Abstract
Aim
To determine whether a dose‐dependent effect in the stimulation of gut hormone release (plasma cholecystokinin [CCK], active glucagon‐like peptide‐1 [aGLP‐1] and peptide tyrosine tyrosine [PYY]) is found for the natural sweetener erythritol.
Materials and Methods
Twelve healthy, lean volunteers received solutions with 10, 25 or 50 g erythritol, or tap water enriched with 13C‐sodium acetate on four study days via a nasogastric tube in this randomized (active treatments), placebo‐controlled, double‐blind, cross‐over trial. Blood samples and breath samples (13C‐sodium acetate method for measurement of gastric emptying [GE]) were taken at regular intervals, and sensations of appetite and gastrointestinal symptoms were rated.
Results
We found (a) a dose‐dependent stimulation of CCK, aGLP‐1 and PYY, and slowing of GE, (b) no effect on blood glucose, insulin, motilin, glucagon or glucose‐dependent insulinotropic polypeptide, (c) no effect on blood lipids and uric acid, and (d) no abdominal pain, nausea or vomiting.
Conclusions
Solutions with 10 and 50 g of erythritol stimulated gut hormone release. Emptying of erythritol‐containing solutions from the stomach was slower compared with placebo. There was no effect on plasma glucose, insulin, glucagon, blood lipids or uric acid. All doses were well tolerated.
Keywords: appetite‐related sensations, blood lipids, erythritol, gastric emptying, gastrointestinal symptoms, gut hormones, natural sweeteners, uric acid
1. INTRODUCTION
The prevalence of type 2 diabetes has approximately doubled globally over the past 30 years 1 and it is estimated that its prevalence will increase further by 54% until 2030. 2 The increase is particularly large in developing countries and among children and adolescents. 1 , 3 Traditionally, sucrose is the main ingredient used for sweetening food and beverages. Various studies have shown that excessive sugar consumption is associated with a whole range of negative health effects including overweight and diabetes, and the World Health Organization recommends that intake should be limited. 4 , 5 Low‐calorie sweeteners (LCSs) have gained in popularity over recent decades. However, reports of the effect of LCSs on body weight, abdominal adiposity, glucose homeostasis and gut microbiota are inconsistent. 6 This could be attributed to the fact that LCSs are a very heterogeneous group and there is growing evidence that the various LCSs need to be studied separately as individual substances rather than as a group. 7
Erythritol is a non‐calorific sugar alcohol occurring naturally in smaller amounts in fruits and vegetables but which is commercially produced by yeast fermentation of glucose. Once ingested, a large proportion of erythritol (60%‐90%) is absorbed and excreted unchanged by the kidneys. 8 A smaller portion enters the pentose‐phosphate pathway and is degraded via erythronate. 9
Using a cross‐over, double‐blind, placebo‐controlled design, we recently showed that intragastric administration of a solution containing 75 g erythritol resulted in a significant increase in plasma concentrations of cholecystokinin (CCK) and active glucagon‐like peptide‐1 (aGLP‐1), whereas insulin and plasma glucose remained unaffected. Erythritol‐containing solutions emptied significantly slower from the stomach compared with tap water (placebo). 10 Overduin et al. showed that substituting sucrose with erythritol leads to equal stimulation of gut hormone release and similar levels of satiety. If given as a preload to an ad libitum test meal, subsequent calorie intake was equal as well. 11 Furthermore, in a pilot study in patients with type 2 diabetes, replacing sugar with erythritol improved endothelial function and reduced central aortic stiffness. 12 Nonetheless, in this context, possible side effects should be studied: osmotic effects can cause discomfort in some individuals in the case of rapid overconsumption. The concentrations we used in our preliminary study (75 g erythritol) led to bloating and diarrhoea in 60%‐70% of all subjects. 10 Furthermore, other sweeteners such as fructose led to a rapid increase of blood lipids and a rise in uric acid, 13 , 14 which have been associated with obesity and metabolic syndrome. 15 Therefore, nutrients leading to an increase in uric acid or blood lipids should be avoided. At least, in theory, triglyceride synthesis might be affected by ingestion of polyols: the glycerol backbone—which is found in triglycerides—is a product of the pentose‐phosphate pathway. In this way, the intake of sugar alcohols might increase glycerol concentrations that might end up in triglyceride synthesis. Only a two studies have examined the effects of erythritol intake on uric acid and blood lipids in humans to date. 16 , 17
The primary outcome of the current study was to determine whether a dose‐dependent effect in the stimulation of gut hormone release such as CCK, aGLP‐1 and peptide tyrosine tyrosine (PYY) could be observed for erythritol, and secondary outcomes included the speed of emptying of the solutions from the stomach, and glucagon, motilin and glucose‐dependent insulinotropic polypeptide (GIP) secretions not previously studied. Possible side effects (e.g. gastrointestinal [GI] symptoms, impact on uric acid and blood lipid concentrations) were also investigated. We chose lower doses that could be used in everyday life. The sweetness of the highest dose (50 g erythritol in 300 mL), for instance, corresponds to around 30 g sucrose in 330 mL, as found in sweet beverages.
2. MATERIALS AND METHODS
The study was conducted as a randomized (active treatments), placebo‐controlled, double‐blind, cross‐over trial and was performed in accordance with the principles of the Helsinki Declaration (October 2013 version). The protocol was approved by the local ethical committee (Ethikkommission Nordwest‐ und Zentralschweiz: 2016‐01928) and registered at ClinicalTrials.gov (NCT03039478). The exclusion criteria included substance and alcohol abuse, regular intake of medications (except for oral contraceptives), acute infections, chronic medical illness or illnesses affecting the GI system, a history of food allergies, dietary restrictions or pre‐existing consumption of erythritol on a regular basis.
On four separate occasions, at least 3 days apart and after a 10‐h overnight fast, after taking fasting blood (t = −10 and −1 min) and breath samples (t = −10 min), as well as assessing appetite‐related perceptions and GI symptoms, participants received one of the following test solutions (at t = 0 min) directly into the stomach by use of a nasogastric feeding tube over 2 min: 10, 25 or 50 g erythritol + 50 mg of 13C‐sodium acetate dissolved in 300 mL tap water or 300 mL tap water + 50 mg of 13C‐sodium acetate (placebo). The active treatments were given in a completely randomized order. The study participant and the person who carried out all tests, as well as the personnel performing analysis of blood samples, were blinded concerning the content of the intragastric infusion administered. Following administration of the test solution blood samples (after 15, 30, 45, 60, 90, 120 and 180 min for analysis of plasma CCK, aGLP‐1, PYY, GIP, motilin, glucose, insulin and glucagon), and breath samples (after 15, 30, 45, 60, 75, 90, 105, 120, 150, 180, 210 and 240 min for analysis of gastric emptying) were taken. Appetite‐related sensations were assessed immediately after each blood collection; participants were asked to rate GI symptoms at 30, 60, 90, 120, 150, 180 and 240 min after administration of the test solutions. Extra blood samples were taken during the visit, with the highest erythritol load (50 g) for analysis of serum total cholesterol, high‐ and low‐density lipoprotein (HDL and LDL, respectively), triglyceride and uric acid concentrations. Blood samples were collected on ice into tubes and analysed using enzyme‐ or radioimmunoassay. Gastric emptying was determined using a 13C‐sodium acetate breath test 18 : test solutions were labelled with 50 mg of 13C‐sodium acetate, an isotope that is absorbed readily in the proximal small intestine then transported to the liver where it is metabolized to 13CO2, which is then exhaled rapidly and can therefore be used as an indirect marker of gastric emptying. 18 Validated visual analogue scales were used to rate the appetite‐related sensations (hunger, prospective food consumption, satiety and fullness). 19 , 20 Further information on the methodology can be found in Appendix S1.
2.1. Statistical analysis
In our previous work on the effects of 75 g erythritol on GI hormone secretion and gastric emptying, we did find significant results. 10 However, because pharmacological effects are usually not linear with respect to dose, we did not have a reliable estimate of effect size for the current study, so no formal estimate of sample size could be obtained. A sample size of 12 subjects per group was chosen for reasons of comparability and practicability. In this respect, this study is a hypothesis‐generating study that allows descriptive data analysis. Descriptive statistics were used for demographic variables such as age, weight, height and body mass index (BMI). For hormone and glucose profiles, gastric emptying and appetite‐related sensations, incremental values were used to calculate the incremental area under the curve (iAUC) by the trapezoidal rule. Isolated missing values (because of technical problems or being below the detection limit) were replaced by the treatment group median to enable calculation of the iAUC. The maximum and minimum deviations from baseline—iCmax and iCmin, respectively—were determined using baseline‐corrected data. For iAUC calculations, in addition to the total time interval of 180 min, an interval of 60 min is reported because in some variables (CCK and GLP‐1) the main effect was observed during this time period. Linear mixed effects modelling was applied to describe differences between the different treatments (placebo, 10, 25 and 50 g). In the case of significant overall treatment effects, pairwise post hoc within‐subject comparisons were performed using a Šidak multicomparison test. In addition, for the variables of interest (e.g. iAUCs of 0‐60 min for CCK, aGLP‐1 and PYY), the minimum detectable differences were estimated on the basis of the observed data in the current study by a simulation with power analysis and sample size 2020 software (NCSS, LLC, Kaysville, UT, USA) using 1000 iterations per run. The order of treatments was evaluated as a covariate. To explore putative relationships between different gut hormone responses (e.g. CCK, PYY and aGLP‐1) and gastric emptying of the different treatments, the integrated responses (iAUC 0‐60 min) were correlated on an individual basis by linear matrix correlation. The goodness of this correlation was expressed by Pearson's correlation coefficient, R. All statistical analysis was performed using SPSS statistics for windows version 25.0 (IBM, Armonk, NY, USA). Values are reported as means ± standard deviation (SD) and displayed in figures as means ± standard error of mean (SEM). Differences were considered to be statistically significant when p was less than .05.
3. RESULTS
Twelve normal‐weight, healthy individuals (seven men and five women; mean BMI: 21.7 ± 0.4, range: 19.4‐24.0 kg/m2; mean age: 26.2 ± 2.0, range: 18‐40 years) participated in the study (Appendix S2, Figure S1). All the subjects tolerated the study well, and there were no adverse events during the period of the study. Five subjects did not receive placebo treatment and, therefore, complete data for seven (placebo) to 12 participants (all erythritol doses) were available for analysis. There was no order effect for the active treatments. For each measured variable, baseline values were compared between the treatments. None of these variables were statistically significant between the treatments.
3.1. Plasma CCK
There was an overall statistically significant difference comparing the iAUCs for 0‐60 min. Pairwise comparison revealed a statistically significant difference for 25 g erythritol versus placebo, 50 g erythritol versus placebo, 10 versus 25 g erythritol, 10 versus 50 g erythritol and 25 versus 50 g erythritol. Overall statistical significance was also reached for the iAUCs for 0‐180 min. Pairwise comparisons revealed a statistically significant difference for 25 g erythritol versus placebo, 50 g erythritol versus placebo and 10 versus 50 g erythritol. Further, there was an overall statistically significant difference comparing the iCmax values: pairwise significant difference were observed between 10 g erythritol versus placebo, 25 g erythritol versus placebo, 50 g erythritol versus placebo, 10 versus 25 g erythritol and 10 versus 50 g erythritol (Figure 1A and Table 1).
FIGURE 1.
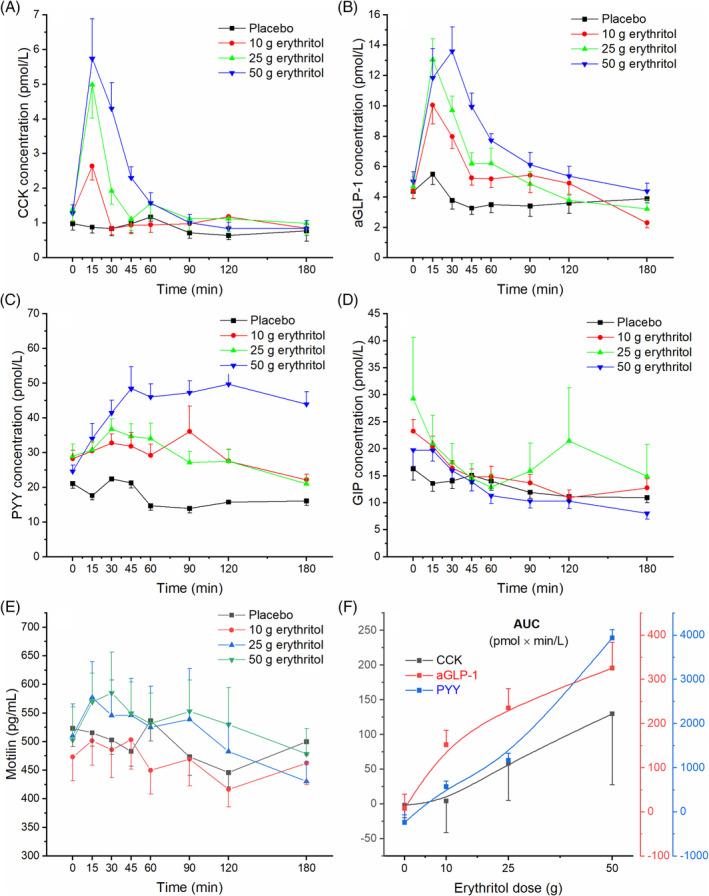
Effect of erythritol on plasma concentrations of gut hormones. A, cholecystokinin (CCK), B, active glucagon‐like peptide‐1 (aGLP‐1), C, peptide tyrosine tyrosine (PYY), D, glucose‐dependent insulinotropic polypeptide (GIP), E, motilin, and F, dose–response evaluation. Data are expressed as mean ± SEM, absolute values are reported. N = 7 (placebo), n = 12 (erythritol treatments). Statistical tests: linear mixed‐effects modeling followed by Šidak post hoc test in case of overall significance. Results of the statistical analysis are shown in Table 1
TABLE 1.
Effect of erythritol on plasma concentrations of cholecystokinin (CCK), active glucagon‐like peptide‐1 (aGLP‐1), peptide tyrosine tyrosine (PYY), glucose‐dependent insulinotropic polypeptide (GIP) and motilin
| Hormones | Placebo, n = 7 | Erythritol 10 g, n = 12 | Erythritol 25 g, n = 12 | Erythritol 50 g, n = 12 | p‐values (overall) | p‐values (post hoc) | |
|---|---|---|---|---|---|---|---|
| CCK | Fasting values (pmol/L) | 1.1 ± 0.9 | 1.3 ± 0.9 | 1.4 ± 1.2 | 1.3 ± 0.8 | NS | |
| iAUC (0‐60 min) (pmol x min/L) | −1.9 ± 18.2 | 4.4 ± 45.3 | 58.9 ± 53.8 | 129.8 ± 101.9 | .001 |
2).001 3).001 4).016 5).001 6).012 |
|
| iAUC (0‐180 min) (pmol x min/L) | −30.6 ± 56.0 | −32.4 ± 142.2 | 29.4 ± 101.1 | 92.9 ± 177.6 | .004 |
2).028 3).007 5).048 |
|
| iCmax (pmol/L) | 0.6 ± 0.5 | 1.5 ± 1.2 | 3.6 ± 2.9 | 4.9 ± 3.7 | .011 |
1).024 2).003 3).002 4).028 5).003 |
|
| Tmax (min) | 57.0 ± 62.1 | 21.3 ± 31.6 | 15.0 ± 0.0 | 20.0 ± 7.4 | NS | ||
| aGLP‐1 | Fasting values (pmol/L) | 4.1 ± 2.2 | 4.4 + 1.6 | 4.7 ± 2.2 | 5.0 ± 2.2 | NS | |
| iAUC (0‐60 min) (pmol x min/L) | 8.0 ± 83.9 | 151.7 ± 114.4 | 235.2 ± 152.0 | 325.4 ± 200.8 | .002 |
1).016 2).001 3).001 5).03 |
|
| iAUC (0‐180 min) (pmol x min/L) | −43.9 ± 210.2 | 162.3 ± 290.0 | 149.9 ± 297.6 | 396.0 ± 342.3 | .002 |
1).011 2).013 3).001 6).036 |
|
| iCmax (pmol/L) | 2.4 ± 2.0 | 7.2 ± 4.2 | 9.1 ± 3.4 | 11.5 ± 5.8 | <.001 |
1).001 2)<.001 3)<.001 |
|
| Tmax (min) | 34.3 ± 64.6 | 31.3 ± 28.2 | 30.0 ± 47.4 | 27.5 ± 8.7 | NS | ||
| PYY | Fasting values (pmol/L) | 91.7 ± 28.1 | 121.8 ± 35.5 | 124.9 ± 51.5 | 106.2 ± 26.2 | NS | |
| iAUC (0‐60 min) (pmol x min/L) | −238.3 ± 1200.0 | 568.5 ± 1550.5 | 1167.6 ± 1906.7 | 3935.0 ± 2264.0 | .001 |
3).025 5).001 6).024 |
|
| iAUC (0‐180 min) (pmol x min/L) | −3359 ± 4219 | 578 ± 5009 | −42.7 ± 7948 | 15 628 ± 7773 | <.001 |
3).002 5)<.001 6)<.001 |
|
| iCmax (pmol/L) | 24.0 ± 16.9 | 76.1 ± 81.0 | 58.9 ± 59.5 | 163.9 ± 80.8 | <.001 |
3).004 5).001 6).010 |
|
| Tmax (min) | 27.9 ± 16,0 | 40.0 ± 28.1 | 42.5 ± 28.5 | 82.5 ± 33.5 | .003 |
3).002 5).019 6).009 |
|
| GIP | Fasting values (pmol/L) | 91.3 ± 45.6 | 115.9 ± 37.3 | 146.0 ± 195.3 | 146.0 ± 195.3 | NS | |
| iAUC (0‐60 min) (pmol x min/L) | −970.9 ± 1440.5 | −1744.9 ± 1872.8 | −3222.6 ± 7365.1 | −1050 ± 1984.4 | NS | ||
| iAUC (0‐180 min) (pmol x min/L) | −4893.9 ± 5873.0 | −8033.4 ± 5893.7 | −1036.5 ± 17 308.1 | −7006.8 ± 7383.5 | NS | ||
| iCmin (pmol/L) | −47.0 ± 37.6 | −69.7 ± 33.9 | −95.2 ± 158.3 | −63.3 ± 47.5 | NS | ||
| Tmin (min) | 105.0 ± 52.7 | 132.5 ± 37.2 | 133.8 ± 54.1 | 152.5 ± 41.4 | NS | ||
| Motilin | Fasting values (pg/mL) | 525.9 + 151.6 | 473.2 + 143.2 | 510.2 + 191.7 | 503.0 + 199.5 | NS | |
| iAUC (0‐60 min) (pg x min/mL) | −750.3 ± 4302.5 | 881 ± 6306 | 2190 ± 6024 | 3143 ± 6268 | NS | ||
| iAUC (0‐180 min) (pg x min/mL) | −5419 ± 8547 | −2381 ± 17 475 | −349 ± 15 857 | 5539 ± 19 957 | NS | ||
| iCmax (pg/mL) | 65.0 ± 54.4 | 106.2 ± 98.3 | 158.3 ± 144.5 | 157.5 ± 132.0 | NS | ||
| iCmin (pg/mL) | −109.6 ± 47.9 | −95.5 ± 90.7 | −113.1 ± 89.7 | −108,1 ± 134.0 | NS | ||
| Tmax (min) | 40.7 ± 26.5 | 52.5 ± 53.3 | 41.3 ± 35.1 | 45.0 ± 33.8 | NS | ||
| Tmin (min) | 79.3 ± 43.1 | 71.3 ± 50.0 | 113.8 ± 67.5 | 97.5 ± 69.9 | NS | ||
Note: Data are expressed as mean ± SD and reported from baseline (incremental). Statistical tests: linear mixed‐effects modelling followed by a Šidak post hoc test in case of overall significance.
Abbreviation: iAUC, incremental area under the curve.
1)10 g erythritol versus placebo.
2)25 g erythritol versus placebo.
3)50 g erythritol versus placebo.
4)10 g versus 25 g erythritol.
5)10 g versus 50 g erythritol.
6)25 g versus 50 g erythritol.
3.2. Plasma aGLP‐1
There was an overall statistically significant difference comparing the iAUCs for 0‐60 min. Pairwise comparisons revealed statistically significant differences for 10 g erythritol versus placebo, 25 g erythritol versus placebo, 50 g erythritol versus placebo and for 10 versus 50 g erythritol. Overall statistical significance was also reached for the iAUCs for 0‐180 min. Pairwise comparisons revealed statistically significant differences for 10 g erythritol versus placebo, 25 g erythritol versus placebo, 50 g erythritol versus placebo and 25 versus 50 g erythritol. Further, there was an overall statistically significant difference comparing the iCmax values: pairwise significant differences were observed between 10 g erythritol versus placebo, 25 g erythritol versus placebo and 50 g erythritol versus placebo (Figure 1B and Table 1).
3.3. Plasma PYY
There was an overall statistically significant difference comparing the iAUCs for 0‐60 min. Pairwise comparisons revealed statistically significant differences for 50 g erythritol versus placebo, 10 versus 50 g erythritol and 25 versus 50 g erythritol. Overall statistical significance was also reached for the iAUCs for 0‐180 min. Pairwise comparisons revealed statistically significant differences for 50 g erythritol versus placebo, 10 versus 50 g erythritol and 25 versus 50 g erythritol. Further, there was an overall statistically significant difference comparing the iCmax values: pairwise significant differences were observed between 50 g erythritol versus placebo, 10 versus 50 g erythritol and 25 versus 50 g erythritol (Figure 1C and Table 1).
3.4. Minimum detectable difference
Based on the results of this study, the minimum detectable differences were estimated for the iAUCs for 0‐60 min for CKK, aGLP‐1 and PYY. The minimum detectable difference for CCK was 28 pmol × min/L, for aGLP‐1 it was 127 pmol × min/L and for PYY it was 1803 pmol × min/L, with a corresponding statistical power (95% confidence interval) of 81.3% (78.4%‐83.7%), 80.8% (78.2%‐83.2%) and 80.4% (77.8%‐82.8%), respectively. These data correspond well with the applied statistical tests.
3.5. Dose–response evaluation
For CCK, aGLP‐1 and PYY, the dose–response relationship was evaluated and is shown in Figure 1F. On average, within the first 60 min after administration of erythritol, all these hormones showed a monotonically increasing curvilinear secretion with increasing erythritol dose. An apparent saturation of the stimulating effect was not observed for any of the hormones, therefore no half‐maximal stimulating dose (ED50) could be estimated.
3.6. Plasma GIP, motilin, insulin and glucagon
No effect of erythritol treatment on plasma GIP, motilin, insulin and glucagon concentrations was found: the values of iAUC 0‐60 min, iAUC 0‐180 min, iCmax and iCmin did not show any overall statistically significant differences (GIP/motilin: Figure 1D,E and Table 1; insulin/glucagon: Figure 2B,C and Table 2).
FIGURE 2.
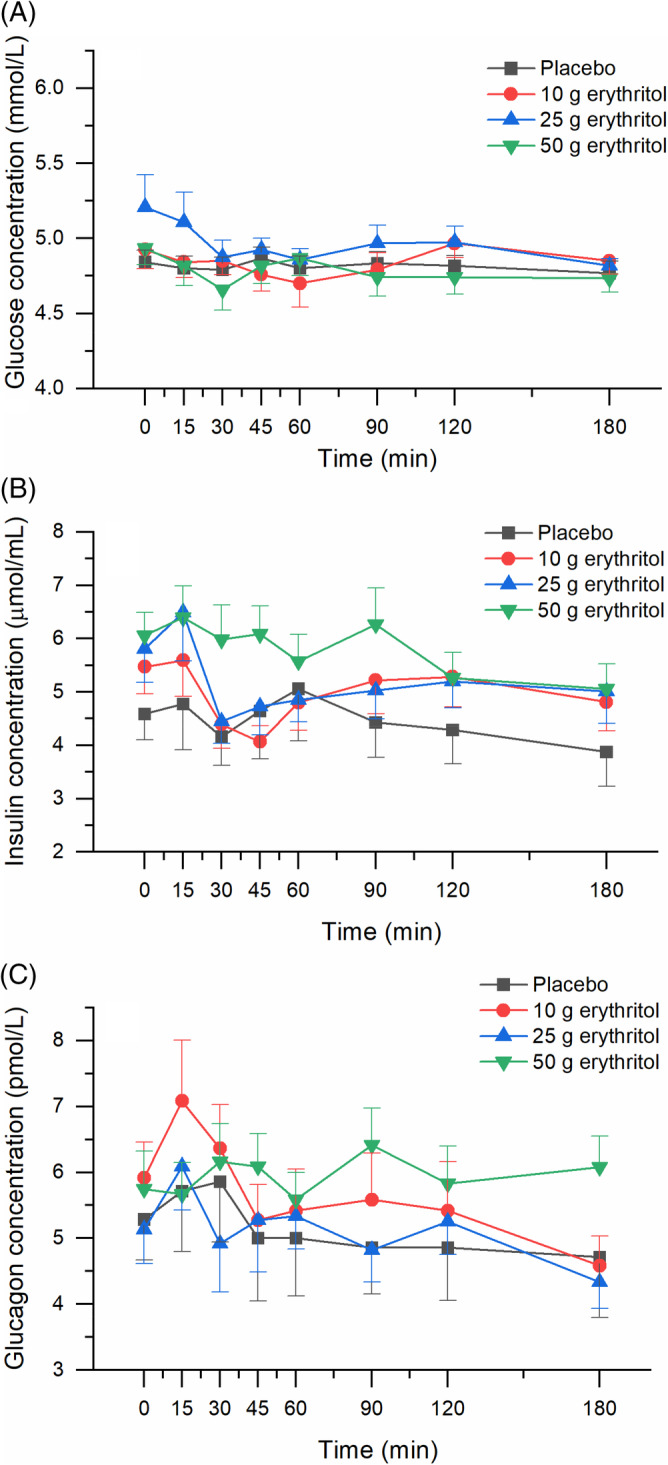
Effect of erythritol on plasma concentrations of glucose, insulin and glucagon. A, Glucose, B, insulin, and C, glucagon. Data are expressed as mean ± SEM, absolute values are reported. N = 7 (placebo), n = 12 (erythritol treatments). Statistical tests: linear mixed‐effects modeling followed by Šidak post hoc test in case of overall significance. Results of the statistical analysis are shown in Table 2
TABLE 2.
Effect of erythritol on plasma concentrations of glucose, insulin and glucagon
| Variables | Placebo, n = 7 | Erythritol 10 g, n = 12 | Erythritol 25 g, n = 12 | Erythritol 50 g, n = 12 | p‐values (overall) | p‐values (post hoc) |
|---|---|---|---|---|---|---|
| Glucose | ||||||
| fasting values (mmol/L) | 4.9 ± 0.4 | 4.9 ± 0.4 | 5.2 ± 0.7 | 4.9 ± 0.4 | NS | |
| iAUC (0‐60 min) (mmol × min/L) | −3.32 ± 4.56 | −6.56 ± 17.18 | −13.38 ± 29.66 | −8.13 ± 9.99 | NS | |
| iAUC (0‐180 min) (mmol × min/L) | −8.46 ± 6.72 | −14.3 ± 45.6 | −48.1 ± 93.8 | −29.5 ± 37.2 | NS | |
| iCmax (mmol/L) | 0.06 ± 0.1 | 0.22 ± 0.22 | 0.15 ± 0.28 | 0.10 ± 0.14 | NS | |
| iCmin (mmol/L] | −0.17 ± 0.08 | −0.41 ± 0.53 | −0.54 ± 0.76 | −0.38 ± 0.24 | .015 | 1).042 |
| Tmax (min) | 38.6 ± 48.9 | 52.2 ± 60.5 | 20.0 ± 29.5 | 25.0 ± 41.6 | NS | |
| Tmin (min) | 91.1 ± 68.4 | 76.3 ± 65.4 | 78.8 ± 77.8 | 55.0 ± 62.1 | NS | |
| Insulin | ||||||
| fasting values (μmol/L) | 4.6 ± 1.3 | 5.5 ± 1.8 | 5.8 ± 2.1 | 6.1 ± 1.5 | NS | |
| iAUC (0‐60 min) (μmol × min/L) | 0.75 ± 68.0 | −40.5 ± 63.4 | −33.6 ± 72.4 | 0.84 ± 89.4 | NS | |
| iAUC (0‐180 min) (μmol × min/L) | −31.8 ± 169.8 | −86.5 ± 165.4 | −122.8 ± 197.6 | −80.8 ± 261.7 | NS | |
| iCmax (μmol/L) | 1.06 ± 1.32 | 1.27 ± 1.46 | 1.45 ± 2.62 | 1.52 ± 1.57 | NS | |
| iCmin (μmol/L) | −1.46 ± 0.82 | −1.92 ± 1.17 | −2.07 ± 1.16 | −1.78 ± 1.22 | NS | |
| Tmax (min) | 15.0 ± 21.2 | 60.0 ± 65.9 | 50.5 ± 46.4 | 28.8 ± 32.9 | NS | |
| Tmin (min) | 92.1 ± 84.2 | 66.3 ± 50.1 | 81.8 ± 56.3 | 86.3 ± 69.2 | NS | |
| Glucagon | ||||||
| fasting values (pmol/L) | 5.3 ± 1.6 | 5.9 ± 1.9 | 5.1 ± 1.8 | 5.8 ± 2.0 | NS | |
| iAUC (0‐60 min) (pmol × min/L] | 8.6 ± 83.6 | 10.0 ± 53.8 | 16.1 ± 105.3 | 11.3 ± 67.4 | NS | |
| iAUC (0‐180 min) (pmol × min/L] | −45.0 ± 294.3 | −73,8 ± 204.3 | −13.4 ± 279.0 | 42.4 ± 237.1 | NS | |
| iCmax (pmol/L) | 1.43 ± 1.38 | 1.96 ± 1.75 | 1.74 ± 1.04 | 1.54 ± 1.12 | NS | |
| iCmin (pmol/L) | −1.50 ± 1.32 | −1.96 ± 1.37 | −1.79 ± 1.51 | −1.62 ± 1.72 | NS | |
| Tmax (min) | 21.4 ± 17.0 | 43.8 ± 53.3 | 17.5 ± 12.5 | 45.0 ± 56.5 | NS | |
| Tmin (min) | 64.3 ± 46.4 | 118.8 ± 62.8 | 81.3 ± 79.0 | 46.3 ± 60.8 | NS |
Note: Data are expressed as mean ± SD and reported from baseline (incremental). Statistical tests: linear mixed‐effects modelling followed by a Šidak post hoc test in case of overall significance.
Abbreviations: iAUC, incremental area under the curve; iCmax, maximum deviation from baseline; iCmin, minimum deviation from baseline.
1)50 g erythritol versus placebo.
3.7. Plasma glucose
Erythritol treatment did not lead to an increase in plasma glucose concentrations. There was an overall statistically significant difference comparing the iCmin values. Pairwise comparison revealed a statistically significant stronger decrease from baseline after 50 g erythritol compared with placebo (Figure 2A and Table 2).
3.8. Gastric emptying
There was an overall statistically significant difference comparing the T50% values. Pairwise comparisons revealed that the time needed to empty 50% of the dose was significantly longer for 50 g erythritol versus placebo (76.4 ± 12.1 vs. 55.5 ± 17.2 min) and 50 versus 10 g erythritol (76.4 ± 12.1 vs. 59.8 ± 19.5 min). Overall statistical significance was also reached for the iAUCs for 0‐60 min. Pairwise comparisons revealed a statistically significant slower emptying of the 10 g erythritol solution compared with placebo, and 50 g compared with 10 g erythritol. Further, there was an overall statistically significant difference comparing the iAUCs for 0‐180 min. Pairwise significant differences were observed between 10 g erythritol versus placebo and 50 g erythritol versus placebo. The goodness of fit parameter R2 was between 0.990 and 0.998 for all treatments (Appendix S2, Figure S2; Table 3).
TABLE 3.
Effect of erythritol on gastric emptying
| Placebo, n = 7 | Erythritol 10 g, n = 12 | Erythritol 25 g, n = 12 | Erythritol 50 g, n = 12 | p‐values, (overall) | p‐values (post hoc) | |
|---|---|---|---|---|---|---|
| AUC (0‐60 min) (dose*min/h) | 896.9 ± 128.1 | 1103.2 ± 185.6 | 1036.9 ± 190.4 | 921.5 ± 107.3 | .009 |
1).026 4).027 |
| AUC (0‐180 min) (dose*min/h) | 1595.6 ± 252.1 | 2048.4 ± 434.1 | 2052.7 ± 485.3 | 2102.5 ± 259.8 | .001 |
1).035 2).061 3).002 |
| Tmax (min) | 19.29 ± 7.3 | 21.25 ± 7.7 | 25.00 ± 7.4 | 41.25 ± 15.8 | .005 |
3).003 4).005 5).019 |
| T50% (time 50% emptied) (min) | 55. 5 ± 17.2 | 59.8 ± 19.5 | 68.1 ± 16.1 | 76.4 ± 12.1 | .005 |
3).038 4).026 |
Abbreviation: AUC, area under the curve.
Data are expressed as mean ± SD and reported from baseline (incremental). Linear mixed‐effects modelling followed by a Šidak post hoc test in case of overall significance.
1)10 g erythritol versus placebo.
2)25 g erythritol versus placebo.
3)50 g erythritol versus placebo.
4)10 g versus 50 g erythritol.
5)25 g versus 50 g erythritol.
3.9. Correlations of CCK, aGLP‐1, PYY and gastric emptying
CCK, aGLP‐1 and PYY were weakly negatively correlated with gastric emptying (R = −0.266, R = −0.145 and R = −0.427, respectively) (Appendix S2, Figure S3 ).
3.10. Appetite‐related sensations
No effect of erythritol treatment on appetite‐related sensations was found: the values of iAUC 0‐60 min, iAUC 0‐180 min and iCmax did not show any overall statistically significant differences (Appendix S2, Figure S4A‐D).
3.11. Blood lipids and uric acid
There was no statistically significant increase from baseline in blood lipids (total cholesterol, LDL, HDL and triglycerides) or uric acid within 120 min after 50 g erythritol. The mean fasting concentration of total cholesterol was 4.3 ± 0.2 mmol/L (Cmax 4.4 ± 0.2 mmol/L), for LDL it was 2.5 ± 0.2 mmol/L (Cmax 2.6 ± 0.1 mmol/L), for HDL it was 1.3 ± 0.1 mmol/L (Cmax 1.4 ± 0.1 mmol/L), for triglycerides it was 0.9 ± 0.1 mmol/L (Cmax 1.0 ± 0.1 mmol/L) and for uric acid it was 316.8 ± 21.2 μmol/L (Cmax 319.3 ± 21.2 μmol/L) (Appendix S2, Figure S5).
3.12. GI symptoms
There was no abdominal pain, nausea or vomiting after either dose. One volunteer had diarrhoea after 10 g of erythritol (max. severity 1.0). In nine participants after 10 g, seven participants after 25 g, and eight participants after 50 g erythritol, a subjective increase in bowel sounds was reported (max. reported severity 1.25, after 50 g), and two volunteers described an increased feeling of bloating after 25 and 50 g erythritol (max. reported severity 1.0). One volunteer had eructation after 10 g (max. reported severity 1.0), and one volunteer described more flatulence after placebo treatment (max. reported severity 1.0) (Appendix S2, Table S1).
4. DISCUSSION
The main results of the study can be summarized as follows: (a) erythritol led to a robust, dose‐dependent stimulation of CCK, aGLP‐1 and PYY release; (b) the emptying of erythritol‐containing solutions from the stomach were slower compared with placebo; (c) erythritol had no effect on motilin or GIP release; (d) erythritol had no effect on blood glucose, insulin or glucagon concentrations; (e) blood lipids and uric acid were not affected by 50 g erythritol ingestion; and (f) doses of up to 50 g erythritol administration were well tolerated.
The GI tract plays a critical role in regulating appetite and satiation. Through gut‐derived signals, information regarding incoming nutrients is conveyed to the various organ systems involved in the regulation of energy homeostasis. Nutrient components are recognized by receptors located on enteroendocrine cells, which stimulate the release of gut hormones such as GLP‐1, PYY, GIP and CCK. Mediated by the release of these hormones, an entire range of satiation mechanisms are activated, including slowing of gastric emptying. 21 , 22 The antidiabetic effects of, for instance, GLP‐1 have been described in several trials 23 , 24 and GLP‐1 receptor agonists or GLP‐1 analogues are increasingly and successfully used as antidiabetic treatments. 25 On human intestinal endocrine L‐cells, co‐localization of GLP‐1, GIP and PYY with the sweet taste receptor T1R2‐T1R3 has been described. 26 , 27 Sweet taste perception might—at least in part—explain sweetener‐mediated gut hormone release. However, observations published by Saltiel et al. suggest that sweet taste receptor activation is not per se a driver for GIP/GLP‐1 secretion. 28
We have recently shown that the non‐calorific, natural sweetener erythritol can stimulate CCK and GLP‐1 release and that the emptying of erythritol‐containing solutions is slower compared with placebo. 10 A recent publication is in line with our findings: replacement of sucrose by erythritol leads to equal stimulation of GLP‐1 and PYY and hunger scores and, if given as a preload to an ad libitum test meal, calorie intake was equal to that after sucrose, indicating that the satiating effect of erythritol is not different from sucrose. 11 With the current study, we substantiate that erythritol induces a marked rise in plasma gut hormones and that the emptying of erythritol‐containing solutions is slower compared with placebo. We expand these findings by showing stimulation of CCK, aGLP‐1 and PYY in a dose‐dependent manner. Other LCSs have no stimulating effect on gut hormone release in vivo. 29 , 30 , 31 We infer from these observations that the ability of sweeteners to stimulate gut hormone release is sweetener‐specific, irrespective of their calorific value. Whether the sweet taste receptor is involved in erythritol‐stimulated gut hormone release has not been examined to date. Gastric emptying is regulated by numerous feedback mechanisms, including the release of gut hormones such as CCK and GLP‐1. 21 , 22 In this trial, erythritol‐containing solutions were emptied slower from the stomach in a dose‐dependent way, which was at least partly mediated via the gut hormones, as we were able to show that CCK, aGLP‐1 and PYY were negatively correlated to gastric emptying. One publication indicated that an increase in duodenal osmolarity by administration of a hyperosmolar saline solution was associated with an increase in CCK, GLP‐1 and PYY concentrations, while GIP and glucagon were not affected. 32 A difference in osmolarity has also been shown to impact the gastric emptying of various carbohydrate‐containing test solutions; however, differences in the rates of gastric emptying were not entirely explained by differences in osmolality. 33 Whether osmolarity also plays a role in erythritol‐stimulated gut hormone release, and if so, then to what extent, is not known at this point. In our trial, the solution with the lowest concentration (10 g) was close to being iso‐osmolar (272.7 mOsmol/L), yet clearly stimulated CCK and GLP‐1 release. We, therefore, assume that osmolarity alone is not responsible for the erythritol‐stimulated release of gut hormones. More research is needed to explore the exact mechanism underlying erythritol‐stimulated gut hormone release. In addition to the erythritol‐induced secretion of CCK, aGLP‐1 and PYY—all known as GI satiation hormones—we investigated the effect of erythritol on plasma motilin concentrations, a further emerging player in the GI control of appetite and food intake. Motilin is secreted by M‐cells found in the upper GI tract and plasma concentrations fluctuate in synchrony with the phases of the migrating motor complex, to reach a peak just before the occurrence of gastric phase III. 34 , 35 , 36 Deloose et al. have shown that motilin‐induced gastric phase III contractions signal hunger and that motilin plasma concentrations are closely associated with interdigestive hunger ratings. 37 In a recent study, we investigated the effect of the calorific sweeteners, glucose and fructose, versus the LCS acesulfam‐K on plasma motilin concentrations, and showed that glucose and fructose, but not acesulfam‐K, inhibited motilin secretion. 31 In the current study, the effect of erythritol on motilin concentrations did not differ from that of tap water. This leads us to the assumption that erythritol occupies a kind of intermediate position between calorific and non‐calorific sweeteners: on one hand, the release of satiating hormones such as GLP‐1, PYY and CCK was stimulated by erythritol ingestion; on the other hand, there was no effect on motilin. Of note, with the current study design, it was not possible to perform gastroduodenal manometry recording to adjust motilin measurements around phase III of the migrating motor complex to ensure high motilin concentrations right before the administration of erythritol. Whether an inhibitory effect on motilin release is absent after the administration of erythritol needs to be addressed further.
In the current study, we only studied the acute effects of erythritol and did not administer a subsequent test meal. It is proposed that the ability observed for erythritol to stimulate gut hormone secretion and to slow gastric emptying might also improve the glycaemic response to a subsequent carbohydrate‐containing meal when erythritol is given as a preload, as has already been shown for several other sweet‐tasting substances such as D‐xylose, 3‐OMG and tagatose/isomalt. 38 , 39 Future studies could address this issue.
A number of studies indicate that sweeteners—whether calorific or non‐calorific—can influence glycaemic control both positively and negatively. 40 , 41 Animal studies conducted with erythritol showed that, when given over a longer period, erythritol exerts antihyperglycaemic effects, possibly by enhancing insulin‐mediated glucose uptake and reducing intestinal glucose absorption in diabetic rats. 42 Another important player in glycaemic control is the pancreatic hormone glucagon—a hormone produced in the α‐cells of the pancreatic islets—which stimulates hepatic glycogenolysis and gluconeogenesis in hypoglycaemic states to restore glucose homeostasis, and in this way counterbalances insulin. The effects of an acute or chronic intake of erythritol on glucagon secretion have not been studied to date. In the current trial, we found no statistically significant effects of different doses of erythritol on plasma glucose, insulin or glucagon concentrations in this group of lean, healthy volunteers. Thus, at least after acute consumption, the ingestion of erythritol does not affect sugar metabolism and therefore could be a helpful sugar alternative for patients with diabetes.
It was not the main objective of this study to examine GI tolerance and establish a threshold value. However, as the only side effects described for erythritol consumption in the literature to date are GI symptoms, we recorded them in detail. In our previous trial, a rather high dose of erythritol (75 g) was used, which led to GI side effects (such as diarrhoea) in about 50% of participants. 10 In the current trial, doses of up to 50 g erythritol in 300 mL were very well tolerated, with subjective increases in bowel sounds being the most common finding (while there was no pain, vomiting or nausea and only one case of mild diarrhoea), which is in line with findings in previous trials recommending doses of up to 0.6‐0.8 g/kg body weight in adults and children. 43 , 44 , 45 In the current trial—as well as in our previous trial with 75 g erythritol—the volunteers were unaccustomed to sugar alcohols and the dose was rapidly applied (over 2 min) in a solution directly into the stomach, which probably causes the greatest possible strain on the GI system. In subjects accustomed to erythritol intake, slower administration, or consumption in solid food, the GI tolerance is even higher. 46 Other potential side effects associated with sweetener consumption are alterations in blood lipids and uric acid, which have been associated with obesity and metabolic syndrome, 15 and nutrients leading to an increase should be avoided. In the current study, we examined uric acid and blood lipid profile after administration of the highest dose of erythritol (50 g) and did not find any changes from baseline values.
Some potential limitations of the present study require consideration. First, we studied acute effects of single bolus doses of erythritol applied in a liquid in normal‐weight subjects who were not used to these substances. Differential effects of long‐term exposure on gastric emptying and stimulation of gut hormone release need to be investigated, as adaptive processes cannot be ruled out. Also, there could be different effects if the substance is administered with a solid meal rather than in a solution. Second, given the lack of previous data investigating the effect of the administered doses of erythritol, no formal estimate of sample size could be obtained and the study was conducted as a pilot, hypothesis‐generating trial. In future trials, a sample size estimation can now be based on the data obtained in the current study. Third, study days in females were not controlled for the influence of the menstrual cycle. Previous studies have shown differences in gastric emptying and in the release of GLP‐1 and PYY (but not CCK) when comparing the follicular and luteal phases. 47 , 48 Finally, while we did not find any impact on blood lipids or uric acid, and only mild GI symptoms even when applying the highest dose of erythritol in normal‐weight healthy volunteers, future trials should probably include the target audience (obese patients with metabolic syndrome) studied over a longer period (chronic intake).
In conclusion, the findings of this trial show that in lean volunteers ingestion of doses between 10 and 50 g of the natural sweetener erythritol stimulated GI hormone release (CCK, aGLP‐1 and PYY) and slowed gastric emptying, both in a dose‐dependent manner. Meanwhile, there was no effect of erythritol ingestion on blood glucose, insulin, glucagon, motilin or GIP release, blood lipids or uric acid concentrations. Given as a single bolus dose in a liquid, doses of up to 50 g erythritol were well tolerated in this trial. This combination of properties makes erythritol an attractive candidate for sugar replacement, in particular in a metabolically vulnerable population—obese and diabetic patients—who are the target group for sugar substitution.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHORS CONTRIBUTIONS
BKW, RP, CB and ACM‐G designed the research; BKW and ACM‐G conducted research; BKW, JD, WV, CWR, LD‐T, JFR, JJH, BH, JT and ACM‐G analysed data; BKW, JD and ACM‐G performed statistical analysis; BKW and ACM‐G wrote the paper; and BKW and ACM‐G had primary responsibility for its final content. All the authors read and approved the final version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14342.
Supporting information
Appendix S1. Supporting information.
Appendix S2. Supporting information.
ACKNOWLEDGEMENTS
We would like to thank Jason Giger and Zinar Arslan (master students), Anke Etter‐Atlass and Sandra Gagliardo (study coordinators), and Joran Tóth (laboratory assistance). This research received no external funding and was financed by St. Clara Research Ltd, a non‐profit research institution, which is part of St. Claraspital Basel.
Wölnerhanssen BK, Drewe J, Verbeure W, et al. Gastric emptying of solutions containing the natural sweetener erythritol and effects on gut hormone secretion in humans: A pilot dose‐ranging study. Diabetes Obes Metab. 2021;23:1311–1321. 10.1111/dom.14342
Funding information This research received no external funding and was financed by St. Clara Research Ltd, a non‐profit research institution, which is part of St. Claraspital Basel.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet. 2011;378(9785):31‐40. [DOI] [PubMed] [Google Scholar]
- 2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4‐14. [DOI] [PubMed] [Google Scholar]
- 3. Pinhas‐Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693‐700. [DOI] [PubMed] [Google Scholar]
- 4. WHO . Guideline: Sugars Intake for Adults and Children. 2015. [PubMed] [Google Scholar]
- 5. Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar‐sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta‐analysis. Diabetes Care. 2010;33(11):2477‐2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler SPG. Low‐calorie sweetener use and energy balance: results from experimental studies in animals, and large‐scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunter SR, Reister EJ, Cheon E, Mattes RD. Low calorie sweeteners differ in their physiological effects in humans. Nutrients. 2019;11(11):2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bornet FR, Blayo A, Dauchy F, Slama G. Plasma and urine kinetics of erythritol after oral ingestion by healthy humans. Regul Toxicol Pharmacol. 1996;24(2 Pt 2):S280‐S285. [DOI] [PubMed] [Google Scholar]
- 9. Hootman KC, Trezzi JP, Kraemer L, et al. Erythritol is a pentose‐phosphate pathway metabolite and associated with adiposity gain in young adults. Proc Natl Acad Sci USA. 2017;114(21):E4233‐E4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolnerhanssen BK, Cajacob L, Keller N, et al. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am J Physiol Endocrinol Metab. 2016;310(11):E1053‐E1061. [DOI] [PubMed] [Google Scholar]
- 11. Overduin J, Collet TH, Medic N, et al. Failure of sucrose replacement with the non‐nutritive sweetener erythritol to alter GLP‐1 or PYY release or test meal size in lean or obese people. Appetite. 2016;107:596‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flint N, Hamburg NM, Holbrook M, et al. Effects of erythritol on endothelial function in patients with type 2 diabetes mellitus: a pilot study. Acta Diabetol. 2014;51(3):513‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson RJ, Nakagawa T, Sanchez‐Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307‐3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jameel F, Phang M, Wood LG, Garg ML. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis. 2014;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson RJ, Nakagawa T, Jalal D, Sanchez‐Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol Dial Transplant. 2013;28(9):2221‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noda K, Nakayama K, Oku T. Serum glucose and insulin levels and erythritol balance after oral administration of erythritol in healthy subjects. Eur J Clin Nutr. 1994;48(4):286‐292. [PubMed] [Google Scholar]
- 17. Ishikawa M, Miyashita M, Kawashima Y, Nakamura T, Saitou N, Modderman J. Effects of oral administration of erythritol on patients with diabetes. Regul Toxicol Pharmacol. 1996;24(2 Pt 2):S303‐S308. [DOI] [PubMed] [Google Scholar]
- 18. Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon‐labeled octanoic acid breath test. Gastroenterology. 1993;104(6):1640‐1647. [DOI] [PubMed] [Google Scholar]
- 19. Blundell J, de Graaf C, Hulshof T, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11(3):251‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38‐48. [DOI] [PubMed] [Google Scholar]
- 21. Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153(1–2):41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rehfeld JF. Incretin physiology beyond glucagon‐like peptide 1 and glucose‐dependent insulinotropic polypeptide: cholecystokinin and gastrin peptides. Acta Physiol. 2011;201(4):405‐411. [DOI] [PubMed] [Google Scholar]
- 23. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology. 2007;132(6):2131‐2157. [DOI] [PubMed] [Google Scholar]
- 24. Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev. 2007;87(4):1409‐1439. [DOI] [PubMed] [Google Scholar]
- 25. Cimmaruta D, Maiorino MI, Scavone C, et al. Efficacy and safety of insulin‐GLP‐1 receptor agonists combination in type 2 diabetes mellitus: a systematic review. Expert Opin Drug Saf. 2016;15(sup 2):77‐83. [DOI] [PubMed] [Google Scholar]
- 26. Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut‐expressed gustducin and taste receptors regulate secretion of glucagon‐like peptide‐1. Proc Natl Acad Sci USA. 2007;104(38):15069‐15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of 'taste' in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15(1):73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saltiel MY, Kuhre RE, Christiansen CB, et al. Sweet taste receptor activation in the gut is of limited importance for glucose‐stimulated GLP‐1 and GIP secretion. Nutrients. 2017;9(4):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steinert RE, Frey F, Topfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr. 2011;105(9):1320‐1328. [DOI] [PubMed] [Google Scholar]
- 30. Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G735‐G739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer‐Gerspach AC, Biesiekierski JR, Deloose E, et al. Effects of caloric and noncaloric sweeteners on antroduodenal motility, gastrointestinal hormone secretion and appetite‐related sensations in healthy subjects. Am J Clin Nutr. 2018;107(5):707‐716. [DOI] [PubMed] [Google Scholar]
- 32. Veedfald S, Wu T, Bound M, et al. Hyperosmolar duodenal saline infusion lowers circulating ghrelin and stimulates intestinal hormone release in young men. J Clin Endocrinol Metab. 2018;103(12):4409‐4418. [DOI] [PubMed] [Google Scholar]
- 33. Shi X, Osterberg KL, Petrie H, Stofan JR, Murray R. Effect of different osmolalities, CHO types, and [CHO] on gastric emptying in humans. Med Sci Sports Exerc. 2017;49(5):1015‐1021. [DOI] [PubMed] [Google Scholar]
- 34. Peeters TL, Vantrappen G, Janssens J. Fasting plasma motilin levels are related to the interdigestive motility complex. Gastroenterology. 1980;79(4):716‐719. [PubMed] [Google Scholar]
- 35. Vantrappen G, Janssens J, Peeters TL, Bloom SR, Christofides ND, Hellemans J. Motilin and the interdigestive migrating motor complex in man. Dig Dis Sci. 1979;24(7):497‐500. [DOI] [PubMed] [Google Scholar]
- 36. Deloose E, Vos R, Corsetti M, Depoortere I, Tack J. Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man. Neurogastroenterol Motil. 2015;27(1):63‐71. [DOI] [PubMed] [Google Scholar]
- 37. Deloose E, Vos R, Janssen P, et al. The motilin receptor agonist erythromycin stimulates hunger and food intake through a cholinergic pathway. Am J Clin Nutr. 2016;103(3):730‐737. [DOI] [PubMed] [Google Scholar]
- 38. Wu T, Zhao BR, Bound MJ, et al. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr. 2012;95(1):78‐83. [DOI] [PubMed] [Google Scholar]
- 39. Wu T, Bound MJ, Zhao BR, et al. Effects of a D‐xylose preload with or without sitagliptin on gastric emptying, glucagon‐like peptide‐1, and postprandial glycemia in type 2 diabetes. Diabetes Care. 2013;36(7):1913‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hunter SR, Reister EJ, Cheon E, Mattes RD . Low calorie sweeteners differ in their physiological effects in humans. Nutrients. 2019;11(11):2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greyling A, Appleton KM, Raben A, Mela DJ. Acute glycemic and insulinemic effects of low‐energy sweeteners: a systematic review and meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2020;1124:1002‐1014. [DOI] [PubMed] [Google Scholar]
- 42. Chukwuma CI, Mopuri R, Nagiah S, Chuturgoon AA, Islam MS. Erythritol reduces small intestinal glucose absorption, increases muscle glucose uptake, improves glucose metabolic enzymes activities and increases expression of Glut‐4 and IRS‐1 in type 2 diabetic rats. Eur J Nutr. 2018;57(7):2431‐2444. [DOI] [PubMed] [Google Scholar]
- 43. de Cock P. In: O'Donnell A, Kearsley W, eds. Erythritol. Sweeteners and Sugar Alternatives in Food Technology. John Wiley & Sons; 2012:215‐241. [Google Scholar]
- 44. Jacqz‐Aigrain E, Kassai B, Cornu C, et al. Gastrointestinal tolerance of erythritol‐containing beverage in young children: a double‐blind, randomised controlled trial. Eur J Clin Nutr. 2015;69(6):746‐751. [DOI] [PubMed] [Google Scholar]
- 45. Storey D, Lee A, Bornet F, Brouns F. Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. Eur J Clin Nutr. 2007;61(3):349‐354. [DOI] [PubMed] [Google Scholar]
- 46. Tetzloff W, Dauchy F, Medimagh S, Carr D, Bar A. Tolerance to subchronic, high‐dose ingestion of erythritol in human volunteers. Regul Toxicol Pharmacol. 1996;24(2 Pt 2):S286‐S295. [DOI] [PubMed] [Google Scholar]
- 47. Campolier M, Thondre SP, Clegg M, Shafat A, McIntosh A, Lightowler H. Changes in PYY and gastric emptying across the phases of the menstrual cycle and the influence of the ovarian hormones. Appetite. 2016;107:106‐115. [DOI] [PubMed] [Google Scholar]
- 48. Brennan IM, Feltrin KL, Nair NS, et al. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP‐1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G602‐G610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.
Appendix S2. Supporting information.
Data Availability Statement
Data available on request from the authors.


