Abstract
The three part, double‐blind, randomized, controlled reSURFACE 1 trial and extension study (NCT01722331) evaluated efficacy and safety of tildrakizumab in adults with moderate to severe plaque psoriasis. Patients with ≥50% improvement from baseline in Psoriasis Area and Severity Index (PASI 50) following treatment with tildrakizumab 100 mg (TIL100) or 200 mg (TIL200) could enter the optional long‐term extension study and continue treatment at the same dose for an additional 192 weeks. This subgroup analysis assessed the long‐term efficacy and safety of tildrakizumab treatment for Japanese patients enrolled in reSURFACE 1 for up to 5 years of treatment. The primary efficacy outcomes were the proportions of patients who maintained PASI 75 and Physician Global Assessment (PGA) clear or minimal with ≥2‐grade reduction from baseline (PGA 0/1) from base study week 64 to extension week 192. Secondary outcomes were the proportion of patients who maintained PASI 90/100 from base study week 64 to extension week 192. Adverse events (AEs) were monitored throughout the study and for up to 20 weeks after the last study visit. Of the 120 Japanese patients who entered the reSURFACE 1 extension study, 43 (79.6%) patients receiving tildrakizumab 100 mg and 58 (87.9%) patients receiving tildrakizumab 200 mg completed the extension study. Of all Japanese patients with PASI 75/90/100 and PGA 0/1 at week 64, 85%/88% receiving TIL100/TIL200 maintained PASI 75, 70%/96% maintained PASI 90, 63%/67% maintained PASI 100, and 68%/72% maintained PGA 0/1 at extension week 192. AEs led to discontinuation in 1.7 patients per 100 patient‐years (P100PY) receiving tildrakizumab 100 mg and 0.8 P100PY receiving tildrakizumab 200 mg. Incidences of severe infections, malignancies, confirmed major adverse cardiac events, and hypersensitivity reactions were low in both treatment groups. Through 5 years of treatment, tildrakizumab maintained efficacy and was well tolerated with low rates of AEs of special interest.
Keywords: biologic therapy, IL‐23p19, psoriasis, Psoriasis Area and Severity Index, tildrakizumab
1. INTRODUCTION
Psoriasis is an immune‐mediated inflammatory disease with an estimated prevalence of 0.34% in Japan; plaque psoriasis accounts for approximately 97% of cases of psoriasis in Japan. 1 Biological therapies using monoclonal antibodies have been developed to treat psoriasis; ten are approved for use in Japan (infliximab, adalimumab, ustekinumab, secukinumab, brodalumab, ixekizumab, guselkumab, risankizumab, certolizumab pegol, and tildrakizumab). 2 , 3 , 4 , 5 An analysis of the Japanese Medical Data Center database claims from 2009 to 2016 found approximately 11% of patients with psoriasis used biologic therapies. 2 Japanese patients report long‐term efficacy as an important attribute of biologic psoriasis treatment. 6 In a real‐world database of Japanese patients, 24‐month treatment persistence rates for first biologic treatment for psoriasis ranged from 41% for infliximab to 72% for ustekinumab. 2
Tildrakizumab is a high‐affinity, humanized, immunoglobulin G1κ, anti‐interleukin (IL)‐23p19 monoclonal antibody. 7 Tildrakizumab is approved in the US, the EU, Australia, and Japan for treatment of plaque psoriasis. 5 , 8 , 9 , 10 In the long‐term extension periods of the global phase 3 reSURFACE 1 and reSURFACE 2 studies, approximately 90% of patients who responded to tildrakizumab after 28 weeks of treatment maintained treatment response through 148 weeks (observed cases), and few patients discontinued treatment during the extension period due to lack of efficacy or adverse events (AEs). 11 This subgroup analysis explored tildrakizumab efficacy and safety in Japanese patients enrolled in reSURFACE 1 through 5 years of treatment.
2. METHODS
2.1. Study design
The reSURFACE 1 trial has been previously described. 7 Briefly, reSURFACE 1 was a three part, double‐blind, randomized, placebo‐controlled, 64‐week phase 3 study with an optional 192‐week extension study conducted at 118 study sites in five countries, including 45 sites in Japan. 7 The study protocol was reviewed and approved by independent ethics committees at each study site. The study was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided informed consent in accordance with International Conference on Harmonisation‐E6 Guideline for Good Clinical Practice and local country and/or cultural practices as applicable.
2.2. Patients
Detailed patient inclusion and exclusion criteria were previously reported. 7 Briefly, adult patients with moderate to severe chronic plaque psoriasis, including Body Surface Area (BSA) involvement ≥10%, Physician Global Assessment (PGA) ≥3, and Psoriasis Area and Severity Index (PASI) ≥12, were eligible to enroll in the reSURFACE 1 base study. Patients who completed the base study through week 64 with at least 50% improvement from baseline in PASI (PASI 50) could enter the optional long‐term extension study and continue treatment at the same dose for an additional 192 weeks.
2.3. Treatments
2.3.1. Base study
In part 1 (weeks 0–12), patients were randomized 2:2:1 to receive tildrakizumab 100 mg, tildrakizumab 200 mg, or placebo by subcutaneous injection at weeks 0 and 4. In part 2 (weeks 12–28), patients receiving tildrakizumab continued to receive the same dose every 12 weeks. Patients randomized to placebo treatment were rerandomized to receive tildrakizumab 100 or 200 mg at weeks 12 and 16 and administered every 12 weeks thereafter. In part 3 (weeks 28–64), patients originally randomized to tildrakizumab treatment who achieved PASI 75 were rerandomized to continue receiving tildrakizumab at the same dose or to placebo treatment through week 64. Patients rerandomized to placebo resumed tildrakizumab treatment at their previous dose upon relapse (50% reduction in maximum PASI response). Patients randomized to receive tildrakizumab 200 mg in part 1 with a partial response to treatment (PASI 50 but not PASI 75) at week 28 continued to receive tildrakizumab 200 mg. Patients with partial response to tildrakizumab 100 mg were rerandomized to treatment with tildrakizumab 100 or 200 mg. Patients rerandomized from placebo to tildrakizumab treatment at week 12 who achieved PASI 50 at week 28 continued to receive tildrakizumab at the same dose. Any patients who did not achieve PASI 50 at week 28 discontinued the study.
2.3.2. Extension study
Patients received tildrakizumab 100 or 200 mg at the same dose as at base study completion every 12 weeks through extension week 192. Study medication was administered in a double‐blind manner until all patients completed the base study and were unblinded after which study medication was administered open‐label during the long‐term extension period.
2.4. Assessments
The PASI and PGA were assessed at extension week 0 and every 12 weeks thereafter to extension week 96, and then every 24 weeks through extension week 192. AEs were monitored throughout the study and for up to 20 weeks after the last study visit.
2.5. Outcomes
The primary efficacy outcomes were the proportion of patients who maintained PASI 75 from week 64 to extension week 192, and the proportion of patients who maintained PGA clear or minimal with ≥2‐grade reduction from baseline (PGA 0/1) from week 64 to extension week 192. Secondary outcomes were the proportions of patients who maintained PASI 90 and PASI 100 from week 64 to extension week 192. Safety was assessed from exposure‐adjusted incidence rates (EAIRs) of AEs, serious AEs (SAEs), and prespecified AEs of special interest (Tier 1). Tier 1 AEs included serious infections, malignancies, nonmelanoma skin cancer (NMSC), melanoma, confirmed extended major adverse cardiac events (MACE), and hypersensitivity reactions.
2.6. Statistical analysis
Efficacy endpoints were analyzed for the full analysis set, defined as all patients who entered the extension study and received ≥1 dose of extension study medication, based on the treatment assigned. Safety endpoints were analyzed for the all subjects as treated analysis set, defined as all patients who entered the extension study and received ≥1 dose of extension study medication, based on the treatment received. Baseline characteristics and efficacy outcomes over time were summarized using descriptive statistics; missing data were not imputed. EAIRs of AEs were calculated in patients per 100 patient‐years (P100PY). Week 64 response rates excluded partial responders rerandomized from tildrakizumab 100 mg to tildrakizumab 200 mg at base study week 28.
3. RESULTS
3.1. Patients
A total of 120 Japanese patients entered the reSURFACE 1 extension study, including 54 patients receiving tildrakizumab 100 mg and 66 patients receiving tildrakizumab 200 mg. Of these, 43 (79.6%) patients receiving tildrakizumab 100 mg and 58 (87.9%) patients receiving tildrakizumab 200 mg completed the extension study. Among patients receiving tildrakizumab 100 mg, four discontinued due to AEs, three due to lack of efficacy, three due to withdrawal by patient, and one due to physician decision (Figure 1). Among patients receiving tildrakizumab 200 mg, five discontinued due to patient decision, two due to AEs, and one due to lack of efficacy (Figure 1). The majority of extension study patients were male (77.5%), and their mean ± standard deviation (SD) age was 48.2 ± 12.2 (Table 1). Prior to the base study, the patients who entered the extension study had mean ± SD PASI score 26.6 ± 11.4 and mean ± SD percent involved BSA 44.5 ± 21.2; PGA prior to the base study was 3 in 57 (47.5%) patients, 4 in 57 (47.5%) patients, and 5 in 6 (5.0%) patients (Table 1).
FIGURE 1.
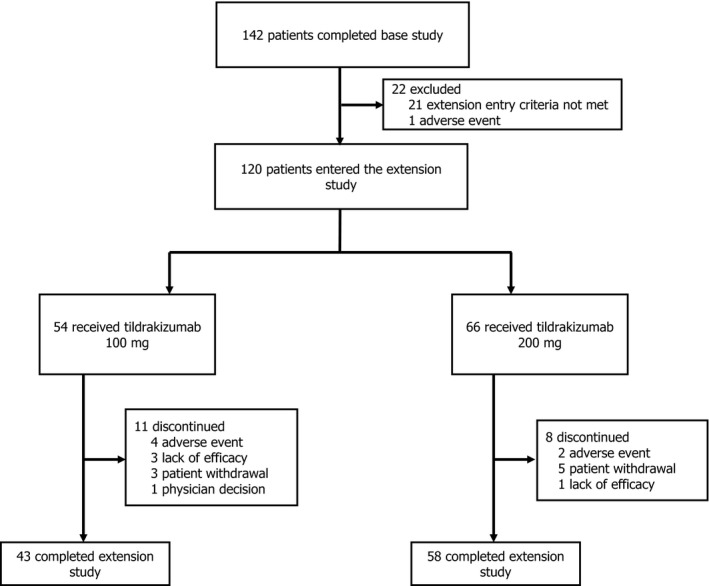
Patient disposition
TABLE 1.
Patient demographics and baseline characteristics of Japanese patients who entered the reSURFACE 1 long‐term extension study
| Patient characteristics | TIL 100 mg (n = 54) | TIL 200 mg (n = 66) | Total (N = 120) |
|---|---|---|---|
| Gender male, n (%) | 40 (74.1) | 53 (80.3) | 93 (77.5) |
| Age (years) | 47.1 ± 12.4 | 49.1 ± 12.1 | 48.2 ± 12.2 |
| Weight (kg) | 67.9 ± 13.0 | 72.1 ± 15.1 | 70.2 ± 14.3 |
| BMI (kg/m2) | 24.6 ± 4.0 | 25.4 ± 4.6 | 25.0 ± 4.4 |
| Baseline disease characteristics | |||
| BSA (%) | 42.1 ± 18.6 | 46.5 ± 23.1 | 44.5 ± 21.2 |
| PASI score | 25.3 ± 8.8 | 27.7 ± 13.1 | 26.6 ± 11.4 |
| PGA score, n (%) | |||
| 3 | 24 (44.4) | 33 (50.0) | 57 (47.5) |
| 4 | 28 (51.9) | 29 (43.9) | 57 (47.5) |
| 5 | 2 (3.7) | 4 (6.1) | 6 (5.0) |
| PsA (yes), n (%) | 8 (14.8) | 12 (18.2) | 20 (16.7) |
| Total cholesterol (mg/dl) | 202.5 ± 41.7 | 197.1 ± 32.2 | 199.5 ± 36.7 |
| LDL cholesterol (mg/dl) | 108.0 ± 28.3 | 113.6 ± 26.9 | 111.1 ± 27.5 |
| HDL cholesterol (mg/dl) | 63.0 ± 15.4 | 57.7 ± 16.5 | 60.1 ± 16.2 |
| Triglycerides (mg/dl) | 159.7 ± 164.3 | 129.3 ± 67.5 | 143.0 ± 121.4 |
| Fasting glucose (mg/dl) | 94.4 ± 13.1 | 97.8 ± 19.4 | 96.3 ± 16.9 |
| Non‐fasting glucose (mg/dl) | 98.2 ± 19.6 | 100.3 ± 24.6 | 99.4 ± 22.5 |
| Mean blood pressure (mmHg) | |||
| Diastolic | 83.0 ± 13.1 | 78.7 ± 11.0 | 80.6 ± 12.1 |
| Systolic | 132.4 ± 16.2 | 129.1 ± 14.5 | 130.6 ± 15.3 |
| Type 2 diabetes, n (%) | 1 (1.6) | 0 | 1 (0.8) |
Data presented as mean ± SD unless otherwise specified.
Abbreviations: BMI, body mass index; BSA, Body Surface Area; HDL, high density lipoprotein; LDL, low density lipoprotein; PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; PsA, psoriatic arthritis; SD, standard deviation; TIL, tildrakizumab.
3.2. Efficacy
3.2.1. Base study completion
At base study week 64, excluding partial responders rerandomized from tildrakizumab 100 mg to tildrakizumab 200 mg at week 28, 89% of patients treated with tildrakizumab 100 mg and 74% treated with tildrakizumab 200 mg achieved PASI 75. The proportion of patients achieving PASI 90 was 56% following treatment with tildrakizumab 100 mg and 46% following treatment with tildrakizumab 200 mg. A PASI 100 was achieved in 33% of patients treated with tildrakizumab 100 mg and 18% treated with tildrakizumab 200 mg. Patients receiving tildrakizumab 100 and 200 mg had PGA 0/1 rates of 78% and 65%, respectively. Proportions of patients with PASI 75, PASI 90, PASI 100, and PGA 0/1 at base study week 64 are displayed in Table 2.
TABLE 2.
Proportions of patients with PASI 75, PASI 90, PASI 100, and PGA 0/1 at base study week 64 and extension study week 192
| Responders | Base study week 64 | Extension study week 192 | ||
|---|---|---|---|---|
|
TIL 100 mg a n = 54 |
n = 57 |
TIL 100 mg n = 44 |
TIL 200 mg n = 58 |
|
| PASI 75, n (%) | 48 (88.9) | 42 (73.7) | 35 (79.5) | 44 (75.9) |
| PASI 90, n (%) | 30 (55.6) | 26 (45.6) | 21 (47.7) | 26 (44.8) |
| PASI 100, n (%) | 18 (33.3) | 10 (17.5) | 11 (25.0) | 9 (15.5) |
| PGA 0/1, n (%) | 42 (77.8) | 37 (64.9) | 26 (59.1) | 36 (62.1) |
Abbreviations: PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; TIL, tildrakizumab.
Includes patients with a partial response at week 28 who continued to receive the same dose.
Excludes patients with a partial response at week 28 in the TIL 100 mg group who were rerandomized to TIL 200 mg. These patients were omitted because they were disproportionately rerandomized to TIL 200 mg versus TIL 100 mg. The response rates including these patients (n = 66 patients total receiving TIL 200 mg at week 64) were PASI 75, 68.2%; PASI 90, 39.4%; PASI 100, 15.2%; PGA 0/1, 59.1%.
3.2.2. Extension study
The proportions of patients with PASI 75, PASI 90, PASI 100, and PGA 0/1 remained stable through extension week 192 (Table 2). At extension week 192, 80% of patients receiving tildrakizumab 100 mg achieved PASI 75, 48% achieved PASI 90, and 25% achieved PASI 100. Among patients receiving tildrakizumab 200 mg, 76% achieved PASI 75, 45% achieved PASI 90, and 16% achieved PASI 100 at week 192. The proportion of patients with PGA 0/1 at week 192 was 59% following treatment with tildrakizumab 100 mg and 62% following treatment with tildrakizumab 200 mg (Table 2).
Among patients with PASI 75 at base study week 64, 33/39 (85%) receiving tildrakizumab 100 mg and 36/41 (88%) receiving tildrakizumab 200 mg maintained response at extension week 192 (Figure 2a). For PASI 90, 19/27 (70.4%) patients treated with tildrakizumab 100 mg and 22/23 (96%) treated with tildrakizumab 200 mg with response at week 64 maintained response at extension week 192 (Figure 2b). A PASI 100 was maintained through extension week 192 in 10/16 (63%) patients receiving tildrakizumab 100 mg and 6/9 (67%) receiving tildrakizumab 200 mg with response at week 64 (Figure 2c). PGA 0/1 was maintained through extension week 192 in 23/34 (68%) patients receiving tildrakizumab 100 mg and 26/36 (72%) receiving tildrakizumab 200 mg with response at week 64 (Figure 2d).
FIGURE 2.
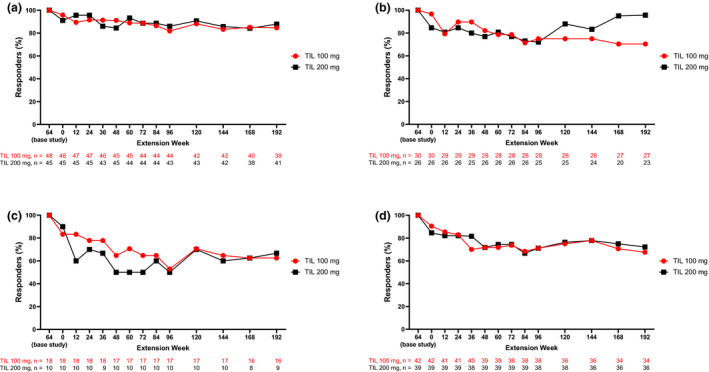
Proportions of patients who maintained base study week 64 (a) PASI 75, (b) PASI 90, (c) PASI 100, and (d) PGA 0/1 through extension week 192. Includes patients who had the indicated response level at week 64. Numbers of patients with data at each time point shown are shown below the graph; percentages are based on patients with data. PASI, Psoriasis Area and Severity Index; PGA 0/1, Physician Global Assessment of clear or minimal with ≥2‐grade improvement from baseline; TIL, tildrakizumab.
Mean percent change from baseline in PASI score was maintained from base study week 64 through extension week 192 for patients in both treatment groups. Among patients receiving tildrakizumab 100 mg, percent change from baseline in PASI was −89.6% at base study week 64 and −84.8% at extension week 192. Among patients receiving tildrakizumab 200 mg, percent change from baseline in PASI was −82.5% at base study week 64 and −82.9% at extension week 192.
3.3. Safety
Through 5 years of treatment, any AEs were reported in 19.3 P100PY receiving tildrakizumab 100 mg and 17.5 P100PY receiving tildrakizumab 200 mg (Table 3). The most common AEs (occurring in ≥5% of patients in one or more treatment groups) included nasopharyngitis, eczema, hypertension, influenza, and arthralgia (Table 4). SAEs occurred in 5.2 P100PY treated with tildrakizumab 100 mg and 3.6 P100PY treated with tildrakizumab 200 mg (Table 3).
TABLE 3.
Summary of AEs and AEs of special interest through 5 years of cumulative exposure
| TIL 100 mg (n = 63) | TIL 200 mg (n = 66) | |
|---|---|---|
| Any AE | 56 (19.3) | 63 (17.5) |
| SAEs | 15 (5.2) | 13 (3.6) |
| Discontinuations due to AEs | 5 (1.7) | 3 (0.8) |
| Deaths due to AEs | 0 | 0 |
| SAEs (≥1) | ||
| Angina pectoris | 1 (0.3) | 1 (0.3) |
| Atrial fibrillation | 1 (0.3) | 0 |
| Hypertensive heart disease | 1 (0.3) | 0 |
| Cataract | 0 | 1 (0.3) |
| Macular fibrosis | 0 | 1 (0.3) |
| Anal fissure | 1 (0.3) | 0 |
| Colitis ischemic | 0 | 1 (0.3) |
| AEs of special interest | ||
| Severe infections | 3 (1.0) | 5 (1.4) |
| Malignancies | 2 (0.7) | 2 (0.6) |
| Nonmelanoma skin cancer | 0 | 0 |
| Melanoma skin cancer | 0 | 0 |
| Confirmed MACE | 4 (1.4) | 1 (0.3) |
| Hypersensitivity reaction | 2 (0.7) | 1 (0.3) |
| Injection site erythema | 0 | 1 (0.3) |
| Injection site pain | 1 (0.3) | 0 |
| Injection site pruritis | 0 | 1 (0.3) |
Data shown as n (n P100PY).
Abbreviations: AE, adverse event; MACE, major adverse cardiovascular event; P100PY, per 100 patient‐years; SAE, serious AE; TIL, tildrakizumab.
TABLE 4.
Summary of most common AEs (occurring in ≥5% of patients in either treatment group) through 5 years of cumulative exposure
| TIL 100 mg (n = 63) | TIL 200 mg (n = 66) | |
|---|---|---|
| Nasopharyngitis | 25 (8.6) | 39 (10.9) |
| Eczema | 10 (3.4) | 8 (2.2) |
| Hypertension | 8 (2.8) | 8 (2.2) |
| Influenza | 6 (2.1) | 10 (2.8) |
| Arthralgia | 3 (1.0) | 10 (2.8) |
| Dermatitis contact | 7 (2.4) | 3 (0.8) |
| Insomnia | 7 (2.4) | 1 (0.3) |
| Pruritis | 6 (2.1) | 5 (1.4) |
| Back pain | 4 (1.4) | 7 (1.9) |
| Urticaria | 5 (1.7) | 4 (1.1) |
| Constipation | 5 (1.7) | 2 (0.6) |
| Psoriasis | 2 (0.7) | 6 (1.7) |
| Skin papilloma | 5 (1.7) | 1 (0.3) |
| Diarrhea | 4 (1.4) | 5 (1.4) |
| Dental caries | 4 (1.4) | 4 (1.1) |
| Abdominal pain upper | 4 (1.4) | 2 (0.6) |
| Seborrheic keratosis | 4 (1.4) | 2 (0.6) |
| Alanine aminotransferase increased | 4 (1.4) | 2 (0.6) |
| Gamma‐glutamyltransferase increased | 4 (1.4) | 1 (0.3) |
| Rhinitis allergic | 4 (1.4) | 1 (0.3) |
| Pharyngitis | 2 (0.7) | 5 (1.4) |
| Acrochordon | 0 | 5 (1.4) |
| Headache | 3 (1.0) | 4 (1.1) |
| Pneumonia | 2 (0.7) | 4 (1.1) |
| Gastroenteritis | 2 (0.7) | 4 (1.1) |
| Large intestine polyp | 1 (0.3) | 4 (1.1) |
| Gastroesophageal reflux disease | 1 (0.3) | 4 (1.1) |
| Spinal osteoarthritis | 1 (0.3) | 4 (1.1) |
| Herpes zoster | 0 | 4 (1.1) |
| Upper respiratory tract infection | 3 (1.0) | 3 (0.8) |
| Bronchitis | 3 (1.0) | 2 (0.6) |
| Conjunctivitis | 3 (1.0) | 2 (0.6) |
| Hyperkeratosis | 3 (1.0) | 2 (0.6) |
| Cellulitis | 3 (1.0) | 1 (0.3) |
| Hyperuricemia | 3 (1.0) | 1 (0.3) |
| Dermal cyst | 3 (1.0) | 0 |
Data shown as n (n P100PY).
Abbreviations: AE, adverse event; P100PY, per 100 patient‐years; TIL, tildrakizumab.
Few patients discontinued due to AEs: 1.7 P100PY receiving tildrakizumab 100 mg and 0.8 P100PY receiving tildrakizumab 200 mg (Table 3). Among patients receiving tildrakizumab 100 mg, patients discontinued due to breast cancer (n = 1), diffuse large B‐cell lymphoma (n = 1), cerebral infarction (n = 1), parkinsonism (n = 1), and asthma (n = 1). Among patients receiving tildrakizumab 200 mg, patients discontinued due to hepatitis E (n = 1), papillary thyroid cancer (n = 1), and cerebellar hemorrhage (n = 1). No deaths were reported.
Incidences of severe infections, malignancies, confirmed MACE, and hypersensitivity reactions were low in both treatment groups. Throughout the base and extension study periods, severe infections occurred in 1.0 P100PY (n = 3) receiving tildrakizumab 100 mg and 1.4 P100PY (n = 5) receiving tildrakizumab 200 mg. Malignancies were reported in 0.7 P100PY of tildrakizumab 100 mg treatment and 0.6 P100PY of tildrakizumab 200 mg treatment (n = 2 each). A total of 1.4 P100PY (n = 4) receiving tildrakizumab 100 mg and 0.3 P100PY (n = 1) receiving tildrakizumab 200 mg experienced confirmed extended MACE. Patients receiving tildrakizumab 100 experienced coronary revascularization (n = 1), hemorrhagic stroke or hemorrhagic change (n = 2), and ischemic stroke (n = 3). One patient receiving tildrakizumab 200 mg experienced coronary revascularization. Hypersensitivity reactions occurred in 0.7 P100PY of treatment with tildrakizumab 100 mg (n = 2) and 0.3 P100PY of treatment with tildrakizumab 200 mg (n = 1). No melanomas or NMSC were reported.
4. DISCUSSION
There are a limited number of phase 3 clinical trials examining long‐term efficacy of biologic agents in Japanese patients with moderate to severe plaque psoriasis. 12 , 13 , 14 This is the first clinical trial assessing tildrakizumab, an anti‐IL‐23p19 monoclonal antibody treatment, in Japanese patients for up to 5 years. Baseline demographics and characteristics for patients in this subanalysis, including age, weight, gender, baseline PASI score, and involved BSA, were comparable to other clinical trials with Japanese patients using IL‐17 (secukinumab, ixekizumab, and brodalumab) and IL‐23 (guselkumab and risankizumab) inhibitors for the treatment of moderate to severe plaque psoriasis. 12 , 13 , 14 , 15 , 16
Most patients with responses at base study week 64 maintained PASI 75, PASI 90, PASI 100 and PGA 0/1 throughout the extension period. Among patients receiving tildrakizumab 100 mg with PASI 75, PASI 90, and PASI 100 at base study week 64, 85%, 70%, and 63% maintained response at extension study week 192, respectively. Among patients receiving tildrakizumab 200 mg with PASI 75, PASI 90, and PASI 100 at base study week 64, 88%, 96%, and 67% maintained response at extension study week 192, respectively. Among patients with PGA 0/1 response at week 64, 68% of patients receiving tildrakizumab 100 mg and 72% of patients receiving tildrakizumab 200 mg maintained response at extension study week 192 in patients.
Biologic use may pose different safety issues depending on the class of inhibitor. Tumor necrosis factor antagonists are associated with increased risk of serious infections including tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections due to other opportunistic pathogens. 17 , 18 , 19 Patients treated with IL‐17 inhibitors displayed increased risk of Candida infections and/or worsening of pre‐existing inflammatory bowel disease during clinical trials. 20 , 21 , 22 , 23 , 24 , 25 , 26 Clinical studies of IL‐23p19 antagonists reported a lower occurrence of specific AEs such as serious infections, malignancies, MACE, and drug hypersensitivity reactions. 16 , 27 , 28 , 29 In the current study, tildrakizumab treatment was generally well tolerated, with low rates of discontinuations, SAEs, and AEs of special interest. Consistent with other clinical trials for the treatment of psoriasis with biologic agents, nasopharyngitis was the most common AE observed in this study. 13 , 14 , 15 , 30 , 31 , 32 Other frequent AEs observed in this and other studies include eczema, hypertension, influenza, arthralgia, pruritis, and contact dermatitis. 13 , 14 , 31
Studies of IL‐17 and IL‐23 inhibitors using different treatment strategies have demonstrated maintenance of PASI 75, PASI 90, and PASI 100 with continuous treatment. 12 , 14 , 15 However, there are few clinical studies evaluating long‐term efficacy and safety in Japanese patients with psoriasis. A clinical trial reporting the 3‐year efficacy and safety results of treatment with 300 mg secukinumab in Japanese patients demonstrated better maintenance of PASI 75 response when patients were treated at fixed, monthly intervals rather than receiving treatment as needed. 14 Another study examining the long‐term efficacy and safety of ixekizumab in Japanese patients with treatment withdrawal and retreatment demonstrated that most patients regained PASI 75, PASI 90, and PASI 100 responses within 12 weeks of retreatment and maintained the response rate between for up to 120 weeks of retreatment. 12 However, direct comparisons between PASI response rates in our study and the rates in these studies should not be made due to differences in study design, procedures, and patient populations.
There are several limitations to the current analysis. Baseline PASI score and BSA of Japanese patients in this study were relatively high, compared to global studies using IL‐23 inhibitors including tildrakizumab. 7 , 11 , 30 Furthermore, the subgroup analysis included relatively small numbers of patients in each treatment group. Comparisons of significant differences in benefits in efficacy between the tildrakizumab 100 and 200 mg groups were not statistically evaluated. However, the PASI 75 response rates at week 64 of 89% and 74% in patients receiving tildrakizumab 100 and 200 mg, respectively, may reflect greater baseline disease severity, including higher BSA, PASI score, and PGA score, in patients randomized to receive tildrakizumab 200 mg compared with those receiving tildrakizumab 100 mg. Additionally, we are the first to present mean improvements in absolute PASI scores over time that were maintained up to week 192 in Japanese patients, measurements that we feel are valuable to physicians and patients during their discussions about drug response, and durability of response.
Through 5 years of treatment, tildrakizumab maintained efficacy and was well tolerated with low rates of AEs of special interest in Japanese patients with moderate to severe plaque psoriasis. Despite a higher burden of disease at baseline, 5‐year efficacy results in Japanese patients were comparable to or slightly higher than the 3‐year efficacy results for the entire study population in reSURFACE 1.
CONFLICT OF INTEREST
Dr. Imafuku has received consulting fees and/or honoraria from AbbVie, Celgene, Eisai, Eli Lilly & Co., Janssen Pharmaceuticals, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Taiho Pharmaceutical, Torii Pharmaceutical, UCB Japan, and Yakuhin. Dr. Nakagawa received consulting fees and/or speaker honoraria from AbbVie, Janssen Pharmaceuticals, Japan Tobacco, Kyowa Hakko Kirin, LEO Pharma, Maruho, Torii Pharmaceutical, and UCB Japan. Dr. Igarashi has received honoraria as a member of an advisory board for AbbVie, Celgene K.K., Eli Lilly Japan K.K., Janssen Pharmaceuticals K.K., Maruho, Novartis Pharma K.K., and Sun Pharma Japan Ltd. Dr. Morita has received research grants, consulting fees, and/or speaker's fees from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly & Co., Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi‐Iko, Nippon Kayaku, Novartis Pharma K.K, Sun Pharmaceutical Industries, Taiho Pharmaceutical, and Torii Pharmaceutical, Ushio. Dr. Okubo has received research grants from Eisai, Maruho, Shiseido, and Torii; and has current consulting/advisory board agreements, and/or is on a speaker's bureau and/or is an investigator on clinical trials with AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen Pharma, Jimro, Kyowa Kirin, LEO Pharma, Maruho, Novartis Pharma, Pfizer, Sanofi, Sun Pharma, Taiho, Tanabe‐Mitsubishi, Torii, and UCB Pharma. Dr. Sano has received research grants from AbbVie, Kaken, Kyowa Hakko Kirin, Maruho, Nihon Kayaku Pola Pharma, Sanofi Aventis, Taiho, and Torii; and speaker's fees from AbbVie, Celgene, Eisai, Eli Lilly & Co., Janssen, Kyowa Hakko Kirin, Maruho, Mitsubishi Tanabe, Torii, Novartis Pharma, Sanofi Aventis, Taiho, and UCB Japan. Dr. Tada has received honoraria for research from AbbVie, Celgene, Eisai, Eli Lilly & Co., Kyowa Hakko Kirin, LEO Pharma, Maruho, Meiji Seika Pharma Co., Taiho Pharmaceutical, and Torii Pharmaceutical; and as a lecturer from AbbVie, Celgene, Eli Lilly & Co., Janssen Pharmaceuticals, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Novartis Pharma K.K., Taiho Pharmaceutical, and UCB. Dr. Nemoto has no COI to disclose in compliance with the guidance released by Japanese Dermatology Association. Dr. Rozzo is an employee of Sun Pharmaceutical Industries, Inc. Dr. Kawamura is an employee of Sun Pharmaceutical Japan, Ltd. Dr. Ohtsuki has received honoraria as a member of an advisory board for AbbVie; Boehringer Ingelheim; Bristol‐Myers Squibb; Celgene K.K.; Eisai Co Ltd; Eli Lilly Japan K.K.; Janssen Pharmaceuticals; Kyowa Hakko Kirin; LEO Pharma; Maruho; Mitsubishi Tanabe; Novartis Pharma K.K.; Pfizer Japan, Inc; and Sun Pharma Japan, Ltd.
ACKNOWLEDGMENTS
The studies were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Analyses were funded by Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA. Medical writing and editorial support were provided by Shavonn Harper, PhD, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc.
REFERENCES
- 1. Kubota K, Kamijima Y, Sato T, Ooba N, Koide D, Iizuka H, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1):e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sruamsiri R, Iwasaki K, Tang W, Mahlich J. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real‐world data study using a claims database. BMC Dermatol. 2018;18(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saeki H, Terui T, Morita A, Sano S, Imafuku S, Asahina A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47(3):201–22. [DOI] [PubMed] [Google Scholar]
- 4. CIMZIA® (certolizumab pegol) now available for patients in Japan living with multiple psoriatic diseases [Press Release]. Brussels, Belgium: UCB; c2007–20 [Cited 2020 June 23]. https://www.ucb.com/_up/ucb_com_presscenter/images/Japan‐PSO‐Approval‐Press‐Release_FINAL.pdf. Accessed 9 Oct 2020. [Google Scholar]
- 5. Sun Pharma announces Japan MHLW approval of ILUMYATM for the treatment of Plaque Psoriasis [Press release]. Mumbai, India: Sun Pharmaceutical Industries Ltd.; [Cited 2020 July 20]. https://jp.sunpharma.com/up_pdf/20200629161219_pdf.pdf. Accessed 9 Oct 2020. [Google Scholar]
- 6. Tada Y, Ishii K, Kimura J, Hanada K, Kawaguchi I. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46(6):466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–88. [DOI] [PubMed] [Google Scholar]
- 8. ILUMYA™ (tildrakizumab‐asmn) injection, for subcutaneous use. Highlights of prescribing information. Cranbury, NJ: Sun Pharmaceutical Industries, Inc.; 2018. [Google Scholar]
- 9. Ilumetri 100 mg solution for injection in pre‐filled syringe (tildrakizumab). Summary of product characteristics. Barcelona, Spain: Almirall, S. A.; 2018. [Google Scholar]
- 10.ILUMYA™ 100 mg/1 mL solution for injection (tildrakizumab). Australian product information. Macquarie Park, NSW: Sun Pharma ANZ Pty Ltd; 2018. [Google Scholar]
- 11. Reich K, Warren RB, Iversen L, Puig L, Pau‐Charles I, Igarashi A, et al. Long‐term efficacy and safety of tildrakizumab for moderate‐to‐severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol. 2020;182(3):605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Umezawa Y, Torisu‐Itakura H, Morisaki Y, ElMaraghy H, Nakajo K, Akashi N, et al. Long‐term efficacy and safety results from an open‐label phase III study (UNCOVER‐J) in Japanese plaque psoriasis patients: impact of treatment withdrawal and retreatment of ixekizumab. J Eur Acad Dermatol Venereol. 2019;33(3):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamaguchi Y, Takatsu N, Ootaki K, Nakagawa H. Long‐term safety of brodalumab in Japanese patients with plaque psoriasis: an open‐label extension study. J Dermatol. 2020;47(6):569–77. 10.1111/1346-8138.15343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okubo Y, Ohtsuki M, Morita A, Yamaguchi M, Shima T, Tani Y, et al. Long‐term efficacy and safety of secukinumab in Japanese patients with moderate to severe plaque psoriasis: 3‐year results of a double‐blind extension study. J Dermatol. 2019;46(3):186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti‐interleukin‐23 monoclonal antibody, for the treatment of moderate to severe plaque‐type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double‐blind, placebo‐controlled study. J Dermatol. 2018;45(9):1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohtsuki M, Fujita H, Watanabe M, Suzaki K, Flack M, Huang X, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. HUMIRA® (adalimumab) injection, for subcutaneous use. Highlights of prescribing information. North Chicago, IL: AbbVie Inc.; 2020. [Google Scholar]
- 18. CIMZIA (certolizumab pegol) for injection, for subcutaneous use. Highlights of prescribing information. Smyrna, GA: UCB, Inc.; 2019. [Google Scholar]
- 19. REMICADE (infliximab) lyophilized concentrate for injection, for intravenous use. Highlights of prescribing information. Horsham, PA: Janssen Biotech, Inc.; 2017. [Google Scholar]
- 20. Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, Xia S, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate‐to‐severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32(9):1507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CEM, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38. [DOI] [PubMed] [Google Scholar]
- 22. Leonardi C, Reich K, Foley P, Torii H, Gerdes S, Guenther L, et al. Efficacy and safety of ixekizumab through 5 years in moderate‐to‐severe psoriasis: long‐term results from the UNCOVER‐1 and UNCOVER‐2 phase‐3 randomized controlled trials. Dermatol Ther. 2020;10(3):431–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papp K, Menter A, Leonardi C, Soung J, Weiss S, Pillai R, et al. Long‐term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE‐1). Br J Dermatol. 2020;183(6):1037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. TALTZ (ixekizumab) injection, for subcutaneous use. Highlights of prescribing information. Indianapolis, IN: Eli Lilly and Company; 2020. [Google Scholar]
- 25. SILIQ™ (brodalumab) injection, for subcutaneous use. Highlights of prescribing information. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2017. [Google Scholar]
- 26. COSENTYX® (secukinumab) for injection, for subcutaneous use. Highlights of prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2020. [Google Scholar]
- 27. Reich K, Griffiths CEM, Gordon KB, Papp KA, Song M, Randazzo B, et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol. 2020;82(4):936–45. [DOI] [PubMed] [Google Scholar]
- 28. Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK‐3222), an anti‐interleukin‐23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo‐controlled trial. Br J Dermatol. 2015;173(4):930–9. [DOI] [PubMed] [Google Scholar]
- 29. Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182(6):1348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blauvelt A, Reich K, Papp KA, Kimball AB, Gooderham M, Tyring SK, et al. Safety of tildrakizumab for moderate‐to‐severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br J Dermatol. 2018;179(3):615–22. [DOI] [PubMed] [Google Scholar]
- 31. Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen Y‐K, et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17. [DOI] [PubMed] [Google Scholar]
- 32. Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group . Efficacy and safety of ustekinumab in Japanese patients with moderate‐to‐severe plaque‐type psoriasis: long‐term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–52. [DOI] [PubMed] [Google Scholar]


