Abstract
Background and purpose
The clinico‐radiological paradox in multiple sclerosis (MS) is well recognized, relevant and yet poorly understood. The suitability of an in vivo model for the clinico‐radiological paradox was tested, using internuclear ophthalmoplegia (INO) and the medial longitudinal fasciculus (MLF).
Methods
In this cross‐sectional study lesions of the MLF were rated by an experienced MS neuroradiologist blinded to all other information. The presence of an INO was objectively determined by a validated infrared oculography protocol (DEMoNS). Clinical information, including the National Eye Institute Visual Function Questionnaire, was obtained.
Results
This study included 202 patients with MS. The clinico‐radiological paradox occurred in 50 patients (25%). This consisted of 45 patients having an INO without an MLF lesion and five patients with an MLF lesion but without an INO. The visual function overall score was related to the presence of an INO (p = 0.016), but not to MLF lesions seen on magnetic resonance imaging (MRI) (p = 0.207). A consensus list of potential causes for the clinico‐radiological paradox was compiled and the MRI images were deposited in a repository.
Conclusion
This study provides an objective and quantitative model to investigate the clinico‐radiological paradox. Our data suggest that pathology of the MLF is more frequently detected and more clinically relevant by infrared oculography than by MLF lesion rating on MRI.
Keywords: eye movements, magnetic resonance imaging, multiple sclerosis, ophthalmoplegia, saccades
This study provides an objective and quantitative model to investigate the clinico‐radiological paradox, by comparing the presence of internuclear ophthalmoplegia and medial longitudinal fasciculus lesions on magnetic resonance imaging.
Abbreviation
- AUC
area under the curve
- EDSS
Expanded Disability Status Scale
- HC
healthy control
- INO
internuclear ophthalmoplegia
- MLF
medial longitudinal fasciculus
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- VDI
versional dysconjugacy index
INTRODUCTION
The clinico‐radiological paradox has been defined as a mismatch between visible lesions on magnetic resonance imaging (MRI) scans from patients with multiple sclerosis (MS) and clinical disability [1, 2]. Essentially, there exist two versions of the clinico‐radiological paradox. The first possibility for a clinico‐radiological paradox arises from more MRI lesions than expected from the clinical assessment. Amongst other factors this may be explained by cortical plasticity [3, 4]. The second possibility for a clinico‐radiological paradox is that there are fewer MRI lesions than expected from the clinical assessment. This could be explained by sub visible white matter pathology or lack of sufficient use of the multimodal imaging capacities MRI provided [5, 6].
The size of the problem has recently been updated in a meta‐analysis [5]. Of 50 studies identified, 32 were included. The studies were published between 1987 and 2014 and were based on cognitive and MRI data from 2050 patients. A key point from this analysis was that there are substantial differences in the methodologies applied and standardization is relevant for future research on the topic [5]. This also applies for spinal cord pathology [2]. One important limitation to all of these studies is that composite clinical scales or cognitive function tests are used which inevitably result in multiple possibilities for MS lesion location and involvement of cortical networks capable of plasticity.
There is a need to overcome these limitations to appropriately investigate a fundamental problem in MS research [2, 5]. It was reasoned that this may be achieved by the use of a well‐defined model with a unique identifiable lesion location and an accurately quantifiable clinical deficit. For this reason internuclear ophthalmoplegia (INO), a common eye movement disorder in MS, was chosen [7, 8, 9, 10]. Whilst an INO is difficult to detect with a clinical examination and easily missed, an INO can be detected and quantified very accurately with infrared oculography [10, 11, 12, 13]. Anatomically the unique lesion location is the medial longitudinal fasciculus (MLF), a pair of short white matter tracts in the periventricular brainstem (Figure S1). Pathological studies [14, 15, 16, 17] have confirmed the sharp anatomical relationship between the presence of ipsilateral MLF involvement and INO: a left sided lesion results in an INO during a rightward saccade (in which the left eye is delayed) and vice versa. Therefore, the aim of this study was to investigate if the INO‐MLF model will permit us to investigate both aspects of the clinico‐radiological paradox. To prevent information bias, a blinded MLF rating in an unselected cohort was required. Next the well‐defined cases of a clinico‐radiological paradox were interrogated for identification of relevant factors and future research.
METHODS
Study design and patient population
This study (study number 2015.227) was approved by the Medical Ethical Committee on Human Research of the Amsterdam University Medical Center. Written informed consent was obtained from all participants prior to inclusion.
For this observational cross‐sectional study, MS patients and healthy controls (HCs) were included from the Amsterdam MS cohort, an ongoing observational cohort of the Amsterdam University Medical Center [18, 19, 20]. The HC group was only used for the INO detection method [10] and comparison of eye movement values. Eye movement results on saccadic and fixational abnormalities of this cohort were previously reported [10, 21]. Participants were at least 18 years of age. All MS patients had to fulfil clinical and radiological criteria for a diagnosis of clinically definite MS [22]. The disease course was described as relapsing–remitting, secondary progressive or primary progressive [23]. Inclusion and exclusion criteria were as described before [10, 18, 19, 20].
Clinical and ophthalmological assessment
All assessments (clinical, infrared oculography and MRI) were performed in the same sequence on the same day.
The disease duration was calculated in years from the first MS symptom. The Expanded Disability Status Scale (EDSS) score [24] was determined by a certified examiner to assess the level of disability. History of symptomatic MS associated optic neuritis was based on a consensus protocol [25]. Visual acuity (high and low contrast) was assessed using Sloan letter charts (100% and 2.5%, respectively) [26]. Spectral‐domain optical coherence tomography (Spectralis, Heidelberg Engineering) was performed in all subjects as described previously, including quality control criteria [27, 28]. Global peri‐papillary retinal nerve fibre layer thickness and macular ganglion cell and inner plexiform layer thickness of both eyes were obtained. The vision‐related quality of life was assessed with the National Eye Institute Visual Function Questionnaire (NEI‐VFQ‐25) [29, 30]. In addition, a published in‐house questionnaire for complaints specific to problems with eye movements was included [10]. This questionnaire records complaints of double vision, blurred vision, focusing problems, oscillopsia and changing lines whilst reading [10].
Infrared oculography
Eye movements were recorded and analysed using the validated DEMoNS protocol [11]. In brief, the Eyelink 100 Plus eye tracker was used for measuring eye movements at a frequency of 1000 Hz. Participants were seated in front of a display monitor and, after a nine‐point calibration and validation procedure, they had to follow instructions for several different fixational and saccadic tasks [11]. For this study, the pro‐saccadic task was used. This task includes 60 saccades from the centre of the screen to an eccentric location 8° or 15° of visual angle left or right from the centre. The presence of an INO was based on a systematic assessment of two versional dysconjugacy index (VDI) parameters in HC and MS patients as described previously [10]. The VDI describes the ratio of the abducting eye to the adducting eye. In brief, these were the VDI area under the curve (AUC) of the saccadic trajectory and the VDI peak velocity divided by amplitude (Pv/Am) of 15° saccades. All MS patients who exceeded either the VDI AUC (1.174) threshold or the VDI Pv/Am (1.180) threshold were recorded as having an INO. Each INO case was further classified into unilateral leftward, unilateral rightward or bilateral [10].
Magnetic resonance imaging acquisition and MLF rating
Magnetic resonance imaging was acquired with a 3 T whole body scanner (GE Signa, HDx T) using an eight‐channel phased‐array coil. T2 and proton density weighted images were analysed by a specialist MS neuroradiologist (ES) who was blinded to the INO detection. MRI images were systematically reviewed for MLF lesions. In the case of doubtful MLF lesions, consensus was achieved with a second reviewer (AP), also blinded for the INO detection. As a post hoc analysis, all cases with a clinico‐radiological paradox were inspected again, to identify possible causes for the discrepancy between presence of INO and MLF lesions.
The MRI images of the cases with a clinico‐radiological paradox are available for inspection via a data repository (https://doi.org/10.17026/dans‐zc3‐ttru).
Statistical analyses
All analyses were performed in Stata (StataCorp. 2015, Stata Statistical Software: Release 14, StataCorp LP). Data were reviewed visually and statistically for normality. Descriptive statistics were provided for the entire cohort and also for the predefined subgroups with a clinico‐radiological paradox.
The frequency distributions of INO detection with infrared oculography and MLF lesions on MRI were compared in a 2 × 2 contingency table. Independent t tests (Gaussian data) and non‐parametric tests (non‐Gaussian data) were used for the comparison of characteristics between patients with and without a clinico‐radiological paradox. The chi‐squared test was used for categorical data. Linear and logistic regression analyses were used for comparing clinical characteristics between INO and non‐INO patients and between MS patients with and without an MLF lesion, adjusted for age and sex. p values lower than 0.05 were regarded as significant.
RESULTS
Study population
In total, 226 MS patients and 61 HCs were recruited to this study. Of these, 16 MS patients and three HCs had to be excluded based on the quality control of the eye movement data [10, 11] and eight MS patients because no MRI data were available. The demographic and clinical characteristics of the included participants are summarized in Table 1. Patients had a mean disease duration of 20.9 (±8.5) years and the majority (63%) had a relapsing–remitting disease course.
TABLE 1.
Demographic and clinical characteristics of the healthy controls and MS patients
| MS patients | Healthy controls | p value | |||
|---|---|---|---|---|---|
|
All N = 202 |
Paradox N = 50 |
No paradox N = 152 |
N = 58 | Paradox vs. non‐paradox | |
| Sex (N, female) | 136 (67%) | 28 (56%) | 108 (71%) | 31 (53%) | 0.049 |
| Age (years) | 54.2 (±10.8) | 58.0 (±1.2) | 53.0 (±0.9) | 52.4 (±9.1) | 0.005 |
| Disease duration (years) | 20.9 (±8.5) | 25.2 (±1.3) | 19.5 (±0.6) | NA | <0.001 |
| EDSS (median, IQR, total range) | 3.5 (2.8, 0.0–8.5) | 4.0 (3.0) | 3.5 (2.0) | NA | 0.022 |
| Disease type | |||||
| RRMS (N) | 127 (63%) | 23 (46%) | 104 (68%) | NA | |
| SPMS (N) | 53 (26%) | 21 (42%) | 32 (21%) | NA | 0.008 |
| PPMS (N) | 17 (8%) | 6 (12%) | 11 (7%) | NA | |
| Unclassifiable | 5 (2%) | 0 (0%) | 5 (3%) | NA | |
| HCVA (best eye) a | 56.4 (±7.0) | 54.9 (±6.1) | 56.9 (±7.2) | NA | 0.092 |
| LCVA (best eye) b | 30.9 (±12.1) | 29.6 (±13.7) | 31.3 (±11.6) | NA | 0.457 |
| Optic neuritis (N) c | 91 (49%) | 27 (57%) | 64 (46%) | NA | 0.176 |
Abbreviations: EDSS, Expanded Disability Status Scale score; HCVA, high‐contrast visual acuity; IQR, interquartile range; LCVA, low‐contrast visual acuity; MS, multiple sclerosis; N, number; NA, not applicable; PP, primary progressive; RR, relapsing–remitting; SP, secondary progressive.
HCVA data missing for 15 patients.
LCVA data missing for 54 patients.
Conclusive evidence on optic neuritis missing for 16 patients.
Internuclear ophthalmoplegia and MLF lesions
Of the total 202 MS patients who were included, 66 (33%) had an INO detected with infrared oculography. An MLF lesion was found in 26 (13%) patients. In Figure 1, examples of different types of MRI lesions and corresponding recordings with infrared oculography are shown.
FIGURE 1.
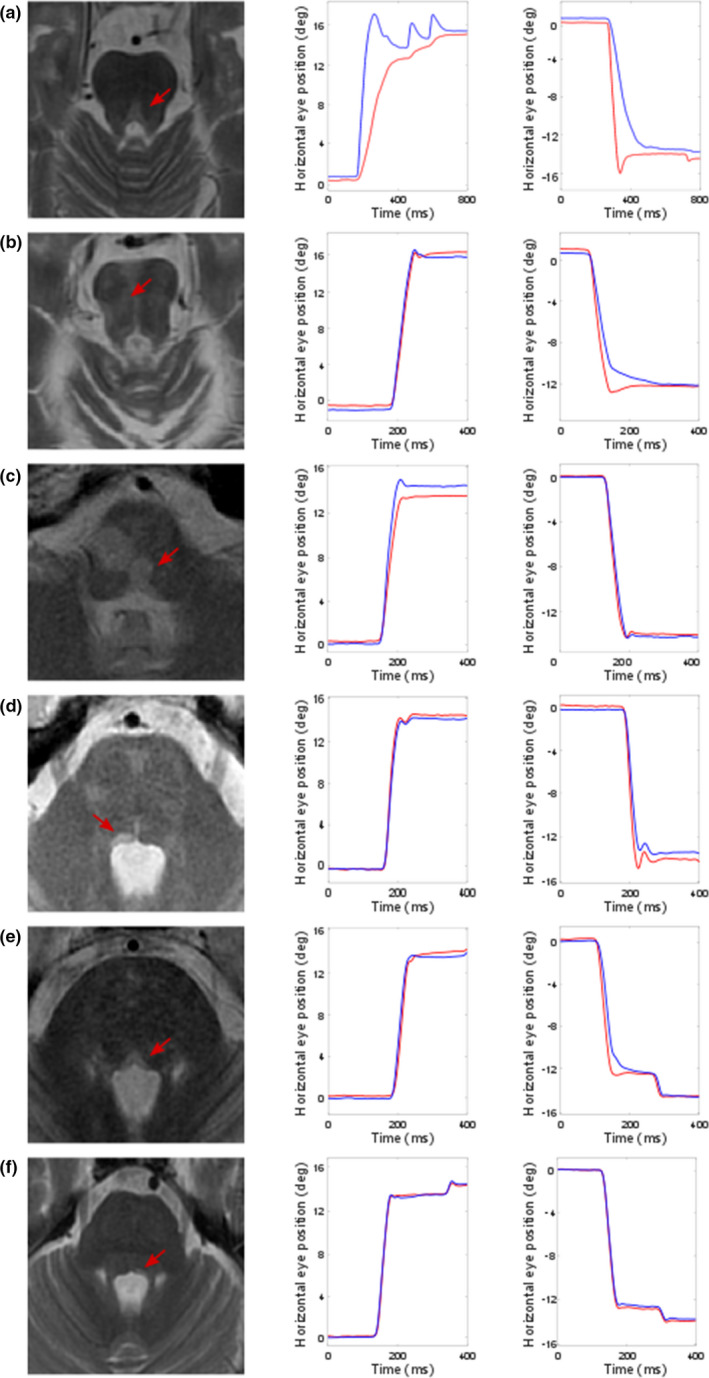
Examples of medial longitudinal fasciculus (MLF) lesions and recordings with infrared oculography. MLF lesions (arrow) on MRI with corresponding recordings of the patient of a rightward (left graph) and a leftward (right graph) saccade measured with infrared oculography. The X‐axis represent the time in milliseconds (ms), the Y‐axis the horizontal eye position in degrees (deg) of visual angle. The blue line represents the right eye, the red line represents the left eye. VDI values mentioned below represent mean VDI area under the curve (AUC) values of 15° saccades. (a) A clearly visible bilateral MLF lesion was found in the brainstem. This patient showed an evident bilateral INO, with a VDI AUC of 1.90 for rightward saccades and 1.53 for leftward saccades. (b) A large lesion was found in the right pons, with MLF involvement. This patient showed a leftward INO, with a VDI AUC of 1.10 for rightward saccades and 1.25 for leftward saccades. (c) A bilateral lesion was found in the lower pons to upper medulla oblongata, with MLF involvement. Based on the detection criteria, a mild bilateral INO was found. The leftward INO is not clearly visible on visual inspection of the recordings (right graph) and was only just above the threshold of detection with the VDI PvAm, not with the VDI AUC. The VDI AUC was 1.26 for rightward saccades and 1.12 for leftward saccades. (d) A clear right sided MLF lesion was found in the mid pons. This patient had a leftward INO, with a VDI AUC of 0.93 for rightward saccades and 1.32 for leftward saccades. (e) A bilateral MLF lesion was found in the lower pons. This patient demonstrated only a leftward INO, with a VDI AUC of 1.14 for rightward saccades and 1.20 for leftward saccades. (f) A lesion in the lower pons rated as left sided MLF lesion was found. In this patient there was no INO with infrared oculography measurement (VDI AUC of 1.05 for rightward saccades and 1.06 for leftward saccades). In retrospect, the lateral location of the lesion may just bypass the MLF. [Colour figure can be viewed at wileyonlinelibrary.com]
In Table 2 the frequency distribution is shown of the relationship between INO detection and MLF lesions. In summary, 50 (25%) patients showed a clinico‐radiological paradox. The majority of this group (n = 45) consisted of patients having an INO without an MLF lesion. In the subgroup (n = 26) of patients with an MLF lesion, 21 (81%) showed an INO with infrared oculography. The majority (n = 15, 71%) of these INOs was bilateral. Of the six unilateral INOs with an MLF lesion, five (83%) showed a lesion that was restricted to the involved side and one showed a bilateral MLF lesion.
TABLE 2.
INO detected with infrared oculography compared to MLF lesion on MRI
| MLF lesion | Total | ||||
|---|---|---|---|---|---|
| Yes | No | ||||
| INO detected | Yes | Number | 21 | 45 | 66 |
| % within MLF lesion | 80.8% | 25.6% | |||
| % within INO detected | 31.8% | 68.2% | |||
| No | Number | 5 | 131 | 136 | |
| % within MLF lesion | 19.2% | 74.4% | |||
| % within INO detected | 3.7% | 96.3% | |||
| Total | Number | 26 | 176 | 202 | |
The percentages accompanying the number of patients indicate either the percentage of the number in the corresponding column (MLF lesion yes or no) or the percentage of the number in the corresponding row (INO detected yes or no).
Abbreviations: INO, internuclear ophthalmoplegia; MLF, medial longitudinal fasciculus; MRI, magnetic resonance imaging.
In Table 1 the patients with a mismatch between INO and MRI findings (paradox cases) and with congruent INO and MRI findings (non‐paradox cases) were compared. The paradox MS cases had a significantly older age, higher disease duration and EDSS and more frequently a progressive disease course. The post hoc inspection of MRI images of the paradox cases revealed multiple hypotheses on the underlying contributing factors of the clinico‐radiological paradox (Table 3).
TABLE 3.
Consensus revision of individual cases with a clinico‐radiological paradox for the INO‐MLF
| Potential factors | Likelihood for contribution | |
|---|---|---|
| INO−MLF+ | INO+MLF− | |
| Technical and MRI‐related factors | ||
| 1. Lesion below MRI resolution | − | + |
| 2. MRI artefacts | + | + |
| 3. Age of lesion (acute more visible than chronic) | − | + |
| 4. Pure demyelination | − | + |
| 5. Pure axonal degeneration | − | + |
| 6. T1 black hole | − | + |
| 7. Astrogliosis | − | + |
| 8. Pre‐symptomatic lesion | + | − |
| 9. Remyelinated lesion | + | − |
| 10. Suboptimal image quality or image sequences | + | + |
| Anatomical | ||
| 1. Anatomical variation of MLF pathway | + | + |
| 2. Lesion closely adjacent to, but not interfering with, MLF | + | − |
| 3. Remote effect of adjacent lesions (e.g., by oedema, cytotoxic causes, conduction block) | − | + |
| 4. Aberrant blood vessel | − | + |
| 5. Altered conduction due to vestibular input to the MLF a | − | + |
| 6. Diffuse MS pathology | − | + |
| Other pathology | ||
| 1. Specific MLF autoimmunity (e.g., antibodies) | − | + |
| 2. Thin peri‐MLF lesion (analogous to perineuritis) | − | + |
| 3. Peri‐CSF space leukoaraiosis (perfusion disturbances) | − | + |
| 4. Other pathology (e.g., small cavernoma, pulse waves from blood vessels, vascular loops) | − | + |
| 5. Other causes for conduction block in MLF | − | + |
| Other | ||
| 1. Borderline INO cases, physiological variation in movement of adducting to abducting eye | + | − |
The list compiled summarizes factors which are speculated as potentially interesting for investigating one or both sides of the paradox (indicated by a + sign).
Abbreviations: CSF, cerebrospinal fluid; INO, internuclear ophthalmoplegia; INO+MLF−, internuclear ophthalmoplegia detected, no MLF lesion on MRI; INO−MLF+, no internuclear ophthalmoplegia detected, MLF lesion on MRI; MLF, medial longitudinal fasciculus; MRI, magnetic resonance imaging; MS, multiple sclerosis.
Efferent fibres of the vestibular system reach the nuclei of the three cranial nerves involved in eye movements via the MLF to enable visual fixation with auto locomotion. Disrupted vestibular input (e.g., due to a lesion in this pathway) might therefore influence conduction through the MLF.
Versional dysconjugacy index distribution
In Figure 2 the distributions of both the VDI AUC and VDI Pv/Am are shown for the HC group, the MS subgroup without an MLF lesion and the MS subgroup with an MLF lesion. The VDI values were significantly higher in the subgroup with an MLF lesion than in the subgroup without an MLF lesion. The VDI values of both MS subgroups were significantly higher than in the HC group. When investigating the subgroup of MS patients with an INO detected with infrared oculography, the patients with an MLF lesion showed a mean VDI AUC of 1.42 (±0.03) and a mean VDI Pv/Am of 1.80 (±0.09). Both were significantly higher (p < 0.001) than in patients without an MLF lesion; this group showed a mean VDI AUC of 1.22 (±0.03) and a mean VDI Pv/Am of 1.27 (±0.04).
FIGURE 2.
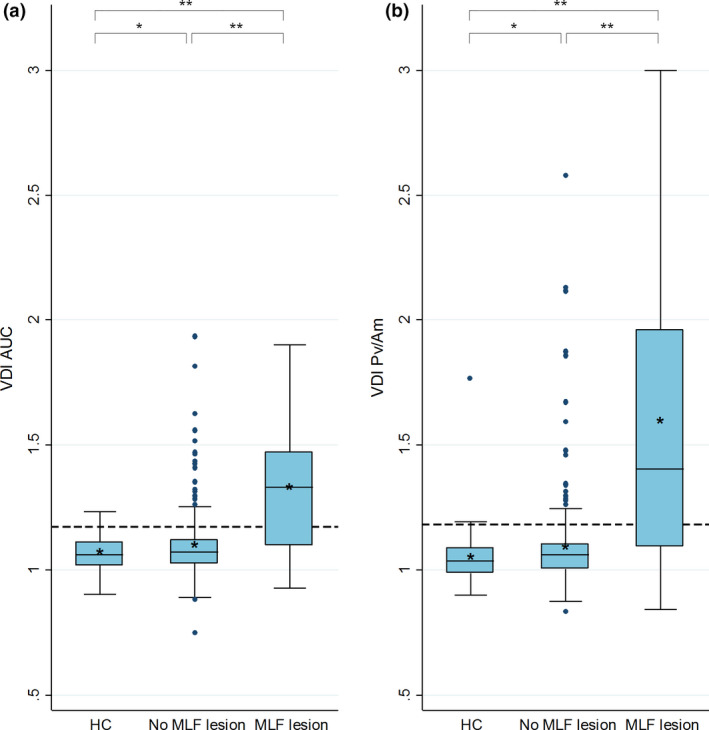
Versional dysconjugacy index (VDI) distribution of the HC group and MS subgroups. Box‐and‐whisker plots of the VDI of the healthy control group, MS patients without a medial longitudinal fasciculus (MLF) lesion and MS patients with an MLF lesion on MRI. Both leftward and rightward VDI of every subject was included. The boxes extend from the 25th to the 75th percentile; the horizontal line in the box is plotted at the median and the asterisk indicates the mean. The dashed line indicates the INO detection threshold of both VDIs. *p < 0.05; **p < 0.001. (a) VDI area under the curve (AUC). VDI detection threshold at 1.174. (b) VDI peak velocity divided by amplitude (Pv/Am). VDI detection threshold at 1.180. [Colour figure can be viewed at wileyonlinelibrary.com]
Comparisons of clinical characteristics
In Table 4, clinical characteristics are compared between (i) the subgroups of MS patients with and without an INO detected with infrared oculography and (ii) the subgroups of MS patients with and without an MLF lesion on MRI. Disease duration was significantly higher in the subgroup with an INO compared to the group without an INO (β = 4.0 years, p = 0.001). EDSS score was higher in both the subgroup with an INO (difference in median 1.0, p = 0.008) and the subgroup with an MLF lesion (difference in median 1.3, p = 0.002). When looking at the overall score of the vision‐related quality of life questionnaire, a significantly lower score was found in the subgroup with an INO compared to the subgroup without an INO (difference in median 1.9, p = 0.016). In addition, complaints of double vision and difficulties with visual focusing were more frequently found in the subgroup of patients with an INO, but not in the subgroup with an MLF lesion (see Table 4).
TABLE 4.
Clinical characteristics of subgroups of MS patients
| INO detected | MLF lesion | |||||
|---|---|---|---|---|---|---|
| Yes | No | p value | Yes | No | p value | |
| N = 66 | N = 136 | N = 26 | N = 176 | |||
| Disease duration (years) | 23.6 (±9.1) | 19.6 (±7.9) | 0.001 | 20.7 (±1.3) | 20.9 (±0.7) | 0.875 |
|
EDSS score (median (IQR)) |
4.0 (3.0) | 3.0 (2.0) | 0.008 | 4.8 (2.0) | 3.5 (2.0) | 0.002 |
| HCVA (best eye) a | 55.2 (±6.8) | 57.0 (±7.1) | 0.219 | 53.5 (±9.4) | 56.8 (±0.5) | 0.037 |
| LCVA (best eye) b | 29.3 (±12.7) | 31.7 (±11.9) | 0.276 | 25.6 (±10.2) | 31.6 (±12.2) | 0.062 |
| Optic neuritis history (N, %) c | 31 (50%) | 60 (48%) | 0.588 | 12 (52%) | 79 (48%) | 0.837 |
| pRNFL thickness (mean ODS, µm) d | 81.5 (±12.9) | 85.5 (±13.1) | 0.111 | 78.5 (±7.7) | 85.0 (±13.5) | 0.082 |
| mGCIPL thickness (mean ODS, µm) d | 74.9 (±15.4) | 78.7 (±12.8) | 0.161 | 73.8 (±12.0) | 77.9 (±14.0) | 0.293 |
| VFQ‐25 overall score (median, IQR) | 89.1 (11.2) | 91.7 (9.3) | 0.016 | 89.4 (16.6) | 91.4 (11.9) | 0.207 |
| Complaints double vision (N, %) | 23 (35%) | 24 (18%) | 0.013 | 5 (19%) | 42 (24%) | 0.616 |
| Complaints focusing SO (N, %) | 33 (50%) | 40 (30%) | 0.005 | 10 (38%) | 63 (36%) | 0.807 |
| Complaints focusing MO (N, %) | 41 (62%) | 40 (30%) | <0.001 | 14 (54%) | 67 (39%) | 0.148 |
p values are the result of linear or logistic regression analyses adjusted for age and sex (except the comparison of disease duration, which was only adjusted for sex). Bold numbers indicate significant differences between the subgroups.
Abbreviations: EDSS, expanded disability status scale; HCVA, high‐contrast visual acuity; INO, internuclear ophthalmoplegia; IQR, interquartile range; LCVA, low‐contrast visual acuity; mGCIPL, macular ganglion cell and inner plexiform layer; MLF, medial longitudinal fasciculus; MO, moving objects; MS, multiple sclerosis; N, number; ODS, right and left eye; pRNFL, peri‐papillary retinal nerve fibre layer; SO, stationary objects; VFQ‐25, Visual Functioning Questionnaire.
HCVA data missing for 15 subjects.
LCVA data missing for 54 subjects.
Conclusive evidence on optic neuritis missing for 16 patients.
Optical coherence tomography (OCT) data missing for 77 subjects.
DISCUSSION
To the best of our knowledge, this is the first study that investigated MLF lesions in an unselected group of MS patients in a blinded manner. The clinico‐radiological paradox was observed in 50 (25%) cases, mainly caused by an absence of MLF lesions in 45 INO MS patients. This might suggest that the pathology of the MLF is more frequently identified by INO detection with infrared oculography than by MLF rating on MRI.
This study provided more insight in the variation in locations of MLF lesions, as shown in Figure 2. The MLF is a tiny structure extending from the lower pons to the midbrain (Figure S1). The most typical location is a clearly visible lesion in the mid dorsal pons crossing the midline which causes a bilateral INO. It is more challenging to deal with unilateral lesions the location of which can be just adjacent to the MLF. Particularly difficult are those lesions which are located more ventral and rostral where the MLF approaches the oculomotor nuclei. Even though this was taken into account in the blinded MRI rating, the post hoc MRI inspection revealed 11 INO cases with an MRI lesion in close proximity to the MLF, which might suggest that within the MLF a miniscule degree of anatomical variation of the ascending fibres from the abducens nucleus and/or a disruption of vestibular input may exist (Table 3).
The finding of more disability and progressive disease type in the patients with a clinico‐radiological paradox suggests that pure axonal degeneration in the MLF could play a role (Table 3). Likewise, pure demyelination, which is difficult to detect with MRI [31] is a plausible alternative. Finally, astrogliosis, a key pathology in the MS brain, remains a distinct possibility [32]. Then cerebrovascular comorbidity in MS needs to be considered as a reason for the clinico‐radiological paradox, which is supported by the higher age of paradox MS cases (Table 1). On MRI inspection, evidence for this was only found in one case.
Medial longitudinal fasciculus lesions were also visible in mild INO cases; however, within the group of INO patients the VDI values were higher in the subgroup with MLF lesions than in the subgroup without MLF lesions, indicating a more severe adduction delay. Furthermore, the majority of MLF lesions were detected in bilateral INOs. This suggests that more evident MLF lesions can be seen in both bilateral and more severe INO cases. Therefore, it is plausible that some of the milder INOs in which no MLF lesion was found do have a very small MLF lesion that is not detectable on MRI. In the study by Frohman et al. [33], all 58 MS patients with INO were found to have evidence of an abnormality in the region of the MLF on proton density weighted images sequences (by non‐blinded rating). This study included a selected group of chronic and clinically evident INO cases, confirmed by infrared video oculography. Therefore, this study supports our finding that MLF lesions can be more easily found in more severe (and therefore probably clinically evident) INO cases. In the study of Frohman et al. no correlation was found between the severity of the INO (as indicated by the VDI peak velocity and peak acceleration) and the presence or absence of an MLF lesion by any imaging technique. However, the above mentioned inclusion criteria could contribute to the absence of such a correlation in this study.
Five cases were identified in whom a lesion was rated as an MLF lesion but without an INO detected with infrared oculography. All of these INOs had VDI values relatively far below the thresholds for detection (VDI AUC below 1.10), which makes it less likely that there was a small, not detected, INO. Possibly anatomical variation of the MLF pathway could exist and perhaps in retrospect the MLF was not involved in some of these cases, as could be the case with the example in Figure 1f. This emphasizes the difficulty of in vivo investigation of the MLF, especially without clinical information. Another possible explanation could be an episode of clinical INO in the past, with complete functional recovery. It is not yet known if such complete recovery (without even a slight adduction delay on infrared oculography measurement) occurs in INO patients.
Magnetic resonance imaging assessment of the MLF is not limited by lesion rating on 3 T images. Other techniques and sequences such as ultra‐high field MRI, diffusion tensor imaging, magnetization‐transfer imaging and T1/T2 ratio are developing and have been the subject of investigation for the past 10 years [34, 35, 36]. Therefore, a limitation of this study is that our results apply only to the paradox that exists between ‘regular’ MRI imaging and clinical findings and cannot be extrapolated to methods that assess microstructural white matter damage in a more sensitive manner. Furthermore, our MLF lesion ratings primarily rely on the review of the MRI scans by one specialist neuroradiologist; only doubtful cases were discussed with a second reviewer. The availability of the MRI scans in a repository will allow inspection of the scans by different readers.
Another limitation of our study is that there is no gold standard for our INO detection. As discussed before, a gold standard is not available as clinical assessment is inaccurate for the diagnosis [13]. Therefore, different parameters and thresholds in differentiating MS patients and HCs for the INO detection with infrared oculography were systematically compared [10]. This detecting method needs validation, which can only be achieved by hypothesis testing (instead of criterion validity, which requires a gold standard), of which the comparison with MLF lesions represents one approach. As expected, an INO was found in almost all MS patients with an MLF lesion. On top of this, INOs detected with infrared oculography showed more visual complaints and a lower visual functioning compared to non‐INOs. This was absent or less evident in the group of patients with MLF lesions compared to patients without MLF lesions. This suggests that the functional definition of an INO, as detected with our standardized infrared oculography method, is clinically relevant. Another part of the validation of our method represents the extent to which the method can detect changes over time. Longitudinal data of our cohort will be available in the next few years. Furthermore, a relevant change in INO can be observed after a single dose of fampridine, as shown in our recent placebo‐controlled trial [37]. This is in line with the acknowledgement in the past few years that accurate measures of the visual system are expected to be able to predict and evaluate therapeutic efficacy more sensitively than MR brain imaging [38, 39]. Finally, infrared oculography is non‐invasive, all our protocols are available open‐source and measurement devices are increasingly commercially available (with declining costs), which makes it suitable for a clinical setting.
Taken together, this study shows that the INO‐MLF model is suitable for a very targeted, and clinically relevant, interrogation of the clinico‐radiological paradox. The unique structure–function relation of these short white matter tracts and the high‐precision measurement by infrared oculography can help an understanding of injury and recovery of white matter pathways. This makes it an ideal candidate target for testing new neuroprotective and remyelination treatment strategies. For further insight, discussion on this topic and multi‐rater assessment, the MRI images of the paradox cases are available in a data repository. Finally, the structural MRI data in this study support the utility of the standardized method of INO detection with infrared oculography as a sensitive functional outcome measure that is relevant for a patient and usable for (selection of patients in) clinical trials.
CONFLICT OF INTERESTS
J.A. Nij Bijvank: receives research support from the Dutch MS Research Foundation, grant number 18‐1006. E. Sanchez, L.J. Balk, D. Coric, I. Davagnanam, H.S. Tan and L.J. van Rijn: report no conflicts of interest. B.M.J Uitdehaag: has received consultancy fees from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva. A. Petzold: reports personal fees from Novartis, Heidelberg Engineering, Zeiss, grants from Novartis, outside the submitted work; and is part of the steering committee of the OCTiMS study which is sponsored by Novartis and the Angio‐OCT steering committee which is sponsored by Zeiss. He does not receive compensation for these activities.
AUTHOR CONTRIBUTIONS
Jenny Annemieke Nij Bijvank: conceptualization (equal); data curation (lead); formal analysis (lead); investigation (lead); methodology (equal); project administration (equal); software (lead); validation (lead); visualization (lead); writing original draft (lead). E. Sánchez Aliaga: data curation (equal); formal analysis (equal); investigation (equal); writing review and editing (equal). Lisanne Johanna Balk: conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing review and editing (equal). Danko Coric: data curation (equal); investigation (equal); methodology (equal); writing review and editing (equal). Indran Davagnanam: conceptualization (equal); validation (equal); writing review and editing (equal). H. S. Tan: conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal); writing review and editing (equal). Bernard M. J. Uitdehaag: conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal); writing review and editing (equal). L. J. van Rijn: conceptualization (equal); investigation (equal); methodology (equal); resources (equal); software (equal); supervision (equal); writing review and editing (equal). Axel Petzold: conceptualization (equal); investigation (equal); methodology (equal); software (equal); supervision (equal); writing review and editing (equal).
Supporting information
Fig S1
Supplementary Material
ACKNOWLEDGEMENT
This research was supported by Stichting MS Research.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. Data include deidentified participant data, statistical files and codes. Written proposals will be assessed by a representative of each cohort study and a decision made about the appropriateness of the use of data. A data sharing agreement will be put in place before any data will be shared. Contact the corresponding author for information. The measurement and analysis protocol is available on https://www.protocols.io/view/demons‐protocol‐for‐measurement‐and‐analysis‐of‐ey‐ruad6se. The MRI images of the cases with a clinico‐radiological paradox are available upon request for inspection via a data repository (https://doi.org/10.17026/dans‐zc3‐ttru).
REFERENCES
- 1. Truyen L, van Waesberghe JH, van Walderveen MA, et al. Accumulation of hypointense lesions (‘black holes’) on T1 spin‐echo MRI correlates with disease progression in multiple sclerosis. Neurology. 1996;47(6):1469‐1476. [DOI] [PubMed] [Google Scholar]
- 2. Fouad K, Popovich PG, Kopp MA, Schwab JM. The neuroanatomical‐functional paradox in spinal cord injury. Nat Rev Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stampanoni Bassi M, Gilio L, Buttari F, et al. Remodeling functional connectivity in multiple sclerosis: a challenging therapeutic approach. Front Neurosci. 2017;11:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barkhof F. The clinico‐radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002;15(3):239‐245. [DOI] [PubMed] [Google Scholar]
- 5. Mollison D, Sellar R, Bastin M, et al. The clinico‐radiological paradox of cognitive function and MRI burden of white matter lesions in people with multiple sclerosis: a systematic review and meta‐analysis. PLoS One. 2017;12(5):e0177727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barkhof F, van Walderveen M. Characterization of tissue damage in multiple sclerosis by nuclear magnetic resonance. Philos Trans R Soc Lond B Biol Sci. 1999;354(1390):1675‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Müri RM, Meienberg O. The clinical spectrum of internuclear ophthalmoplegia in multiple sclerosis. Arch Neurol. 1985;42:851‐855. [DOI] [PubMed] [Google Scholar]
- 8. Meienberg O, Müri R, Rabineau PA. Clinical and oculographic examinations of saccadic eye movements in the diagnosis of multiple sclerosis. Arch Neurol. 1986;43:438‐443. [DOI] [PubMed] [Google Scholar]
- 9. Downey DL, Stahl JS, Asiri RB, et al. Saccadic and vestibular abnormalities in multiple sclerosis. Ann N Y Acad Sci. 2002;956:438‐440. [DOI] [PubMed] [Google Scholar]
- 10. Nij Bijvank JA, Rijn LJ, Balk LJ, Tan HS, Uitdehaag BMJ, Petzold A. Diagnosing and quantifying a common deficit in multiple sclerosis: internuclear ophthalmoplegia. Neurology. 2019;92(20):e2299‐e2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nij Bijvank JA, Petzold A, Balk LJ, et al. A standardized protocol for quantification of saccadic eye movements: DEMoNS. PLoS One. 2018;13(7):e0200695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ventre J, Vighetto A, Bailly G, Prablanc C. Saccade metrics in multiple sclerosis: versional velocity disconjugacy as the best clue? J Neurol Sci. 1991;102:144‐149. [DOI] [PubMed] [Google Scholar]
- 13. Frohman TC, Frohman EM, O'Suilleabhain P, et al. Accuracy of clinical detection of INO in MS: corroboration with quantitative infrared oculography. Neurology. 2003;61(6):848‐850. [DOI] [PubMed] [Google Scholar]
- 14. Ross AT, DeMyer WE. Isolated syndrome of the medial longitudinal fasciculus in man. Anatomical confirmation. Arch Neurol. 1966;15(2):203‐205. [DOI] [PubMed] [Google Scholar]
- 15. Cogan DG, Kubik CS, Smith WL. Unilateral internuclear ophthalmoplegia; report of 8 clinical cases with one postmortem study. AMA Arch Ophthalmol. 1950;44(6):783‐796. [PubMed] [Google Scholar]
- 16. Harrington RB, Hollenhorst RW, Sayre GP. Unilateral internuclear ophthalmoplegia. Report of a case including pathology. Arch Neurol. 1966;15(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 17. Kim JS. Internuclear ophthalmoplegia as an isolated or predominant symptom of brainstem infarction. Neurology. 2004;62(9):1491‐1496. [DOI] [PubMed] [Google Scholar]
- 18. Eijlers AJ, Meijer KA, Wassenaar TM, et al. Increased default‐mode network centrality in cognitively impaired multiple sclerosis patients. Neurology. 2017;88(10):952‐960. [DOI] [PubMed] [Google Scholar]
- 19. Schoonheim MM, Hulst HE, Brandt RB, et al. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology. 2015;84(8):776‐783. [DOI] [PubMed] [Google Scholar]
- 20. Steenwijk MD, Geurts JJ, Daams M, et al. Cortical atrophy patterns in multiple sclerosis are non‐random and clinically relevant. Brain. 2016;139(Pt 1):115‐126. [DOI] [PubMed] [Google Scholar]
- 21. Nij Bijvank JA, Petzold A, Coric D, et al. Quantification of visual fixation in multiple sclerosis. Invest Ophthalmol Vis Sci. 2019;60(5):1372‐1383. [DOI] [PubMed] [Google Scholar]
- 22. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):293‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907‐911. [DOI] [PubMed] [Google Scholar]
- 24. Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444‐1452. [DOI] [PubMed] [Google Scholar]
- 25. Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. 2014;10(8):447‐458. [DOI] [PubMed] [Google Scholar]
- 26. Balcer LJ, Baier ML, Cohen JA, et al. Contrast letter acuity as a visual component for the multiple sclerosis functional composite. Neurology. 2003;61:1367‐1373. [DOI] [PubMed] [Google Scholar]
- 27. Balk LJ, Coric D, Nij Bijvank JA, Killestein J, Uitdehaag BM, Petzold A. Retinal atrophy in relation to visual functioning and vision‐related quality of life in patients with multiple sclerosis. Mult Scler. 2018;24(6):767‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tewarie P, Balk L, Costello F, et al. The OSCAR‐IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25‐item National Eye Institute visual function questionnaire. Arch Ophthalmol. 2001;119:1050‐1058. [DOI] [PubMed] [Google Scholar]
- 30. Noble J, Forooghian F, Sproule M, Westall C, O'Connor P. Utility of the National Eye Institute VFQ‐25 questionnaire in a heterogeneous group of multiple sclerosis patients. Am J Ophthalmol. 2006;142(3):464‐468. [DOI] [PubMed] [Google Scholar]
- 31. Schmierer K, Scaravilli F, Barker GJ, Gordon R, MacManus DG, Miller DH. Stereotactic co‐registration of magnetic resonance imaging and histopathology in post‐mortem multiple sclerosis brain. Neuropathol Appl Neurobiol. 2003;29(6):596‐601. [DOI] [PubMed] [Google Scholar]
- 32. Petzold A, Eikelenboom MJ, Gveric D, et al. Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain. 2002;125(Pt 7):1462‐1473. [DOI] [PubMed] [Google Scholar]
- 33. Frohman EM, Zhang H, Kramer PD, et al. MRI characteristics of the MLF in MS patients with chronic internuclear ophthalmoparesis. Neurology. 2001;57(5):762‐768. [DOI] [PubMed] [Google Scholar]
- 34. Sakaie K, Takahashi M, Dimitrov I, et al. Diffusion tensor imaging the medial longitudinal fasciculus in INO: opportunities and challenges. Ann N Y Acad Sci. 2011;1233:307‐312. [DOI] [PubMed] [Google Scholar]
- 35. Sakaie K, Takahashi M, Sagiyama K, et al. Injury to a specific neural pathway detected by ultra‐high‐field MRI. Neurology. 2014;82(2):182‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakaie K, Takahashi M, Remington G, et al. Correlating function and imaging measures of the medial longitudinal fasciculus. PLoS One. 2016;11(1):e0147863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanhai KMS, Nij Bijvank JA, Wagenaar YL, et al. Treatment of internuclear ophthalmoparesis in multiple sclerosis with fampridine: a randomized double‐blind, placebo‐controlled cross‐over trial. CNS Neurosci Ther. 2019.25(6):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andorra M, Nakamura K, Lampert EJ, et al. Assessing biological and methodological aspects of brain volume loss in multiple sclerosis. JAMA Neurol. 2018;75(10):1246‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andorra M, Alba‐Arbalat S, Camos‐Carreras A, et al. Using acute optic neuritis trials to assess neuroprotective and remyelinating therapies in multiple sclerosis. JAMA Neurol. 2019.77(2):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material
Data Availability Statement
Data are available upon reasonable request. Data include deidentified participant data, statistical files and codes. Written proposals will be assessed by a representative of each cohort study and a decision made about the appropriateness of the use of data. A data sharing agreement will be put in place before any data will be shared. Contact the corresponding author for information. The measurement and analysis protocol is available on https://www.protocols.io/view/demons‐protocol‐for‐measurement‐and‐analysis‐of‐ey‐ruad6se. The MRI images of the cases with a clinico‐radiological paradox are available upon request for inspection via a data repository (https://doi.org/10.17026/dans‐zc3‐ttru).


