MA). Whole‐brain voxel‐wise logistic regression was then conducted to identify brain areas whose connectedness was associated with incidence of memory events. Finally, to validate these normative results, a supplemental connectivity analysis was performed using a disease‐specific connectome assembled from 12 AD‐DBS patients with available preoperative rsfMRI imaging.
2.5. Connectomic overlap with canonical memory networks
To explore how these findings related to the relevant human literature, we analyzed the spatial overlap between our DBS‐induced flashback functional connectivity network and (1) brain structures identified through meta‐analysis as evoking memory events when stimulated 5 ; and (2) brain regions whose BOLD response is associated with memory as per Neurosynth (http://neurosynth.org) meta‐analyses of published task‐based fMRI studies. 32 For the former, we selected bilateral probabilistic regions‐of‐interest (ROIs) using a standardized atlas (Harvard‐Oxford cortical‐subcortical atlas) in MNI space (Figure 4 A). 33 For the latter, we used meta‐analytic association maps of voxels linked to autobiographical memory and memory retrieval across 84 and 228 pre‐existing fMRI studies, respectively. To assess whether overlap with these entities was non‐random, we permuted the voxels in the DBS‐induced flashback connectivity network 1000 times and determined the extent of each permutation's overlap with the aforementioned ROIs and meta‐analytic association maps. As an additional validation, we used the Neurosynth “decoder” to identify behavioral functional networks—derived from all available fMRI meta‐analyses—with the greatest spatial similarity to the flashback network. 32 , 34
FIGURE 4.
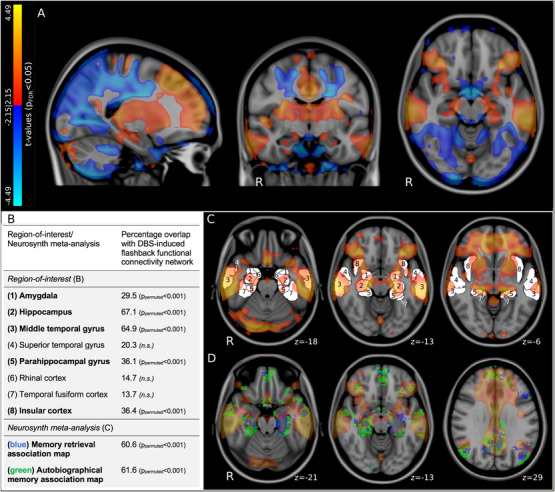
Connectomic mapping of flashback phenomena and comparison with memory‐inducing ROIs and regions involved in autobiographical memory. (A) Results of voxel‐wise normative functional connectivity analysis showing significantly (P FDR < 0.05) positively (red) and negatively (blue) connected voxels associated with memory flashbacks are overlaid on sagittal (left), coronal (middle), and axial (right) T1‐weighted MNI152 brain slices. Flashback‐inducing stimulation was linked to greater connectivity to the bilateral lateral temporal lobes, medial temporal lobes, prefrontal regions, cingulate cortex, and insular cortex. (B) Table stating percentage overlap of ROIs at which electrical stimulation reportedly induces acute memory events (see C) and Neurosynth meta‐analytic association maps (see D) with the DBS‐induced flashback connectome (P FDR < 0.05). The P value indicating the likelihood that this overlap is random given 1000 permutations of the flashback connectome is also provided (P permutation). (C, D) Axial T1‐weighted MNI152 brain slices showing DBS‐induced flashback connectome (warm colors) with (C) ROI outlines (white; labeled—see B), and (D) Neurosynth meta‐analytic association maps (green and blue—see B). FDR = false discovery rate; MNI = Montreal Neurological Institute; n.s. = not significant; ROI = region of interest
2.6. Statistics
All statistical analyses were performed using R (v3.4.4; https://www.r-project.org) and RMINC (https://github.com/Mouse-Imaging-Centre/RMINC). The pROC (version 1.16.2) package was used to calculate the receiver‐operating characteristic (ROC) curve and the e1071 (version 1.7‐3) package was used for the support vector machine (or SVM) analysis. Whole‐brain correction for multiple comparisons was performed using the false discovery rate (FDR; voxel‐wise threshold of P FDR < 0.05). To strengthen any voxel‐wise VTA mapping results and address the presence of non‐independent observations in our data, we also conducted a non‐parametric permutation analysis at the cluster level. Following a previously described approach, the clinical score associated with each VTA was randomly shuffled across all lead‐contact combinations, creating 10,000 new permuted data sets. Summary Q statistics were obtained for each data set and the summary statistic magnitudes of the actual voxel‐wise map and the permuted maps were compared to discern the validity of the observed results. 35 , 36 , 37
3. RESULTS
Of the 39 patients included for analysis, 18 (46%) patients experienced flashback phenomena at least once, whereas 21 patients (54%) never experienced flashbacks. Baseline demographic characteristics were similar between these two groups of patients (Table 1 ). In total, 43 “memory‐yes” and 343 “memory‐no” observations were sampled, and a separate VTA was created for each observation. Stratification of “memory‐yes” and “memory‐no” VTAs by contact and stimulation voltage revealed that stimulation delivered from the more dorsal three contacts (contacts “1‐3”) on occasion produced acute memory events, while stimulation at the ventral‐most contact (contact “0”) never did (Figure 2 A). There was a significant effect of contact on voltage, with mean voltage increasing incrementally as stimulation moved dorsally (P < 0.001, voltage at contacts 0‐3 [ventral to dorsal, mean±standard deviation]: contact 0: 5.16±1.22 volts, contact 1: 5.66±1.61 volts; contact 2: 6.36±1.99 volts; contact 3: 7.57±2.26 volts). This likely reflected a greater tendency for stimulation to evoke unpleasant autonomic side‐effects at ventral contacts (thus limiting the voltage tolerated), which were in proximity to hypothalamic nuclei. 7 Voltage was significantly lower for “memory‐yes” compared to “memory‐no” VTAs both overall (mean±standard deviation, “memory‐yes”: 5.67±2.01 volts; “memory‐no”: 6.36±2.05 volts, P < 0.01) and individually for contacts 2 (mean±standard deviation, “memory‐yes”: 5.63±1.86 volts; “memory‐no”: 6.54±2.00 volts, P < 0.05) and 3 (mean±standard deviation, “memory‐yes”: 6.14±2.29 volts; “memory‐no”: 7.81±2.18 volts, P < 0.01) (Figure 2 B).
FIGURE 2.
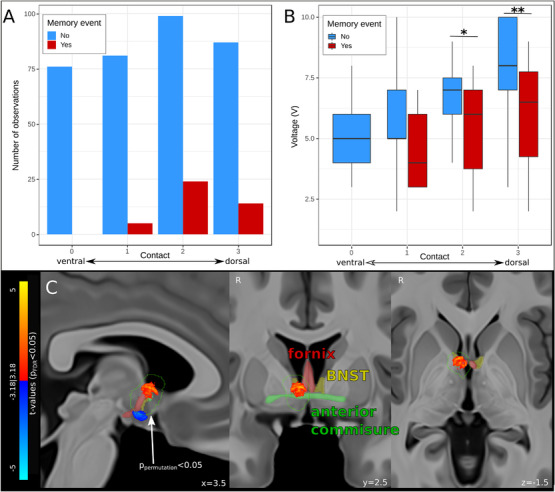
Results of voxel‐wise VTA mapping of flashback‐inducing stimulation. (A) Bar graph showing the number of stimulations (count) at each contact (ventral to dorsal, “0′” to “3′”) that did (red) or did not (blue) produce acute memory events. Note that no memory events were reported during stimulation of the ventral‐most contact. (B) Boxplot showing the minimal voltage (memory event, red) and maximal voltage (no memory event, blue) for the stimulations at each contact. (C) Results of voxel‐wise VTA mapping show voxels significantly (P FDR < 0.05) positively (warm colors) and negatively (cool colors) associated with memory flashbacks. Only the dorsal cluster of significant voxels lay within a region (outlined in green) established as non‐random by non‐parametric permutation testing (P permutation < 0.05, n = 10,000 permutations). The fornix (red), BNST (yellow), and anterior commissure (green) are overlaid in faded colors on sagittal (left), coronal (middle), and axial (right) T1‐weighted MNI152 brain slices. BNST = bed nucleus of stria terminalis; FDR = false discovery rate; MNI = Montreal Neurological Institute; VTA = volume of tissue activated; *P < 0.05; **P < 0.01
3.1. Whole‐brain voxel‐wise VTA analysis
Using whole‐brain voxel‐wise logistic regression to investigate the association of VTA location and memory events, we identified two significant clusters (each voxel passed FDR correction at P FDR < 0.05): a dorsal cluster in the anterior diencephalon, impinging on the column of the fornix, septal region, bed nucleus of stria terminalis (BNST), and anterior commissure, associated with greater likelihood of memory events; and a ventral cluster in the hypothalamus associated with a lower likelihood of memory events (Figure 2C; Table S3). To confirm that these results were not driven by patient‐specific characteristics of individuals who reported memory flashbacks, a linear mixed‐effect model analysis with subject as random variable (repeated measure design) was performed, looking only at patients who had at least one memory event. In these patients, we compared each setting that elicited flashbacks with a matched setting at the same contact, just below in voltage, that did not elicit flashbacks. This supplementary analysis, which used threshold‐free cluster enhancement 38 (TFCE; voxel threshold of P Bonferroni < 0.0001) for multiple comparisons correction, reaffirmed the results of the whole sample analysis, identifying a nearly identical cluster of voxels to be significantly associated with memory flashbacks (Figure S1). Only the dorsal cluster lay within a region that was shown by non‐parametric permutation testing to be non‐random (P permutation < 0.05, n = 10,000 permutations).
3.2. Support‐vector machine classification
SVM modeling reinforced the role of the fornix, BNST, and anterior commissure in eliciting memory flashbacks upon electrical stimulation. A model using stimulation voltage, volume overlap (continuous) with BNST, and impingement (binary) on fornix and anterior commissure was found to be most successful at classifying VTAs (Figure 3 ). This model achieved 72% accuracy (true‐negative rate: 0.68, false‐negative rate: 0.24, false‐positive rate: 0.32, true‐positive rate: 0.76) and 77% area under the receiver‐operating characteristic (ROC) curve (AUC) compared to chance performance (50%). The addition of other components like the septal region, other diencephalic structures, baseline Alzheimer's Disease Assessment Scale‐Cognitive Subscale scores, or demographic features (eg, age or sex) did not improve performance. The alternative model, which disregarded anatomic structure involvement and used only voltage and electrode contact, performed more poorly (67% accuracy; true‐negative rate: 0.46, false‐negative rate: 0.11, false‐positive rate: 0.54, true‐positive rate: 0.89; 67% AUC). Ten‐fold cross‐validation of the best model classified VTAs with 71% accuracy (true‐negative rate: 0.64, false‐negative rate: 0.22, false‐positive rate: 0.36, true‐positive rate: 0.78) (Figure S2).
FIGURE 3.
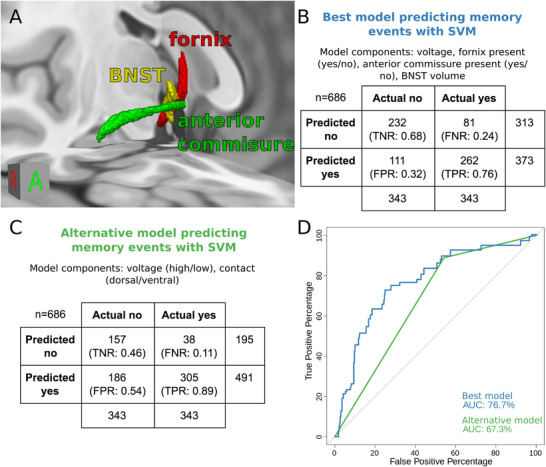
SVM modeling of flashback‐inducing stimulation. (A) Three‐dimensional rendering of fornix, BNST, and anterior commissure on a T1‐weighted MNI152 brain. (B) Confusion matrix summarizing classification performance of the best SVM, which incorporated voltage as well as VTA impingement on fornix, BNST, and anterior commissure. (C) Confusion matrix summarizing classification performance of an alternative SVM that used voltage and contact as components. (D) ROC curves and AUC summary of the best model (see B) and alternative model (see C). AUC = area under the ROC curve; BNST = bed nucleus of stria terminalis; FNR = false‐negative rate; FPR = false‐positive rate; MNI = Montreal Neurological Institute; ROC = receiver‐operating characteristic; SVM = support‐vector machine; TNR = true‐negative rate; TPR = true‐positive rate
3.3. Connectomic mapping of flashback phenomena
Whole‐brain voxel‐wise logistic regression of the VTA‐specific connectivity maps identified a number of brain areas whose connectedness was associated with memory events. Flashback‐inducing stimulation was linked to significantly (each voxel passed FDR correction at P FDR < 0.05) greater connectivity to the bilateral lateral and medial temporal lobes, prefrontal regions, cingulate cortex, and insular cortex (Figure 4A ). These same regions were also significantly related to flashback‐inducing stimulation when a disease‐specific connectome was used, corroborating our normative results (Figure S3).
3.4. Connectomic overlap with canonical memory networks
The extent of overlap between this DBS‐induced flashback connectivity profile and previously reported memory‐eliciting ROIs and memory‐related meta‐analytic association maps was then calculated. As verified by permutation testing (n = 1000 permutations), considerable non‐random overlap was observed between the flashback connectome and several ROIs (amygdala, hippocampus, middle temporal gyrus, parahippocampal gyrus, and insular cortex) as well as both the “memory retrieval” and “autobiographical memory” association maps (Figure 4 B‐D). Using the Neurosynth “decoder” we identified the top five most similar behavioral networks to be “autobiographical” (r = 0.24), “episodic” (r = 0.20), “retrieval” (r = 0.17), “autobiographical memory” (r = 0.17), and “episodic memory” (r = 0.17).
4. DISCUSSION
Using VTA modeling and normative functional connectomics, we uncovered brain areas and networks likely involved in previously reported stimulation‐induced flashback phenomena. 7 To our knowledge, this is the first systematic experimental analysis of deep subcortical stimulation causing acute reminiscences in humans. This marks a meaningful step beyond modern causal evidence, which has by necessity derived primarily from animal work or case series of human brain lesions. In doing so, this study marks a rare addition to the research legacy of classical 19th and 20th experiments 8 , 9 , 10 , 11 , 12 that meticulously elucidated the relationship between various brain areas and memory responses through direct intraoperative stimulation of the exposed cortex.
The region of the antero‐dorsal diencephalon emerged as important for inducing memory flashbacks; insights derived from machine learning moreover suggested that BNST, 39 , 40 fornix, 15 , 41 , 42 and anterior commissure, 43 , 44 , 45 in particular, contributed to these events. The fact that a model incorporating overlap with these structures performed better than an alternative model that relied solely on stimulation voltage and contact—specifically avoiding false‐positive identification of flashbacks—emphasizes that the occurrence of memory events cannot be explained fully by stimulation intensity or relative depth, instead being more accurately predicted by “hitting” specific neuroanatomical substrates. All three of these structures have been implicated extensively in memory function. 14 , 15 , 19 , 39 , 40 , 41 , 42 , 43 , 44 , 45 Of interest, the volume of VTA overlap appeared to make a difference with respect to flashback induction for the gray matter structure (BNST) but not for the two white matter structures. This may reflect the continuous nature of white matter axons, and the notion that impingement on a circumscribed cross‐section of a given bundle will propagate along its extent.
Through normative rsfMRI mapping, we found that flashback‐associated VTAs were preferentially connected to a wider brain network that primarily comprised the medial and lateral temporal lobes, prefrontal regions, insular cortex, and cingulate areas. These same regions are implicated in autobiographical memory recall by prior brain stimulation work 5 and functional neuroimaging studies, 32 as well as in a recent normative mapping analysis of brain lesions causing amnesia. 19 Indeed, the BNST, fornix, and anterior commissure are known to be intimately structurally connected with the medial and lateral temporal lobes. 13 , 16 , 18 This, coupled with the converging evidence described here, places these structures at the heart of this putative recall network and suggests that they may be ideally suited to evoke autobiographical memory percepts. Future prospective studies in humans should follow‐up on this line of research, seeking to clarify more specific roles for each structure and working to disambiguate their necessity or sufficiency with regard to flashbacks.
This study does have some limitations. For one, the collection of behavioral flashback data may have been affected by other AD phenomena such as delusions or disorientation. Other limitations relate to the neuroimaging methods employed. Finite element method VTA modeling was used to estimate the size and shape of the electrical fields generated by DBS. Although this approach utilized standard space tissue segmentations and conductivity values to approximate the extent of the electrical field, it remains a simplification of the manner in which electrical stimulation interfaces with the brain. Nonetheless, this method has been used in several recent publications 25 , 46 and has been shown to predict clinical improvement in out‐of‐sample data. 36 In addition, our connectomic analysis was performed primarily using normative data and thus may have omitted certain idiosyncrasies of patient‐ or pathology‐specific functional connectivity. This disadvantage is partially offset by a number of clear advantages of normative data, however. Unlike imaging obtained in patients, which is frequently of suboptimal quality, normative data gathered through initiatives such as the Brain Genomics Superstruct Project offer superior spatial resolution and signal‐to‐noise ratio. 32 , 47 , 48 Moreover, we were able to replicate our main connectivity results using a disease‐specific connectome derived from a subset of our AD‐DBS patients who had preoperative rsfMRI data, suggesting that these findings hold true in this specific population. This fits with recent work by Wang and colleagues 49 that compared the ability of healthy normative, disease‐specific, and patient‐specific connectomes to predict Parkinson disease DBS treatment response, finding that each connectome identified a similar whole‐brain pattern that significantly related to optimal outcome.
In sum, insights from VTA modeling, machine learning, and normative functional connectomics indicate that BNST, fornix, and anterior commissure are key local substrates of flashbacks evoked during fornix region DBS, and that flashback‐inducing stimulation interacts with a distributed brain network previously implicated in autobiographical memory retrieval. These findings might provide the basis for future work investigating therapies to stabilize or improve memory in patients with dementia.
AUTHOR CONTRIBUTIONS
Jürgen Germann, Gavin J.B. Elias, and Alexandre Boutet conceived the experiment. Wissam Deeb, Bryan Salvato, Leonardo Almeida, Kelly D. Foote, Paul B. Rosenberg, David F. Tang‐Wai, David A. Wolk, Anna D. Burke, Stephen Salloway, Marwan N. Sabbagh, M. Mallar Chakravarty, Gwenn S. Smith, Constantine G. Lyketsos, Michael S. Okun, and Andres M. Lozano collected the data. Andreas Horn performed MR image registration and electrode localization. Alexandre Boutet and Keshav Narang constructed the VTAs. Aaron Loh constructed the patient specific connectome. Jürgen Germann and Gavin J.B. Elias performed the data analysis. Jürgen Germann, Gavin J.B. Elias, and Clemens Neudorfer wrote the manuscript. All authors edited the manuscript. Andres M. Lozano supervised the project.
FUNDING
This work was supported by the RR Tasker Chair in Functional Neurosurgery at University Health Network (AML), the Canadian Institutes of Health Research (reference # 164235: GJBE), and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG NE 2276/1‐1: CN; DFG grant 410169619: AH).
COMPETING INTERESTS
AML is scientific director of Functional Neuromodulation and reports consultant fees from Medtronic, Abbott, and Boston Scientific. CGL reports consultant fees from Avanir, Eli Lilly, and the NFL benefits office. MSO reports consultant fees from the American Academy of Neurology, Peerview, WebMD/Medscape, and MedEdicus. PBR reports consultant fees from GLG, Leerink, Otsuka, Avanir, Bionomics, ITI, IQVIA, and the US Food and Drug Administration. SS reports research support and consultant fees from Biogen, Lilly, Eisai, Genentech, Roche, Novartis, and Avid. DAW reports consultant fees from Lilly, Merck, Jannsen, GE Healthcare, and Neuronix. MNS reports consultant fees from Allergan, Biogen, Roche, Genentech, Cortexyme, Bracket, and Sanofi. The other authors have nothing to disclose.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Germann J, Elias GJB, Boutet A, et al. Brain structures and networks responsible for stimulation‐induced memory flashbacks during forniceal deep brain stimulation for Alzheimer's disease. Alzheimer's Dement. 2021;17:777–787. 10.1002/alz.12238
DATA AVAILABILITY STATEMENT
The data supporting these analyses are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119‐123. [DOI] [PubMed] [Google Scholar]
- 2. Laxton AW, Tang‐Wai DF, McAndrews MP, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010;68:521‐534. [DOI] [PubMed] [Google Scholar]
- 3. Leoutsakos J‐MS, Yan H, Anderson WS, et al. Deep brain stimulation targeting the Fornix for mild Alzheimer Dementia (the ADvance Trial): a two year follow‐up including Results of Delayed Activation. J Alzheimers Dis. 2018;64:597‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lozano AM, Fosdick L, Chakravarty MM, et al. A Phase II study of Fornix deep brain stimulation in mild Alzheimer's disease. J Alzheimers Dis;2016:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curot J, Busigny T, Valton L, et al. Memory scrutinized through electrical brain stimulation: a review of 80 years of experiential phenomena. Neurosci Biobehav Rev. 2017;78:161‐177. [DOI] [PubMed] [Google Scholar]
- 6. Curot J, Roux F‐E, Sol J‐C, Valton L, Pariente J, Barbeau EJ. Awake craniotomy and memory induction through electrical stimulation: why are penfield's findings not replicated in the modern era?. Neurosurgery. 2020;87:E130‐E137 . 10.1093/neuros/nyz553. [DOI] [PubMed] [Google Scholar]
- 7. Deeb W, Salvato B, Almeida L, et al. Fornix‐region deep brain stimulation‐induced Memory Flashbacks in Alzheimer's Disease. N Engl J Med. 2019;381:783‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrier D. The Functions Of The Brain. London: Smith, Elder & Co.; 1876. [Google Scholar]
- 9. Ferrier D. The croonian lectures on cerebral localisation. Br Med J. 1890;1:1349‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foerster O. The cerebral cortex in man. Lancet. 1931;221:309‐312. [Google Scholar]
- 11. Penfield W. Memory mechanisms. Arch Neurol Psychiatry. 1952;67:178. [DOI] [PubMed] [Google Scholar]
- 12. Penfield W, Rasmussen T. The cerebral cortex of man: a clinical study of localization of function. JAMA. 1950;144:1412. [Google Scholar]
- 13. DeVito JL, Jr WhiteLE. Projections from the fornix to the hippocampal formation in the squirrel monkey. J Comp Neurol. 1966;127:389‐398. [DOI] [PubMed] [Google Scholar]
- 14. Botez‐Marquard T, Botez MI. Visual memory deficits after damage to the anterior commissure and right fornix. Arch Neurol. 1992;49:321‐324. [DOI] [PubMed] [Google Scholar]
- 15. Douet V, Chang L. Fornix as an imaging marker for episodic memory deficits in healthy aging and in various neurological disorders. Front Aging Neurosci. 2014;6:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peltier J, Verclytte S, Delmaire C, Pruvo J‐P, Havet E, Le Gars D. Microsurgical anatomy of the anterior commissure: correlations with diffusion tensor imaging fiber tracking and clinical relevance. Neurosurgery. 2011;69:ons241‐ons246; discussion ons246–7. [DOI] [PubMed] [Google Scholar]
- 17. Berti A, Arienta C, Papagno C. A case of amnesia after excision of the septum pellucidum. J Neurol Neurosurg Psychiatry. 1990;53:922‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staniloiu A, Markowitsch HJ. A rapprochement between emotion and cognition: amygdala, emotion, and self‐relevance in episodic‐autobiographical memory. Behav Brain Sci. 2012;35:164‐166. [DOI] [PubMed] [Google Scholar]
- 19. Ferguson MA, Lim C, Cooke D, et al. A human memory circuit derived from brain lesions causing amnesia. Nat Commun. 2019;10:3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mishkin M. A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci. 1982;298:83‐95. [DOI] [PubMed] [Google Scholar]
- 21. Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): mRI methods. J Magn Reson Imaging. 2008;27:685‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piolino P, Desgranges B, Eustache F. Episodic autobiographical memories over the course of time: cognitive, neuropsychological and neuroimaging findings. Neuropsychologia. 2009;47:2314‐2329. [DOI] [PubMed] [Google Scholar]
- 25. Horn A, Reich M, Vorwerk J, et al. Connectivity predicts deep brain stimulation outcome in Parkinson's disease. Ann Neurol 2017;82:67‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horn A, Li N, Dembek TA, et al. Lead‐DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage. 2019;184:293‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neudorfer Clemens, Germann Jürgen, Elias Gavin J. B., Gramer Robert, Boutet Alexandre, Lozano Andres M. (2020) A high‐resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Scientific Data, 7 (1), 10.1038/s41597-020-00644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Joutsa J, Shih LC, Horn A, et al. Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol. 2018;84:153‐157. [DOI] [PubMed] [Google Scholar]
- 29. Elias GJB, Giacobbe P, Boutet A, et al. Probing the circuitry of panic with deep brain stimulation: connectomic analysis and review of the literature. Brain Stimul. 2020;13:10‐14. [DOI] [PubMed] [Google Scholar]
- 30. Boutet A, Jain M, Elias GJB, et al. Network basis of seizures induced by deep brain stimulation: literature review and connectivity analysis. World Neurosurg. 2019;132:314‐320. [DOI] [PubMed] [Google Scholar]
- 31. Mithani K, Boutet A, Germann J, et al. Lesion network localization of seizure freedom following MR‐guided laser interstitial thermal ablation. Sci Rep. 2019;9:18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large‐scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968‐980. [DOI] [PubMed] [Google Scholar]
- 34. Rubin TN, Koyejo O, Gorgolewski KJ, Jones MN, Poldrack RA, Yarkoni T. Decoding brain activity using a large‐scale probabilistic functional‐anatomical atlas of human cognition. PLoS Comput Biol. 2017;13:e1005649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eisenstein SA, Koller JM, Black KD, et al. Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Ann Neurol. 2014;76:279‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dembek TA, Roediger J, Horn A, et al. Probabilistic sweet spots predict motor outcome for deep brain stimulation in Parkinson disease. Ann Neurol. 2019;86:527‐538. 10.1002/ana.25567. [DOI] [PubMed] [Google Scholar]
- 37. Dembek TA, Barbe MT, Åström M, et al. Probabilistic mapping of deep brain stimulation effects in essential tremor. Neuroimage Clin. 2017;13:164‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83‐98. [DOI] [PubMed] [Google Scholar]
- 39. Goode TD, Maren S. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn Mem. 2017;24:480‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goode TD, Acca GM, Maren S. Threat imminence dictates the role of the bed nucleus of the stria terminalis in contextual fear. Neurobiol Learn Mem. 2020;167:107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. D'Esposito M, Verfaellie M, Alexander MP, Katz DI. Amnesia following traumatic bilateral fornix transection. Neurology. 1995;45:1546‐1550. [DOI] [PubMed] [Google Scholar]
- 42. Tsivilis D, Vann SD, Denby C, et al. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834‐842. [DOI] [PubMed] [Google Scholar]
- 43. Lewine JD, Doty RW, Astur RS, Provencal SL. Role of the forebrain commissures in bihemispheric mnemonic integration in macaques. J Neurosci. 1994;14:2515‐2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doty RW, Overman WH, Negrão N. Role of Forebrain Commissures in Hemispheric Specialisation and Memory in Macaques. Structure and Function of Cerebral Commissures;1979:333‐342. 10.1007/978-1-349-03645-5_26. [DOI] [Google Scholar]
- 45. Kucharski D, Burka N, Hall WG. The anterior limb of the anterior commissure is an access route to contralateral stored olfactory preference memories. Psychobiology. 1990;18:195‐204. [Google Scholar]
- 46. Baldermann JC, Melzer C, Zapf A, et al. Connectivity profile predictive of effective deep brain stimulation in obsessive‐compulsive disorder. Biol Psychiatry. 2019;85:735‐743. [DOI] [PubMed] [Google Scholar]
- 47. Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Glasser MF, Smith SM, Marcus DS, et al. The Human Connectome Project's neuroimaging approach. Nat Neurosci. 2016;19:1175‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Q, Akram H, Muthuraman M, et al. Normative vs. patient‐specific brain connectivity in Deep Brain Stimulation. Neuroimage 2020;224:117307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The data supporting these analyses are available from the corresponding author upon reasonable request.


