Summary
Individually, tissue and soluble markers involved in the programmed cell death protein 1/programmed death‐ligand (PD‐1/PD‐L) axis have been described as biomarkers with clinical value in classical Hodgkin lymphoma (cHL). In the context of the success of immune checkpoint blockade therapy in cHL, it is interesting to discover whether plasma levels of proteins in the PD‐1/PD‐L axis are a reflection of expression by the corresponding tissue. Paired tissue and plasma samples of cHL patients were collected and analysed for PD‐1, PD‐L1 and PD‐L2 levels. In addition, vascular endothelial growth factor (VEGF) and CD83, molecules regarded to influence the expression of PD‐1, PD‐L1 and/or PD‐L2, were included. PD‐L1 was upregulated in the plasma of cHL patients compared to healthy controls and correlated well with several clinical parameters. Strong PD‐L1 expression in the tumour microenvironment contributed to high soluble (s)PD‐L1 levels, although there was no direct correlation between plasma PD‐L1 levels and total expression of PD‐L1 in corresponding cHL tissue. Interestingly, we observed a positive correlation between VEGF and PD‐1 levels in both tissue and plasma. In conclusion, although PD‐L1 is a promising soluble biomarker in cHL, its levels do not reflect the total tissue expression. Future studies focusing on PD‐L1 as a predictor for immune checkpoint treatment response, should include both biopsy and plasma samples.
Keywords: Hodgkin lymphoma, immune checkpoint, PD‐1, PD‐L1, plasma biomarkers, VEGF
Introduction
In recent years, drugs that block programmed cell death‐1 (PD‐1) have shown high efficacy in relapsed and refractory classical Hodgkin lymphoma (cHL) patients, with overall response rates of 65–87%. 1 Programmed death‐ligand (PD‐L)1 and PD‐L2 are often overexpressed by the cHL tumour cells, called Hodgkin‐Reed Sternberg (HRS) cells, due to amplification of the 9p24·1 genomic region. There is also expression of these molecules on immune cells such as tumour associated macrophages (TAMs). 2 , 3 , 4
Several studies have investigated the clinical value of molecules involved in the PD‐1/PD‐L axis in primary cHL patients. In HL tissue, high expression of PD‐L1 on HRS cells has been associated with advanced stage of the disease and worse prognosis. 5 , 6 High proportions of PD‐L1+ TAMs in the HL tumour microenvironment (TME) were more prevalent in patients with low serum albumin, bulky disease, B symptoms and a high International Prognostic Score (IPS). 3 In addition, high proportions of PD‐1+ tumour‐infiltrating lymphocytes and the presence of PD‐L1+ TAMs in the TME have been associated with inferior outcome. 3 , 7 Interestingly, upon relapse after standard first‐line treatment, the percentage of PD‐1+ tumour‐ infiltrating lymphocytes, PD‐L1+ TAMs and PD‐L1+ HRS cells were significantly increased in comparison to primary biopsies. 8 Moreover, upon progression on PD‐1 blockade treatment, the number of PD‐1+ T cells was higher and the number of PD‐L1+ TAMs was lower, whereas expression of PD‐L1 by HRS cells seemed unaffected compared to pre‐treatment tissue samples. 9
Besides being expressed on the cell membrane PD‐1, PD‐L1 and PD‐L2 can also be present as soluble proteins in the blood. 10 This suggests that blood could be used as a surrogate for tissue to determine expression of these molecules. The advantages of using blood‐based compared to tissue biomarkers are the non‐invasive collection method, the opportunity to obtain sequential samples for treatment response and the possibility of identifying relapses at an early time point. Indeed, higher levels of soluble (s)PD‐L1 were observed in patients with cHL compared to controls. 11 , 12 Elevated levels of sPD‐L1 at diagnosis were significantly associated with B symptoms, advanced disease stage, bulky disease, high erythrocyte sedimentation rate and low serum albumin. 13 Patients with higher levels of sPD‐L1 had significantly shorter progression‐free survival (PFS) compared to those with low levels of sPD‐L1. 11 In contrast, no association was found between clinical parameters and sPD‐1. 13 Although both tissue expression and soluble levels have shown a prognostic impact for the proteins of the PD‐1/PD‐L axis, the correlation between soluble levels and tissue expression was not studied in paired tissue and plasma.
The levels of PD‐1, PD‐L1 or PD‐L2 are regulated by VEGF and possibly linked to CD83. VEGF induced PD‐1 expression on CD8+ T cells in mouse models of colon cancer. 14 In HL, VEGF is expressed by both HRS cells and TAMs. 15 High VEGF levels have been implicated in HL progression and correlated with high PD‐L1 and PD‐L2 expression by HRS cells. 16 , 17 CD83 plays a role in antigen presentation and cellular interaction following lymphocyte activation. CD83 is frequently expressed by HRS cells, which can lead to expression of CD83 on T cells via trogocytosis. 18 , 19 CD83+ CD4+ T cells showed a significantly higher expression of PD‐1 compared to CD83− CD4+ T cells in a co‐culture model of a CD83+ HL cell line with T cells. This suggests, a possible co‐regulation of these molecules, although a direct causal relation has not been proven. 19
Although membrane bound and soluble PD‐1, PD‐L1 and PD‐L2 have been studied individually, it is currently unknown which biomarker is the most promising and whether the immune checkpoint levels in the blood of cHL patients can be used as a surrogate for expression of these molecules by the tumour and infiltrating immune cells. This study, for the first time, addresses this association by comparing the soluble immune checkpoint levels in paired tissue and plasma samples in newly diagnosed cHL patients.
Materials and methods
Patient inclusion and characteristics
Plasma was collected from cHL patients in the University Medical Center Groningen (UMCG) between January 2006 and June 2011. 20 In total, 69 newly diagnosed patients were included. Patient characteristics are summarised in Table I. Median age of the patients was 37 years (range 16–66) and there were slightly more females (57%) than males. Most patients had the nodular sclerosis subtype (65%) and early stage disease (stage I–II) (64%). Metabolic tumour volume (MTV) was determined as described previously 20 and could be obtained for 40 patients. This cohort and data on MTV, soluble thymus‐ and activation‐regulated chemokine (sTARC) and sCD163 have been published previously. 20 , 21 Healthy controls were selected from a previously published age and gender‐matched cohort. 20 Patients were compared with 26 controls [median age 30 years (range 21–61), 59% females]. Permission for this study was obtained from the institutional review board of the UMCG and all participating patients and healthy controls signed informed consent.
Table I.
Patient characteristics.
| Characteristics |
Plasma cHL patients (n = 69) n (%) |
FFPE cHL patients (n = 26) n (%)* |
|---|---|---|
| Median age (range) | 37 (16–66) | 33 (16–60) |
| Female | 39 (57) | 16 (62) |
| Histology | ||
| NS | 45 (65) | 17 (65) |
| MC | 7 (10) | 4 (15) |
| LR | 4 (6) | 1 (4) |
| cHL NOS | 13 (19) | 4 (15) |
| Ann Arbor stage | ||
| I–II (early stage) | 44 (64) | 17 (65) |
| III–IV (advanced stage) | 25 (36) | 9 (35) |
| B‐symptoms present | 27 (39) | 12 (46) |
| Bulky disease | 22 (32) | 9 (35) |
cHL, classical Hodgkin lymphoma; FFPE, formalin fixed paraffin embedded; NS, Nodular sclerosis; MC, mixed cellularity; LR, lymphocyte rich; NOS, not otherwise specified.
FFPE samples were available for 26 of 69 patients.
Immunohistochemistry
Formalin fixed paraffin embedded (FFPE) tissue samples could be retrieved from 26 patients that were representative of the total patient group of 69 (Table I). Immunohistochemistry (IHC) for PD‐1 (clone: NAT105; Ventana Medical Systems; AZ, USA) and PD‐L1 (clone: E1L3N; Cell Signaling Technology; MA, USA) was performed using an automated cell stainer (Ventana Benchmark Ultra; Ventana Medical Systems). IHC for TARC, CD163, PD‐L2, VEGF and CD83 were performed according to standard laboratory procedures. Briefly, slides were deparaffinised with xylene and heat induced‐antigen retrieval was performed using a 10 mM citrate buffer at pH 6·0 (for TARC and CD163), a 1 mM ethylene diamine tetracetic acid (EDTA) buffer at pH 8·0 (for CD83 and VEGF) or a 10 mM tris‐hydroxymethyl‐aminomethane (Tris)/1 mM EDTA buffer at pH 9·0 (for PD‐L2). Primary antibodies were used against TARC (polyclonal; 1:50; R&D Systems; MN, USA), CD163 (clone: 10D6; 1:100; Leica Biosystems; Wetzlar Germany), PD‐L2 (clone: D7U8C; 1:50; Cell Signaling Technology), CD83 (clone: F5, sc‐55535; 1:50; Santa Cruz Biotechnology; TX, USA) or VEGF (polyclonal; RB‐9031‐R7 1:2; ThermoFisher Scientific; MA, USA). Primary antibodies were detected using appropriate secondary and tertiary horseradish peroxidase (HRP) conjugated antibodies and visualised with diaminobenzidine (DAB). TARC and CD163 were included as markers for tumour cells and TAMs respectively.
Staining of HRS cells and TME was evaluated manually by an experienced haematopathologist (AD). HRS cell positivity for TARC, PD‐L1, PD‐L2, CD83 and VEGF was scored as negative (<25% of tumour cells positive), heterogeneous (25–75% of tumour cells positive) or positive (>75% of tumour cells positive). The TME was scored as negative (−), positive (+) or strong positive (++). Next, slides were scanned by a Hamamatsu NanoZoomer 2.0 HT slide scanner (Hamamatsu City, Japan) at 40× magnification and the percentage of positive pixels was determined using the positive pixel count algorithm in the Aperio ImageScope program (version 11). Areas with only necrosis, sclerosis or other technical artefacts (e.g. tissue folding) were excluded from the analysis. For the positive pixel count algorithm, a hue value of 0·1 and a hue width of 0·5 were used. A colour saturation threshold of 0·04 was used. For PD‐1, the intensity threshold was set at 125 to exclude non‐specific background staining, for the other analyses 220 was used. The number of positive pixels was divided by the total number of pixels (negative and positive) in the analysed area, and multiplied by 100, to obtain the percentage of positive pixels.
Enzyme‐linked immunosorbent assay
Levels of sTARC, sCD163, sPD‐1, sPD‐L1, sPD‐L2, sVEGF (R&D systems) and sCD83 (Sino Biological; Beijing, China) were measured with a sandwich enzyme‐linked immunosorbent assay (ELISA), according to the manufacturer’s instructions.
Statistical analysis
We used non‐parametric tests for analysis because of the skewed distribution of the biomarker levels in the plasma samples. Differences between two groups were evaluated by a two‐tailed Mann‐Whitney U test. To determine linear correlation coefficients (rho) between two different markers, a Spearman’s rank correlation test was used. The correlation was considered strong when the rho value was >0·7, moderate when the rho value was between 0·5 and 0·7 and weak when the rho value was between 0·3 and 0·5. Rho values below 0·3 were considered as not or very weakly correlated. Bonferroni multiple testing correction was performed on analyses concerning our main research questions and a statistically significant difference was considered when the P value was <0·01. For all secondary or explorative analyses P < 0·05 was considered significant. Statistical analyses were performed using GraphPad Prism, version 8.04 (GraphPad Software, San Diego, CA, USA).
Results
Expression of PD‐L1, PD‐L2 and PD‐1 in cHL tissue
Expression of molecules involved in the PD‐1/PD‐L axis was analysed on FFPE tissue of 26 cHL patients (Fig 1A–D). Expression of PD‐L1 and PD‐L2 in at least 25% of the HRS cells was observed in 10 out of 26 (38%) and eight of 26 (31%) of cases respectively (Table SI). Half of the PD‐L1 positive cases also expressed PD‐L2. Overall, PD‐L1 and/or PD‐L2 positivity in HRS cells was observed in 13 of 26 (50%) cases. Expression of PD‐L1 and PD‐L2 was observed in the TME in 18 of 26 (69%) and 15 of 26 (58%) of cHL cases, most likely expressed by TAMs. Combined, PD‐L1 and PD‐L2 expression in HRS cells and/or TAMs was observed in 22/26 (85%) and 19 of 26 (73%) of cHL cases respectively. PD‐1 was solely expressed in the TME and the number of positive cells was in general low. PD‐1 expression was rarely present in the T cells directly rosetting around HRS cells. Total positivity scores for PD‐L1, but not PD‐L2, defined by the positive pixel count algorithm showed a moderate correlation with both the tumour cell marker TARC and the TAM marker CD163 (P = 0·0042, rho = 0·611 and P = 0·0012, rho = 0·5992 respectively) (Figure S1). The total percentage PD‐L1 in the tissue correlated weakly with the percentage PD‐L2 (P = 0·04, rho = 0·4052), but both were not correlated with the percentage PD‐1 (Fig 1E).
Fig 1.
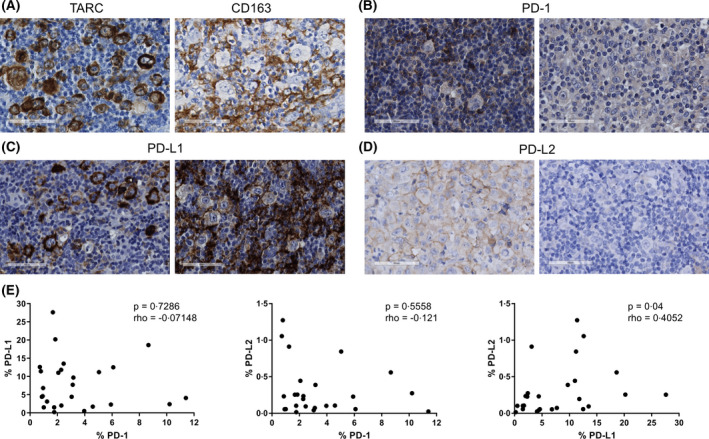
Representative examples of the immunohistochemical staining of thymus‐ and activation‐regulated chemokine (TARC), CD163, programmed cell death protein 1 (PD‐1), programmed death‐ligand (PD‐L1) and PD‐L2. (A) A representative case staining positive for TARC in the Hodgkin‐Reed Sternberg (HRS) cells and a representative case showing CD163 staining in the tumour associated macrophages (TAMs). (B) A representative case with (left panel) and without (right panel) PD‐1+ cells in the rosettes. (C) A representative case staining positive for PD‐L1 in the HRS cells, but not in the tumour microenvironment (TME) (left panel) and a case without PD‐L1 staining in the tumour cells, but with PD‐L1 positive cells in the TME (right panel). (D) A representative case with (left panel) and without (right panel) PD‐L2 staining in the HRS cells. (E) Correlations between the percentages of positive pixels of PD‐1 with both PD‐L1 and PD‐L2 and between PD‐L1 and PD‐L2. The correlation analyses were performed using a Spearman’s rank correlation test. A P value <0·05 was considered to be statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Plasma levels of PD‐L1, PD‐L2 and PD‐1 in cHL patients
Plasma of 69 cHL patients and 26 healthy controls was analysed for soluble levels of PD‐L1, PD‐L2 and PD‐1 (Fig 2A). Levels of sPD‐L1, were significantly higher in cHL patients compared to controls (P < 0·0001). There was no significant difference between sPD‐1 or sPD‐L2 levels in patients compared to controls. Levels of soluble markers were compared with clinical characteristics. Significantly higher levels of sPD‐L1 were observed in patients with advanced stage disease (P = 0·0412), B symptoms (P = 0·0152), high IPS (P = 0·0012) and low albumin levels (P = 0·0004), but not with bulky disease (Figure S2). sPD‐L1 levels were also moderately correlated with MTV (P = 0·0001, rho = 0·5813). Both sPD‐1 and sPD‐L2 were not significantly associated with clinical characteristics (Table SII). In addition, sPD‐L1 had a weak correlation with sTARC (P = 0·0045, rho = 0·3504) and sPD‐L2 with sCD163 (P = 0·026, rho = 0·2782) (Figure S3). No significant correlations were observed when sPD‐1, sPD‐L1 and sPD‐L2 were correlated with each other (Fig 2B). Together, these results indicate that PD‐L1 of the PD‐1/PD‐L axis proteins is the most promising soluble biomarker for cHL.
Fig 2.
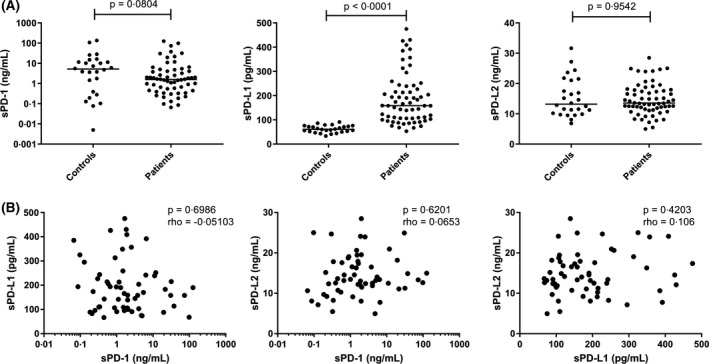
Comparison of plasma levels of programmed cell death protein 1 (PD‐1), programmed death‐ligand (PD‐L)1 and PD‐L2 between classical Hodgkin lymphoma patients and healthy controls. Plasma levels of (A) soluble (s)PD‐1 (ng/ml) (left panel), PD‐L1 (pg/ml) (middle panel) and sPD‐L2 (ng/ml) (right panel) were compared between patients and controls using a Mann‐Whitney U test and used a P value of <0·01 as the cut‐off for significance. (B) Pairwise correlations between the levels of sPD‐1, sPD‐L1 and sPD‐L2 using a Spearman’s rank correlation test with a P value cut‐off of <0·05 for significance.
Tissue expression versus plasma levels
To determine if plasma levels of soluble markers were representative of the expression in the paired tissues, both total percentage of positive pixels and manual IHC evaluation results were used to compare with plasma levels (Table SI). As a control, we also evaluated the percentage of positive pixels for the tumour cell specific marker TARC, and the TAM marker CD163. There was a strong correlation between the percentage of TARC in the tissue and sTARC levels (P < 0·0001, rho = 0·7209), while tissue and plasma levels for CD163 and PD‐L1 were not correlated (Fig 3A). To correct for complete tumour volume in the patient, we also calculated the staining area relative to the MTV. This clearly improved the correlation for TARC (P < 0·0001, rho = 0·9142) and for CD163 (P = 0·0023, rho = 0·701). However, correction for MTV did not yield significant results for PD‐L1 (Fig 3B). No significant correlations were found for PD‐L2 or PD‐1 (Table SIII). For PD‐L1, manual IHC results showed that tumour cell positivity did not correspond with high sPD‐L1 levels (Fig 3C). In contrast, strong TME positivity was associated with higher sPD‐L1 levels (Fig 3D). Interestingly, cases with strong positivity for PD‐L1 in the TME were usually negative for PD‐L1 in HRS cells (Fig 3E). No association was found for PD‐L2 (data not shown), which was also expressed in HRS cells and TME.
Fig 3.
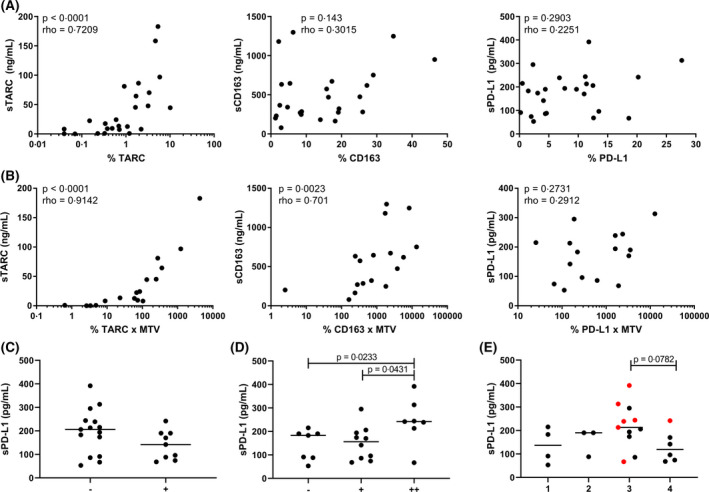
Comparison between tissue expression and plasma levels. (A) The percentage of positive pixels for thymus‐ and activation‐regulated chemokine (TARC), CD163 and programmed death‐ligand 1 (PD‐L1) in tissue of classical Hodgkin lymphoma patients was correlated with the levels of soluble (s)TARC, sCD163 and sPD‐L1 in the plasma using a Spearman’s rank correlation test. (B) Correlation between the metabolic tumour volume (MTV) adjusted pixel percentages of TARC, CD163 and PD‐L1 with the plasma levels of sTARC, sCD163 and sPD‐L1 using a Spearman’s rank correlation test. For A and B, a P value of <0·01 was considered to be statistically significant. (C) Hodgkin Reed‐Sternberg (HRS) cells in each case were scored as negative (<25%) or positive (25% or above) for PD‐L1 and divided in groups accordingly. Levels of sPD‐L1 in the negative and positive group are displayed. (D) The tumour microenvironment (TME) of each case was scored as negative (−), positive (+) or strong positive (++) for PD‐L1 and divided in groups accordingly. Levels of sPD‐L1 in each group are displayed. (E) The evaluation of the HRS cells and TME were combined and divided in four groups: HRS‐TME‐ (group 1); HRS+TME− (group 2); HRS−TME+ (group 3) and HRS+TME+ (group 4). In red cases with a strong positive (++) TME are indicated. Two groups were compared using a Mann‐Whitney U test. For C, D and E, a P value of <0·05 was considered to be statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
The effect of VEGF and CD83 on the PD‐1/PD‐L axis
Expression of VEGF by HRS cells was observed in 20 of 26 (77%) cases and in the TME in all 26 (100%) cases (Fig 4A). HRS cells expressed CD83 in 22 of 26 (85%) cases and CD83 was almost exclusively expressed by HRS cells (Fig 4B). In plasma, the levels of both sVEGF and sCD83 were significantly increased in cHL patients compared to healthy controls (P = 0·0001 and P < 0·0001 respectively) (Fig 4C). To support a possible link between VEGF or CD83 and the PD‐1/PD‐L axis, we correlated levels of these two proteins to PD‐1, PD‐L1 and PD‐L2. A borderline significant association was observed between the percentage of tissue staining positive for VEGF and PD‐1 (P = 0·0740; rho = 0·3563) and a significant correlation was found between sPD‐1 and sVEGF in plasma (P = 0·0072, rho = 0·3722), although both correlations were weak (Fig 4D). Additional correlation analyses performed, including all CD83 analyses, were not significant (Table SIV).
Fig 4.
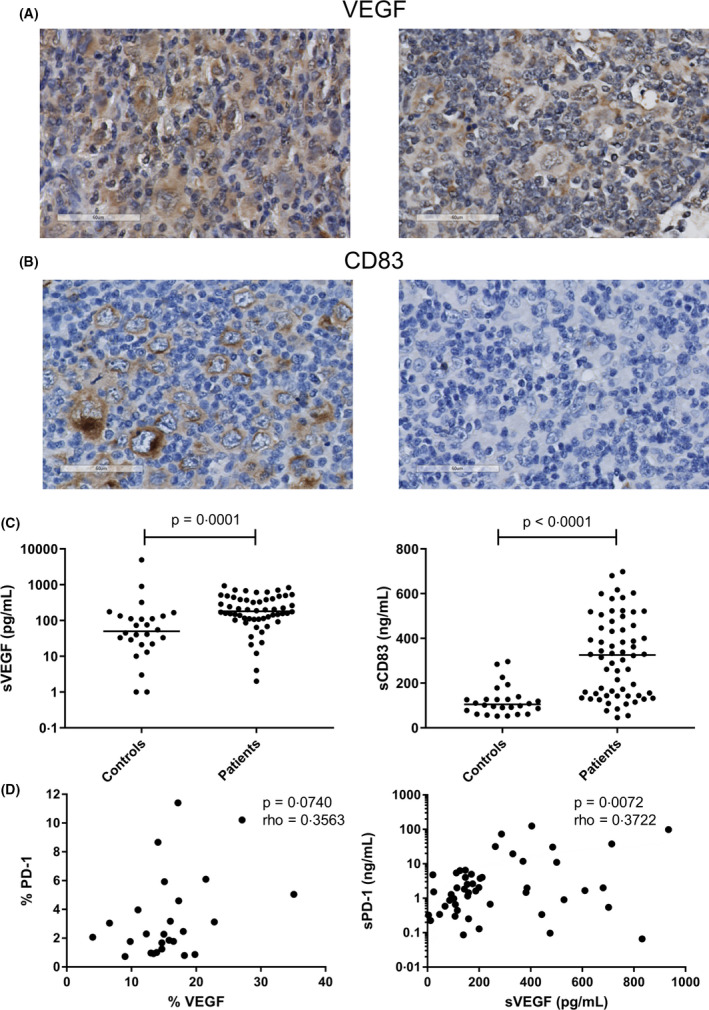
Effect of vascular endothelial growth factor (VEGF) and CD83 on the programmed cell death protein 1/programmed death‐ligand (PD‐1/PD‐L) axis. (A) Representative cases staining positive (left panel) and negative (right panel) for VEGF in the Hodgkin Reed‐Sternberg (HRS) cells. (B) Representative cases staining positive (left panel) and negative (right panel) for CD83 in the HRS cells. (C) Plasma levels of soluble (s)VEGF (pg/ml) (left panel) and sCD83 (ng/ml) (right panel) were compared between patients and controls using a Mann‐Whitney U test. (D) Correlation of VEGF and PD‐1 in both tissue (left panel) and plasma (right panel) using a Spearman’s rank correlation test. A P value of <0·05 was considered to be statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Immune checkpoint blockade therapy is extremely successful in relapsed and refractory HL patients, with high overall response rates. However, complete responses are scarce (9–22%) and median PFS is limited (11–15 months). 1 It is, therefore, important to identify patients who would benefit from this treatment at an early stage. Both tissue and soluble markers involved in the PD‐1/PD‐L axis have been associated with HL disease activity. 3 , 5 , 6 , 7 , 11 , 13 As the availability of tissue biopsies, especially at relapse, is often limited, the use of blood‐based biomarkers to predict treatment response would be more broadly applicable, cost effective and patient friendly. Therefore, this study aimed to investigate if soluble proteins involved in the PD‐1/PD‐L axis were representative of tissue expression.
We showed that plasma sPD‐L1 is the most promising biomarker to accurately reflect cHL disease activity. sPD‐L1 levels were significantly higher in cHL patients compared to controls and the levels were associated with several clinical parameters, confirming the results described previously by da Silva et al. in a smaller cohort of patients. 13 Although sPD‐L1 was associated with several adverse prognostic markers in our cohort, we were unable to determine the prognostic value of sPD‐L1 levels on PFS as described previously, 11 due to a limited number of relapses. In contrast, sPD‐L2 and sPD‐1 were not upregulated in cHL patients and were not associated with clinical characteristics. In tissue, PD‐L1 expression was observed in HRS cells and/or TME in the majority of cases. PD‐L1 expression on >25% of HRS cells was observed in 38% of cases, which is comparable to studies by Hollander et al. 3 , 8 but lower in comparison to other studies. 17 , 22 This might be due to the use of different cut‐off values to define PD‐L1 positivity on HRS cells. Interestingly, PD‐L1 expression in the TME and not in the HRS cells contributed to high sPD‐L1 levels. Despite this positive association, we did not observe a direct correlation between sPD‐L1 levels and the percentage of positive pixels for PD‐L1 in total tissue. This is probably due to the major contribution of PD‐L1 positive macrophages to soluble PD‐L1 levels; these cells are not always tumour specific and can also be found in other tissues. Interestingly, the levels of sTARC, a well‐known blood‐based biomarker for HL, 20 , 23 , 24 , 25 did show a strong correlation with the percentage of pixels positive for TARC, both with and without adjustment for MTV. In addition, for CD163, described previously as a disease response biomarker in HL by some studies, 21 , 26 a strong correlation was observed between the MTV‐adjusted percentage of pixels staining positive for CD163 and the levels observed in plasma, validating the approach as correct.
Two molecules, VEGF and CD83, have recently been described that might influence the expression of PD‐1, PD‐L1 and PD‐L2. In this study we confirmed the increased levels of both sVEGF and sCD83 in cHL patients compared to controls. 16 , 19 , 27 The positive correlation between VEGF and PD‐1 in both tissue and plasma supports the in vitro experiments showing that VEGF induced PD‐1 expression on CD8+ T cells. 14 The previously observed association between VEGF, PD‐L1 and PD‐L2, as described by Koh et al., could not be confirmed. This might be explained by the differences in the approaches, being limited to the expression of VEGF and PD‐1 ligands in HRS cells or, as in our study, in total tissue sections including the TME. 17 For CD83 we confirmed the expression by HRS cells in the majority of cases (>25% in 85% of cases). 19 Previously, it was also described that CD83+ CD4+ T cells expressed higher levels of PD‐1 compared to CD83− CD4+ T cells upon in vitro co‐culture of HL cell lines and T cells, suggesting a role of CD83 in up regulating PD‐1. However, we did not find a correlation in HL tissue, probably due to of the low number of CD83+ cells present in the TME. Taken together, this points towards a role for VEGF, but not CD83, in regulating the PD‐1/PD‐L axis.
Some limitations of our present study include its exploratory nature and the low number of matched tissue and plasma samples. Future studies are warranted to confirm our findings. Those studies should include both tissue biopsies and plasma samples to assess which biomarker most accurately predicts response to immune checkpoint blockade treatment. The most promising markers, based on our data, are TARC and PD‐L1. TARC can be used as a response marker and an indication of the tumour load. In addition, it would be interesting to monitor soluble PD‐L1 levels during immune checkpoint inhibitor treatment and compare this with the predictive value of PD‐L1 expression status in tissue biopsies obtained before the start of treatment, especially focusing on TME positivity.
In conclusion, both tissue and plasma PD‐L1 levels are promising cHL biomarkers. Although strong TME positivity for PD‐L1 seems to be the main contributor to high sPD‐L1 levels, we could not find a direct correlation between the total levels in the tissue and plasma. Interestingly, VEGF levels correlated with the PD‐1 levels in both tissue and plasma, supporting a potential role for VEGF in regulating PD‐1. Future studies should focus on identifying biomarkers that accurately predict immune checkpoint blockade treatment response, including relapse biopsy samples.
Conflict of interest
All authors declare no conflicts of interest.
Author contributions
JV, AD and LV designed the study. JV, AD, LV, AvdB and WJP wrote the manuscript. ZNDA, JV and LV performed the ELISA analysis. JV, LV and AD performed the IHC analysis. All authors read and approved the final manuscript.
Supporting information
Table SI. Immunohistochemistry scores and soluble levels of PD‐1, PD‐L1 and PD‐L2 in paired samples of cHL patients.
Table SII. Association of plasma levels with clinical characteristics.
Table SIII. Correlation between tissue expression as scored by the percentage of positive pixels and levels as measured in plasma.
Table SIV. The effect of VEGF and CD83 on the PD‐1‐PD‐L axis.
Fig S1. Relationship among the percentage of positive pixels for TARC and CD163 with the percentage of positive pixels for PD‐L1 and PD‐L2.
Fig S2. Comparison of sPD‐L1 plasma levels with clinical parameters.
Fig S3. Relationship among the levels of sTARC and sCD163 with sPD‐L1 and sPD‐L2 in plasma of cHL patients.
Acknowledgements
JV was funded by the Graduate School of Medical Sciences, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
References
- 1. Merryman RW, Armand P, Wright KT, Rodig SJ. Checkpoint blockade in Hodgkin and non‐Hodgkin lymphoma. Blood Adv. 2017;1(26):2643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD‐1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B‐cell lymphoma. Blood. 2010;116(17):3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hollander P, Kamper P, Smedby KE, Enblad G, Ludvigsen M, Mortensen J, et al. High proportions of PD‐1(+) and PD‐L1(+) leukocytes in classical Hodgkin lymphoma microenvironment are associated with inferior outcome. Blood Adv. 2017;1(18):1427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, et al. Topological analysis reveals a PD‐L1‐associated microenvironmental niche for Reed‐Sternberg cells in Hodgkin lymphoma. Blood. 2017;130(22):2420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD‐L1 and PD‐L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol. 2016;34(23):2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paydas S, Bagir E, Seydaoglu G, Ercolak V, Ergin M. Programmed death‐1 (PD‐1), programmed death‐ligand 1 (PD‐L1), and EBV‐encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94(9):1545–52. [DOI] [PubMed] [Google Scholar]
- 7. Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death‐1+ tumor‐infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40(12):1715–22. [DOI] [PubMed] [Google Scholar]
- 8. Hollander P, Amini RM, Ginman B, Molin D, Enblad G, Glimelius I. Expression of PD‐1 and PD‐L1 increase in consecutive biopsies in patients with classical Hodgkin lymphoma. PLoS One. 2018;13(9):e0204870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasse S, Reddemann K, Diepstra A, Oschlies I, Schnitter A, Borchmann S, et al. Programmed cell death protein‐1 (PD‐1)‐expression in the microenvironment of classical Hodgkin lymphoma at relapse during anti‐PD‐1‐treatment. Haematologica. 2019;104(1):e21–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu X, Lang J. Soluble PD‐1 and PD‐L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8(57):97671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo X, Wang J, Jin J, Chen H, Zhen Z, Jiang W, et al. High Serum Level of Soluble Programmed Death Ligand 1 is Associated With a Poor Prognosis in Hodgkin Lymphoma. Transl Oncol. 2018;11(3):779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jalali S, Price‐Troska T, Bothun C, Villasboas J, Kim HJ, Yang ZZ, et al. Reverse signaling via PD‐L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 2019;9(3)):22. 10.1038/s41408-019-0185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. da Silva PB, Real JM, Ferreira LRP, Esteves GH, Brito FDN, Baiocchi OCG. Soluble PD‐1 and PD‐L1 as potential biomarkers for classical Hodgkin lymphoma. Hematol Oncol. 2018;36(4):709–12. [DOI] [PubMed] [Google Scholar]
- 14. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF‐A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doussis‐Anagnostopoulou IA, Talks KL, Turley H, Debnam P, Tan DC, Mariatos G, et al. Vascular endothelial growth factor (VEGF) is expressed by neoplastic Hodgkin‐Reed‐Sternberg cells in Hodgkin's disease. J Pathol. 2002;197(5):677–83. [DOI] [PubMed] [Google Scholar]
- 16. Rueda A, Olmos D, Villareal V, Torres E, Pajares BI, Alba E. Elevated vascular endothelial growth factor pretreatment levels are correlated with the tumor burden in Hodgkin lymphoma and continue to be elevated in prolonged complete remission. Clin Lymphoma Myeloma. 2007;7(6):400–5. [DOI] [PubMed] [Google Scholar]
- 17. Koh YW, Han JH, Yoon DH, Suh C, Huh J. PD‐L1 expression correlates with VEGF and microvessel density in patients with uniformly treated classical Hodgkin lymphoma. Ann Hematol. 2017;96(11):1883–90. [DOI] [PubMed] [Google Scholar]
- 18. Ju X, Silveira PA, Hsu WH, Elgundi Z, Alingcastre R, Verma ND, et al. The Analysis of CD83 Expression on Human Immune Cells Identifies a Unique CD83+‐Activated T Cell Population. J Immunol. 2016;197(12):4613–25. [DOI] [PubMed] [Google Scholar]
- 19. Li Z, Ju X, Lee K, Clarke C, Hsu JL, Abadir E, et al. CD83 is a new potential biomarker and therapeutic target for Hodgkin lymphoma. Haematologica. 2018;103(4):655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plattel WJ, van den Berg A, Visser L, van der Graaf AM, Pruim J, Vos H, et al. Plasma thymus and activation‐regulated chemokine as an early response marker in classical Hodgkin's lymphoma. Haematologica. 2012;97(3):410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plattel WJ, Alsada ZN, van Imhoff GW, Diepstra A, van den Berg A, Visser L. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin‐1, sCD163 and sCD30 with TARC. Br J Haematol. 2016;175(5):868–75. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka Y, Maeshima AM, Nomoto J, Makita S, Fukuhara S, Munakata W, et al. Expression pattern of PD‐L1 and PD‐L2 in classical Hodgkin lymphoma, primary mediastinal large B‐cell lymphoma, and gray zone lymphoma. Eur J Haematol. 2018;100(5):511–7. [DOI] [PubMed] [Google Scholar]
- 23. Weihrauch MR, Manzke O, Beyer M, Haverkamp H, Diehl V, Bohlen H, et al. Elevated serum levels of CC thymus and activation‐related chemokine (TARC) in primary Hodgkin's disease: potential for a prognostic factor. Cancer Res. 2005;65(13):5516–9. [DOI] [PubMed] [Google Scholar]
- 24. Sauer M, Plutschow A, Jachimowicz RD, Kleefisch D, Reiners KS, Ponader S, et al. Baseline serum TARC levels predict therapy outcome in patients with Hodgkin lymphoma. Am J Hematol. 2013;88(2):113–5. [DOI] [PubMed] [Google Scholar]
- 25. Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, et al. Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140(5):527–36. [DOI] [PubMed] [Google Scholar]
- 26. Jones K, Vari F, Keane C, Crooks P, Nourse JP, Seymour LA, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19(3):731–42. [DOI] [PubMed] [Google Scholar]
- 27. Giles FJ, Vose JM, Do KA, Johnson MM, Manshouri T, Bociek G, et al. Clinical relevance of circulating angiogenic factors in patients with non‐Hodgkin's lymphoma or Hodgkin's lymphoma. Leuk Res. 2004;28(6):595–604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Immunohistochemistry scores and soluble levels of PD‐1, PD‐L1 and PD‐L2 in paired samples of cHL patients.
Table SII. Association of plasma levels with clinical characteristics.
Table SIII. Correlation between tissue expression as scored by the percentage of positive pixels and levels as measured in plasma.
Table SIV. The effect of VEGF and CD83 on the PD‐1‐PD‐L axis.
Fig S1. Relationship among the percentage of positive pixels for TARC and CD163 with the percentage of positive pixels for PD‐L1 and PD‐L2.
Fig S2. Comparison of sPD‐L1 plasma levels with clinical parameters.
Fig S3. Relationship among the levels of sTARC and sCD163 with sPD‐L1 and sPD‐L2 in plasma of cHL patients.


