Abstract
BACKGROUND
Maize varieties that are rich in carotenoids have been developed to combat vitamin A deficiency in Sub‐Saharan Africa. Unfortunately, after harvest, carotenoids degrade and off‐flavor volatiles develop, which affect nutrient intake and consumer acceptance. This study evaluated carotenoid retention and aroma compound stability in provitamin A biofortified maize, variety Pool 8A, as influenced by dry milling and storage in different packaging and temperature conditions.
RESULTS
The lowest amount of total carotenoids was found in flour stored in laminated paper bags at 37 °C (only 16% retention after 180 days), attributable to the high storage temperature and oxygen permeability of the packaging material. No significant effect on carotenoid degradation was found for dry milling, either by rotor mill or freezer mill, but the formation of volatile compounds was significantly (P < 0.05) affected. Volatile compounds such as hexanal, 2‐pentylfuran, 1‐propanol, 2‐heptanone, butyrolactone, limonene, and hexanoic acid were found in different proportions after milling. The highest concentration of hexanal was in flour milled by rotor mill or freezer mill, and stored in laminated paper bags at 37 °C after 180 days, and the lowest concentrations were for flour in aluminium bags and double‐layered polyethylene bags stored at 4 °C.
CONCLUSION
Maize flour stored in double‐layered polyethylene bags had the highest carotenoid retention and aroma stability. Importantly, the use of these bags is economically feasible in low‐income countries. Overall, our results show that effective control of storage conditions is crucial to prevent carotenoid loss and decrease off‐odor formation. © 2020 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: carotenoids; biofortified maize; provitamin a, volatile aroma compounds; Africa
INTRODUCTION
Vitamin A deficiency (VAD) is a nutritional disorder caused by lack of vitamin A intake. Globally, one‐third of children under 5 years are affected by VAD with 48% living in Sub‐Saharan Africa (SSA). 1 Vitamin A deficiency results in preventable vision loss, poor growth, and a vulnerable immune system, potentially leading to an increased risk of infections and premature death. Dependence on starch‐dense crops such as white maize, which contain no retinol activity, as a major food source is the major cause of VAD in SSA. Due to the severity of VAD, biofortification of key staple crops such as maize, cassava, and sweet potatoes is applied to alleviate the problem. 2 More than 30 biofortified maize varieties have been developed through conventional breeding and released in SSA. 3 Biofortified maize is rich in provitamin A carotenoids such as α‐carotene, β‐carotene and β‐cryptoxanthin in addition to elevated contents of the non‐provitamin A carotenoids such as zeaxanthin and lutein. 4
The consumption of biofortified maize enhances the vitamin A status of children in SSA. However, carotenoids are partially degraded during post‐harvest handling, i.e. storage, processing, and cooking. 5 The rate of carotenoid reduction has been associated with the presence of oxygen, heat, light, enzymatic and non‐enzymatic factors, which can be controlled through the improvement of post‐harvest conditions. 4 The degradation rate of carotenoids is influenced by the packaging material, the storage conditions, and processing methods. For instance, Burt et al., 6 Sowa et al., 7 and Simpungwe et al. 8 found 50 to 65% carotenoid loss after 4 to 6 months under traditional storage conditions, showing a significant reduction in the nutritional quality of biofortified maize.
Besides the nutritional concern related to carotenoid loss, the sensory quality of biofortified maize can be affected during post‐harvest storage by changes in the profile of the volatile organic compounds (VOCs). Volatile organic compounds play an important role in the sensory perception of food, having a major impact on food appreciation and acceptance by consumers. The germ of maize is rich in unsaturated fats, which is predisposed to rancidity under storage and processing conditions, resulting in an unpleasant taste and aroma. 9 The oxidation products are mostly VOCs, which are associated with off odors. 9 Changes in the VOCs in maize flour during milling, storage, and processing could contribute to the poor consumer acceptance of biofortified maize, as some consumers perceived the smell to be disagreeable. 10 To date, no information on VOC stability or changes during storage and processing of provitamin A biofortified maize is available. Post‐harvest handling must be optimized to increase acceptance, thus improving the impact on the target population.
This study assessed carotenoid retention and changes in VOCs during 6 months’ storage under different conditions using different milling methods, packaging materials, and temperature conditions.
MATERIALS AND METHODS
Chemicals and standards
Extraction and high‐performance liquid chromatography (HPLC) solvents, i.e. absolute ethanol, butylhydroxytoluene, potassium hydroxide, hexane, ammonium acetate, methanol, 2‐dichloroethane and methyl‐t‐butyl‐ether, were obtained from Sigma‐Aldrich Chemie BV, Zwijndrecht, The Netherlands. The β‐carotene, β‐cryptoxanthin, lutein, zeaxanthin and β‐apo‐8'‐carotenal standards were obtained from Sigma‐Aldrich Chemie BV, Zwijndrecht, The Netherlands and CaroteNature, Münsingen, Switzerland and used for carotenoid identification, calibration curves and determination of extraction efficiency. For VOC identification, C7‐C40 saturated alkanes and hexanal were obtained from Merck KGaA, Darmstadt, Germany.
Biofortified provitamin A maize, milling, and storage
Orange maize variety Pool 8A (at <11% grain moisture) was obtained from the Rwanda Agriculture Board. Maize kernels were milled using two different machines, namely a freezer mill (FM) at low heat (−196 °C) (SPEX samplePrep 6875 freezer/Mill, Metuchen, USA), and a rotor mill (RM) with frictional heat (>50 °C) (Pulverisette 14, Fritsch International; Idar‐Obenstein, Germany). Whole maize flour particle size was made homogenous using a mesh of 0.5 mm. Three different packaging materials (i.e. aluminium pouches, laminated paper bags, and double‐layered polyethylene bags), commonly used to store food products, were tested. The double‐layered polyethylene bag contained two liners folded and sealed separately. Equal samples (10 g) of maize flour were packed in the three types of bags, sealed airtight, and stored at 4 °C in a laboratory refrigerator and at 37 °C in laboratory incubators, Fig. 1. Samples were analyzed for carotenoid content at intervals of 0, 10, 20, 30, 60, 90, 120, 150, and 180 days to cover a 6 month storage period. Moisture content was determined by the American Association of Cereal Chemistry (AACC) method 44‐15A. Samples were stored at −20 °C until analysis.
Figure 1.
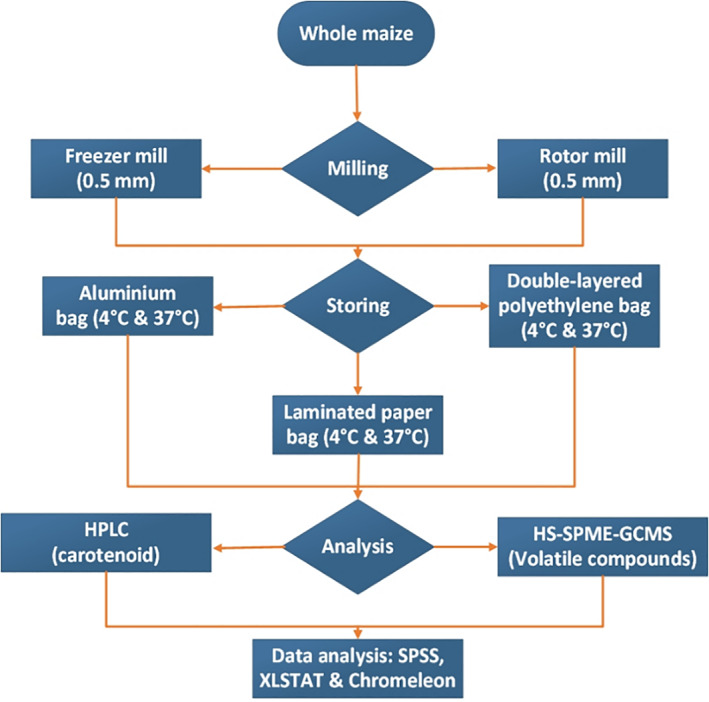
Sampling and experimental scheme of the maize flour storage study. Samples were taken for analysis at intervals of 0, 10, 20, 30, 60, 90, 120, 150, and 180 days.
Carotenoid extraction and quantification by HPLC
Carotenoid extraction was as described by Rosales et al. 11 Briefly, a 600 mg sample was precipitated for 5 min using 6 mL ethanol (containing 0.1% butylated hydroxytoluene) at 85 °C in a water bath before saponification with 500 μL 80% (w/v) KOH solution for 10 min. After saponification, samples were immediately placed in ice followed by the addition of 3 mL of cold deionized water. Carotenoids were extracted 3 times with 3 mL of hexane by centrifugation at 4200 x g for approximately 10 min. The combined hexane layers were dried by vacuum evaporator at 60 °C and 335 mmHg. The extract was resuspended in 2 mL 50:50 methanol:dichloroethane (v/v). Carotenoid extractions and analyses were all performed under red light to prevent degradation. The resuspension was filtered using 0.25 μm. Next, 20 μL of the sample was injected into the Thermo Scientific Dionex UltiMate 3000 HPLC system equipped with a photodiode array detector (Thermo Fisher Scientific, Amsterdam, The Netherlands). The separation was performed using a YMC30 4.6 mm × 250 mm with 5 μm particle size (YMC Europe GmbH, Dinslaken, Germany).
Aroma compounds analysis by GC–MS
Maize flour was analyzed using a Stabilwax DA capillary column (30 m × 0.25 mm ID × 0.25 μm) and Solid Phase Micro‐Extraction (SPME) fiber assembly DVB/CAR/PDMS (Supelco, Bellefonte, PA, USA). An internal standard, viz. perfluorotributylamine (PFTBA, FC43; Thermo Fisher Scientific Inc., Waltham, MA, USA), was used to calibrate the Headspace Solid‐phase Microextraction (HS‐SPME) ‐ Gas Chromatography–Mass Spectrometry (GC‐MS) every 2 weeks. Maize flour (1 g) was placed in a 10 mL glass bottle with crimp caps and incubated for 10 min at 40 °C. The fiber was automatically injected into the injector port of the gas chromatography–mass spectrometry (GC–MS) and desorbed for 10 min. The oven temperature was set at 40 °C for 2 min, increased at 10 °C min−1 to 200 °C and then fixed at 200 °C for 5 min. Split injection was used as the injection method at 225 °C, split ratio 19:1. Helium carrier gas with a flow rate of 1 mL min−1 was used.
Data analysis
The raw data from the HPLC and GC–MS were reprocessed by Thermo Scientific Dionex Chromeleon® 7.2 chromatography data system (CDS) software before further analysis in XLSTAT version 2020.1.1.54525 and IBM SPSS® software version 23, using the Tukey test for pairwise comparison. The aroma compounds were identified by comparing their mass spectra with the National Institute of Standards and Technology (NIST) database, retention indexes from literature as well as the retention time of the standards. For volatile compound quantification, maize flour spiked with hexanal stock solution was used to obtain a calibration curve. Carotenoid peaks were identified by comparing the UV spectra to literature and the retention times of pure carotenoid standards. 12 Provitamin A was calculated as all‐trans βC (β‐carotene) + (1/2) (β‐cryptoxanthin). The total carotenoid content was calculated as the sum of all carotenoids (zeaxanthin + lutein + β‐cryptoxanthin + β‐carotene). The extraction efficiency was calculated based on β‐apo‐8'‐carotenal and moisture content to express concentration on a weight basis.
RESULTS AND DISCUSSION
Carotenoid content of biofortified maize
The carotenoid content of biofortified maize flour processed using two different milling methods is reported in Table 1. The main carotenoids were lutein, zeaxanthin, β‐cryptoxanthin, and β‐carotene. The total carotenoid content of biofortified maize was 23.8 ± 3.7 μg g−1 with β‐cryptoxanthin and zeaxanthin as the major carotenoids, i.e. 8.2 and 9.2 μg g−1, respectively. Carotenoid loss was between 11 to 12% for milling in the rotor mill.
Table 1.
Carotenoid content (μg g−1 dry weight)* of freezer‐milled and rotor‐milled maize flour of biofortified variety Pool 8A grown in Rwanda
| βC | βCX | ZEA | LUT | PVAC | TCC | |
|---|---|---|---|---|---|---|
| Freezer mill | 1.09 ± 0.28a | 8.27 ± 0.11a | 9.21 ± 2.17a | 5.19 ± 1.3a | 5.23 ± 0.25a | 23.76 ± 3.65a |
| Rotor mill | 0.96 ± 0.22a | 7.27 ± 0.52a | 8.20 ± 0.78a | 4.55 ± 0.05a | 4.60 ± 0.04b | 20.99 ± 0.93a |
| % loss** | 11.93 | 12.09 | 10.97 | 12.33 | 12.05 | 11.66 |
Different superscript letters indicate significant differences within columns (Tukey; P < 0.05). Values are mean ± standard deviation of triplicates. βC, β‐carotene; βCX, β‐cryptoxanthin; ZEA, zeaxanthin; LUT, lutein; PVAC, provitamin A carotenoids; TCC, Total carotenoid content.
The percentage of carotenoid loss due to frictional heat in the rotor mill.
Zeaxanthin and β‐cryptoxanthin accounted for 73.6% of the total carotenoids. Total carotenoid content in this study are comparable to the results of Mugode et al. 13 and Taleon et al., 4 who reported a range of 12.8 to 30.8 μg g−1 dry weight. Similarly, Ortiz et al. 14 reported zeaxanthin as the predominant carotenoid (37.9 to 61.3%) in nine maize genotypes. Provitamin A carotenoids accounted for 22.0% of the carotenoids present in biofortified maize. The amount of provitamin A carotenoid is within the range of 0.6 to 12.7 μg g−1 (17.4% to 26.9%) reported by Rosales et al.. 11 Note that the values of the β‐carotene isomers ‐ 15‐cis‐, 13‐cis‐ and 9‐cis ‐ were not included in the provitamin A carotenoid data presented. The isomers ‐ 15‐cis‐, 13‐cis‐ and 9‐cis‐ β‐carotene in biofortified maize were found in the range 0.4 to 1.4 μg g−1, 0.1 to 1.2 μg g−1 and 0.3 to 2.2 μg g−1, respectively, which corroborate the low values of provitamin A carotenoids obtained in the current study. 4 , 14 Mugode et al. 13 and Pixley et al. 3 reported β‐cryptoxanthin to be twofold higher than β‐carotene, but a much higher β‐cryptoxanthin amount of about sevenfold was found in the present study. Varietal differences in the carotenoid content of biofortified maize have been reported widely in the literature. 3 , 4 , 11 , 12 , 13 , 15 Differences in the carotenoid content due to postharvest handling, milling method, extraction and chromatographic separation techniques could also have contributed to the differences in data. Regarding the current research, sun‐drying was applied post‐harvest and the whole kernel was stored at ambient conditions for 3 months before the experiment. This could have reduced the carotenoid content before our experimental treatments. For instance, sundried carotenoid‐rich sweet potato was reported to have a carotenoid retention of 66 to 67%, 16 and an even more severe loss occurred at lower relative humidity. 14 Furthermore, other maize genotypes have been used in previous studies, which might also explain the differences in carotenoid profiles. Varieties with high β‐carotene and β‐cryptoxanthin are desirable for VAD‐affected regions due to the retinol and antioxidant activity. Although β‐cryptoxanthin is usually present in higher amounts in biofortified maize, it has a 50% lower retinol activity equivalent (RAE) than β‐carotene, i.e. the Institute of Medicine (IOM) 17 set a default value of 12 μg g−1 β‐carotene and 24 μg g−1 β‐cryptoxanthin for 1 RAE. Better bioequivalence factors have been found for biofortified maize by Li et al. 18 and Muzhingi et al. 19 In general, the provitamin A carotenoid content of the tested variety was low compared to the global biofortified breeding target of 15 μg g−1, which was established to provide 50% of the estimated average requirement (EAR) of vitamin A, particularly for vulnerable groups such as preschool children. 20
No significant difference (P < 0.05) for the two milling methods was found in lutein, zeaxanthin, β‐cryptoxanthin, β‐carotene and the total carotenoid content, even though they differed in heat production (absence of heat for the freezer mill and presence of heat for the rotor mill). This agrees with the report of Mugode et al., 13 which indicated that β‐cryptoxanthin is stable during milling. However, the provitamin A carotenoid content showed a significant difference (P < 0.05), signifying that the milling method affected provitamin A retention in maize. Milling has been reported to have different effects on the carotenoid profile. Mugode et al. 13 found 60% retention for β‐cryptoxanthin, while Pillay et al. 21 reported a retention of 118.9 to 137.2% of β‐cryptoxanthin in milled maize. Previous workers have attributed carotenoid loss during milling to the effect of frictional heat, light, and oxygen exposure. However, as no significant difference (P < 0.05) was found for the milling method, other factors during postharvest handling, especially the storage conditions, appear to be more important as reported in the next paragraph.
Carotenoid retention during storage
The total carotenoid content and provitamin A carotenoid content are presented in Fig. 2, and Tables S1 and S2. Carotenoid retention after 180 days of storage in aluminium, laminated paper, and double‐layered polyethylene bags at 4 °C and 37 °C was determined to assess the stability. The total carotenoid content of FM maize flour stored at 4 °C was 13.5 to 17.1 μg g−1 dry weight (DW) after 180 days, which is equivalent to a retention index of 53 to 73%. At 37 °C a range of 4.1 to 10.8 μg g−1 DW, which is equal to a retention index of 16 to 42%, was found. The lowest total carotenoid content was in laminated paper bags at 37 °C (16% retention). The laminated paper bags had the highest oxygen permeability of the three tested materials, thus autoxidation coupled with temperature triggered a significant loss in total carotenoid content over time. Considerable degradation of carotenoids in maize during grain and flour storage has been reported. 4 , 13 Bechoff et al. 16 suggested that oxygen is the main cause of β‐carotene degradation in the high carotenoid crop. Ortiz et al. 14 reported 55 to 76% retention of total carotenoid content in three maize varieties after 90 days of storage, suggesting the likelihood of even more degradation after 180 days and proving that varietal differences affect the stability of carotenoids. Other authors reported similar trends. 4 , 13 Generally, the double‐layered polyethylene bags showed better retention, followed by the aluminium bags. Taleon et al. 4 observed that aluminium pouches with oxygen absorber had better retention than double‐layered polyethylene bags during the storage of biofortified maize grain. However, in the present study, no oxygen scavenger or vacuum condition was used. The high retention of double‐layered polyethylene bags was due to better barrier properties resulting from the double liners, which decreased oxygen and light penetration through the package. Although aluminium and double‐layered polyethylene bags preserved the carotenoids better, the air trapped in the bags during sealing might have contributed to the reported degradation. However, vacuum packaging is not a guarantee for preserving carotenoids: a study found that provitamin A rich cassava flour stored under vacuum had a higher loss of carotenoids than without vacuum. 22 Besides, packaging materials that can hold a vacuum are very expensive for the targeted smallholder farmers and resource‐poor households. Double‐layered polyethylene bags have been proposed as a cheaper alternative to reduce maize grain loss during storage in Africa. 23
Figure 2.
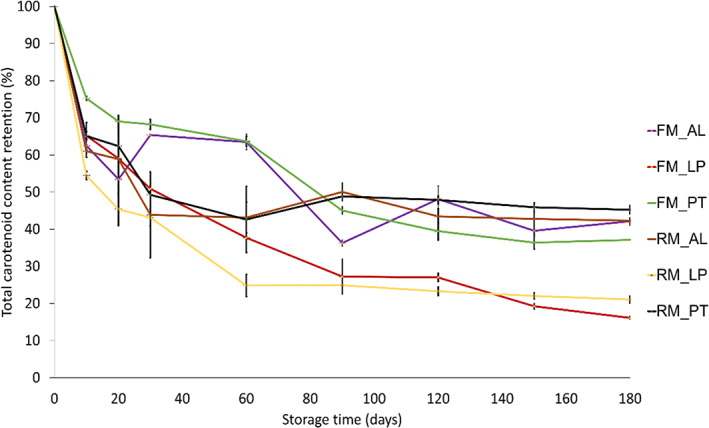
Effect of storage method on total carotenoid content (TCC) retention in maize milled with a FM and a RM using different packaging materials for 180 days at 37 °C. AL – aluminium, LP – laminated pap, PT – double‐layered polyethylene.
For both FM and RM flour, samples stored in aluminium, laminated paper and double‐layered polyethylene bags differed significantly (<0.01) in total carotenoid content between 4 °C and 37 °C. However, no significant differences were found within either storage temperature, except for flour in the laminated paper bag at 37 °C that showed a higher loss in carotenoids. Regarding storage temperature, our results show that storage at 4 °C improves provitamin A and total carotenoid retention. The same was observed for biofortified maize samples stored at 4 °C and 55 °C in the study of Ortiz et al. 14 Similarly, Sowa et al. 7 showed that storage at −22 °C stopped degradation almost completely. Temperature is known to speed up deterioration reactions such as oxidation, which is why it was anticipated that a lower storage temperature would retain carotenoids better.
Freezer mill (FM) flour (from a cryogenic mill) was expected to be more stable during storage because of a lower exposure to heat during milling but this effect was not found. A possible explanation is that the endogenous enzymes in the kernel were not inactivated during freezer milling, resulting in enzymatically induced oxidation of the carotenoids during storage.
Tables S1 and S2 show the retention of β‐carotene and β‐cryptoxanthin during storage for 180 days at different temperatures in different materials. Significant differences in degradation during storage were observed across packaging materials, temperature, and flour type, especially in samples stored at 37 °C. High β‐carotene variability was detected in all packaging materials at 37 °C, indicating poor stability during storage, while at 4 °C better stability was observed.
For FM flour, the lowest retention of β‐carotene was 13% in laminated paper bags at 37 °C (Table S2) and the highest retention was 79% in double‐layered polyethylene bags at 4 °C, Table S1. A similar trend was observed in RM flour, i.e. higher carotenoid retention at 4 °C compared to 37 °C. Sowa et al. 7 reported a high loss of β‐carotene (up to 95%) in maize flour stored at 37 °C for 12 months. The extent of the loss was reported to depend on genotype. Therefore, aside from improving biofortified maize storage and processing conditions, breeding for maize genotypes with better carotenoid stability could enhance retention. As tropical temperatures are detrimental to carotenoid retention, prolonged storage should be avoided as much as possible.
Tables S1 and S2 show the retention of non‐provitamin A carotenoids such as lutein and zeaxanthin during storage for 180 days at different temperatures in different materials. Generally, high retention of both carotenoids was found at 4 °C, but at 37 °C, all of the samples showed a significant decrease in retention. Laminated paper bags retained only 21% of zeaxanthin and 17% of lutein. Sowa et al. 7 reported a similar trend with about 10% retention after 12 months of storage. Overall, these results confirm that the storage of maize flour could have both linear and quadratic degradation kinetics depending on the storage conditions and duration. Other workers have established that carotenoids are better preserved when maize is stored as kernels than when it is stored as flour. 4 , 14 Consequently, milling just before consumption may result in higher carotenoid content.
VOLATILES AND OFF‐ODOR PRODUCTION
Table 2 shows the concentrations of VOCs found in the maize flour after milling with the FM and the RM. Of the 26 volatiles detected, there were six aldehydes (hexanal, heptanal, octanal, nonanal, 2‐octenal, and 2‐heptenal), two aromatics (benzaldehyde and 2‐pentylfuran), five alcohols (ethanol, 1‐propanol, 1‐pentanol, 1‐hexanol and 1‐methoxy‐2‐propanol), five ketones (2‐heptanone, 2‐octanone, 6‐methyl‐5‐heptene‐2‐one, butyrolactone, 3‐octen‐2‐one), one monoterpene (limonene) and three acids (acetic acid, pentanoic acid, and hexanoic acid). Similar lists of compounds have been found for sweet maize, millet, rice, and barley. 24 , 25 , 26 Compounds in the alcohol group had the highest total concentration (1491 to 2157 μg kg−1). The aldehydes ranged from 81 to 94 μg kg−1. Annan et al. 27 reported higher concentrations of aldehydes in raw maize, i.e. 400 μg kg−1 and 7400 μg kg−1 of 2‐nonenal and heptanal, respectively. The authors found four times lower concentrations of alcohols such as 1‐pentanol and 1‐hexanol compared to the current study. A range of 10 to 90 μg kg−1 of benzaldehyde was found in maize extruded at different temperatures and with different levels of moisture content. 28 Generally, studies on maize VOCs are scanty, and the ones that are available 29 , 30 , 31 used peak area for quantification, which makes those results incomparable with our current findings.
Table 2.
Concentrations of volatiles in maize milled with a freezer mill and a rotor mill (μg kg−1)
| Volatile organic compounds | Freezer milled flour | Rotor milled flour | Odor description a |
|---|---|---|---|
| (μg kg−1, mean ± SD) | |||
| Octane | 92.13 ± 1.97 | 81.62 ± 0.49 | alkane, fat, oil, sweet |
| Decane | 340.22 ± 10.32 | 104.04 ± 3.48 | fusel‐like, fruit, sweet |
| Tridecane | 194.11 ± 20.81 | 86.19 ± 1.73 | Alkane |
| Tetrahydrofuran | 347.51 ± 16.27 | 414.28 ± 14.70 | floral, fruit |
| Hexanal | 84.74 ± 0.28 | 92.1 ± 1.80 | grass, fat, herbal |
| Heptanal | 93.29 ± 1.08 | 81.19 ± 0.14 | citrus, dry fish, fat, green |
| Octanal | 83.56 ± 0.23 | 81.47 ± 0.37 | fat, soap, lemon, green |
| Nonanal | 89.5 ± 3.07 | 83.48 ± 0.52 | fat, citrus, green, wax, |
| 2‐Octenal | 81.14 ± 0.13 | 80.96 ± 0.02 | green, nut, fat |
| 2‐Heptenal | 81.82 ± 0.16 | 80.94 ± 0.01 | fat, citrus, rancid |
| Benzaldehyde | 87.7 ± 2.88 | 82.14 ± 0.35 | almond, burnt sugar |
| 2‐pentyl‐Furan | 86.28 ± 0.59 | 82.22 ± 0.61 | green bean, butter |
| Ethanol | 770.08 ± 10.25 | 1149.1 ± 88.57 | alcohol, floral, apple, sweet |
| 1‐Propanol | 81.11 ± 0.08 | 85.23 ± 0.25 | candy, must, pungent, fruit |
| 1‐Pentanol | 87.05 ± 2.32 | 88.01 ± 0.45 | balsamic, fruit, green, yeast |
| 1‐Hexanol | 115.71 ± 2.47 | 109.27 ± 3.96 | resin, flower, green |
| 1‐Methoxy‐2‐propanol | 437.93 ± 3.02 | 726.38 ± 85.38 | Fruit |
| 2‐Heptanone | 150.44 ± 5.83 | 89.92 ± 3.13 | blue cheese, cinnamon, nut |
| Limonene | 84.35 ± 0.88 | 85.78 ± 0.81 | balsamic, citrus, fruit, herb |
| 2‐Octanone | 89.74 ± 0.42 | 82.73 ± 0.48 | herb, butter, resin |
| 6‐Methyl‐5‐heptene‐2‐one | 86.35 ± 0.67 | 85.98 ± 0.36 | Metal |
| Butyrolactone | 192.31 ± 62.9 | 86.18 ± 0.25 | caramel, sweet |
| 3‐Octen‐2‐one | 81.76 ± 0.49 | 80.98 ± 0.00 | herb, butter, resin |
| Acetic acid | 112.64 ± 8.09 | 99.83 ± 0.58 | fruit, pungent, sour, vinegar |
| Pentanoic acid | 94.86 ± 3.77 | 82.53 ± 0.32 | Sweat |
| Hexanoic acid | 83.94 ± 1.02 | 81.91 ± 0.70 | sweat, cheese |
From the compounds found, hexanal was selected for further investigation because it has been used extensively to determine the level of rancidity or off‐odors in cereals. 32 , 33 , 34 Besides, focusing on one compound gives an in‐depth understanding as monitoring trends of formation and degradation of VOCs is complex. Aldehydes and ketones are derived from lipid oxidation, and are therefore useful to assess the deterioration of maize flour aroma.
Figure 3 shows that RM flour had a significantly different (P < 0.05) hexanal content of 92 μg kg−1 compared to 85 μg kg−1 in FM flour. The latter was produced at a temperature below −150 °C. The amount of hexanal in FM might have been present in the kernel before milling. The heat (>50 °C) produced by the rotor mill during milling of the kernel is the key reason for the 8% increase in hexanal production. However, heat production during milling could be of advantage during storage as it may inactivate the endogenous lipase enzyme, whereas in the cryogenic mill the preserved endogenous enzyme could be activated during storage, leading to a product more susceptible to degradation. On the other hand, heat can also expose the maize flour to non‐enzymatic oxidation. 35 Lampi et al. 35 reported that an extrusion temperature of 70 °C was sufficient to deactivate lipase enzymes in oats to give a stable product while a higher temperature could result in non‐enzymatic lipid oxidation.
Figure 3.
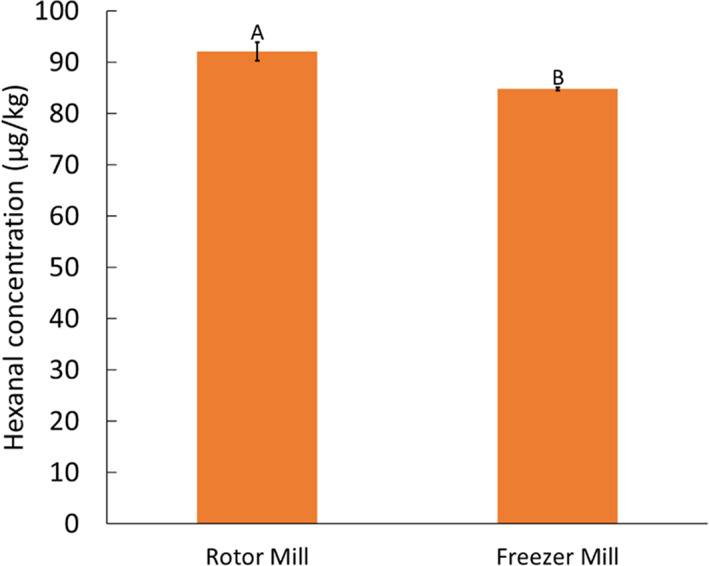
Hexanal production during milling of maize using a RM and a FM. Bars with different letters are significantly different (n = 3, P < 0.05).
Figure 4 shows the hexanal content of maize flour stored with different packaging materials at 37 °C for 180 days. The highest amount of hexanal found in this study was in RM and FM maize flour stored in laminated paper bags at 37 °C, namely 2429 μg kg−1 on day 150 and 2493 μg kg−1 on day 180, respectively. In both cases, significant hexanal production started after 60 days and continued to increase until 180 days of storage, except for LP_FM37C at day 150, which showed a sharp drop in hexanal concentration due to high permeability of the laminated paper bag. Hexanal in all maize flours stored at 4 °C (excluded in the chart) was below 100 μg kg−1 and showed no significant differences throughout the 180 days storage period. In all bags, suppression of hexanal production in both FM and RM flours at low temperature (4 °C) was detected, thus differences at 4 °C between the packaging materials was insignificant. The suppression of hexanal formation during storage at 4 °C is due to the inhibition of the lipid oxidation process. The temperature and nature of packaging material significantly reduced the hexanal formation. This effect is seen for both milling methods but is more obvious in samples stored in laminated paper bags. Considering the packaging materials, the most evident difference was observed at 37 °C between laminated paper bag samples compared to aluminium and double‐layered polyethylene bag samples. Understanding the volatile degradation at 37 °C was relevant because cold storage is not applicable in the daily practice of the relevant populations.
Figure 4.
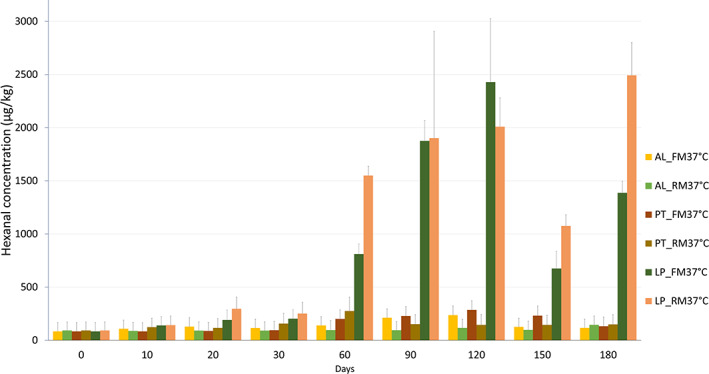
Hexanal production in maize milled with a FM and RM stored using different packaging materials for 180 days at 37 °C. AL – aluminium, LP – laminated paper, PT – Double layered polyethylene.
Annan et al. 27 found 5000 μg kg−1 hexanal in whole maize kernel obtained from a retail outlet in Ghana. While the authors do not mention the duration of storage at room temperature (30 °C) before analysis, the reported hexanal value is twice as high as in our maize flour samples stored at 37 °C for 180 days. Bredie et al. 28 reported a range of 9 to 170 μg kg−1 of hexanal in extruded maize, which corresponds to the values for maize flour stored for 180 days in aluminium bags and double‐layered polyethylene bags in the current research.
In comparison with RM flour, hexanal production was generally high in FM flour, except when stored in laminated paper bags. Increases in hexanal and heptanal were reported in rice during storage at 35 °C, causing the development of a stale flavor. 36 The authors suggested that lipoxygenase‐3 enzyme contributed to aldehyde production after detecting an increased activity of the enzyme. Hexanal, heptanal, octanal, nonanal, and 2‐heptanone have been evaluated in other studies regarding flavor deterioration in oats, wheat, and whey powder. 33 , 37 , 38 Aldehydes have a low flavor threshold value in water, making them very important flavor compounds. Their aromas have been reported to be grassy, fatty, fishy, citrus, rancid, and musty. 33 Heiniö et al. 32 reported that hexanal was perceived as a rancid flavor in oats. Rancidity is caused by lipid oxidation, which can be accelerated by light, heat, or the presence of moisture but, most important, the presence of oxygen. 33 , 39 This implies that the low hexanal formation in the samples stored in the aluminium bags and double‐layered polyethylene bags was due to the low oxygen permeability of the packaging materials. Samples stored in laminated paper bags at 37 °C showed a steady increase in hexanal due to the higher oxygen permeability of these bags. Milling at freezing temperature leaves the endogenous enzymes intact, thereby catalyzing deterioration upon activation at higher storage temperatures. Thermal deactivation of the oxidative enzymes during milling in RM was therefore beneficial to aroma stability during storage.
Furthermore, VOCs in maize can be derived from non‐volatile precursors such as polyphenols, carotenoids, unsaturated fatty acids, sugars and amino acids through autoxidation, thermal breakdown, and enzymatic reactions. Volatile degradation products from carotenoids, such as damascenone, geranylactone, 6‐methyl‐5‐hepten‐2‐one and ‐ionones, were not found in this study. 40 , 41 However, oxidative degradation of carotenoids together with lipoxidase can result in the development of off‐odor compounds. 40 Carotenoid‐based volatiles can contribute to aroma changes if present above the threshold values. Future studies should try to track the presence of these products to ascertain the by‐products of carotenoid degradation in biofortified maize. This is important because studies until now have focused mainly on enzyme‐dependent degradation of unsaturated fatty acids, even with evidence showing that carotenoid‐based changes may affect the aroma profile. 40 , 41
Rotor and hammer mills seem suitable and feasible for maize milling compared to stone and plate mills, which produce 85 °C–90 °C of heat during milling. 14 The type of storage material and the storage temperature play a more important role in the preservation of maize aroma. Double layered polyethylene is a feasible material for storing maize due to a proper stability of carotenoids (Tables S1 and S2) and VOCs (Fig. 4). However, the polyethylene bags pose environmental challenges. Many African countries have banned or reduced the use of plastics bags for this reason. 42 Biodegradable plastic could be a solution only if cost‐effective for smallholder farmers and resource‐poor households.
CONCLUSION
This study assessed the stability of carotenoids during the milling and storage of provitamin A biofortified maize variety Pool 8A. We found β‐carotene, β‐cryptoxanthin, zeaxanthin, and lutein within the range reported by previous studies. No difference in carotenoid retention was detected after milling with a rotor mill and a freezer mill but substantial differences were observed in both flours during storage. Temperature plays an important role in carotenoid retention during postharvest storage because a rapid loss was observed at 37 °C. Our findings stress the need to pay more attention to postharvest handling to retain carotenoids in end products. Storage of maize flour in laminated paper bags showed the worst carotenoid retention while double‐layered polyethylene and aluminium bags showed improved retention. The milling method influenced the formation of off‐odor. Hexanal production was significantly high in laminated paper bags at 37 °C while lower temperatures suppressed the formation. Hexanal was least formed in aluminium bags, followed by polyethylene bags, and laminated paper bags. The rate of carotenoid degradation and formation of sensory defects can be mitigated through the improvement of postharvest handling practices, thereby enhancing food security and consumer acceptance of provitamin A biofortified maize.
Supporting information
Table S1. Effect of storage method on carotenoid retention in maize flour after 180 days at 4 °C
Table S2. Effect of storage method on carotenoid retention in maize flour after 180 days at 37 °C
ACKNOWLEDGEMENTS
The authors wish to thank the Rwanda Agriculture Board, for providing the maize samples. We also appreciate the enthusiastic contribution of Nadine Uwobasa and Fleur de Groot in laboratory analysis.
REFERENCES
- 1. Stevens GA, Bennett JE, Hennocq Q, Lu Y, De‐Regil LM, Rogers L et al., Trends and mortality effects of vitamin a deficiency in children in 138 low‐income and middle‐income countries between 1991 and 2013: a pooled analysis of population‐based surveys. Lancet Glob Health 3:e528–e536 (2015). [DOI] [PubMed] [Google Scholar]
- 2. De Moura FF, Miloff A and Boy E, Retention of provitamin a carotenoids in staple crops targeted for biofortification in Africa: cassava, maize and sweet potato. Crit Rev Food Sci Nutr 55:1246–1269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pixley K, Rojas NP, Babu R, Mutale R, Surles R and Simpungwe E, Biofortification of maize with provitamin A carotenoids, in Carotenoids and Human Health. Humana Press, Totowa, NJ: Springer, pp. 271–292 (2013). [Google Scholar]
- 4. Taleon V, Mugode L, Cabrera‐Soto L and Palacios‐Rojas N, Carotenoid retention in biofortified maize using different post‐harvest storage and packaging methods. Food Chem 232:60–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N et al., Biofortified orange maize is as efficacious as a vitamin a supplement in Zambian children even in the presence of high liver reserves of vitamin a: a community‐based, randomized placebo‐controlled trial. Am J Clin Nutr 100:1541–1550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burt AJ, Grainger CM, Young JC, Shelp BJ and Lee EA, Impact of postharvest handling on carotenoid concentration and composition in high‐carotenoid maize (Zea mays L.) kernels. J Agric Food Chem 58:8286–8292 (2010). [DOI] [PubMed] [Google Scholar]
- 7. Sowa M, Yu J, Palacios‐Rojas N, Goltz SR, Howe JA, Davis CR et al., Retention of carotenoids in biofortified maize flour and β‐cryptoxanthin‐enhanced eggs after household cooking. ACS Omega 2:7320–7328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simpungwe E, Dhliwayo T, Palenberg M, Taleon V, Birol E, Oparinde A et al., Orange maize in Zambia: crop development and delivery experience. Afr J Food Agric Nutr Dev 17:11973–11999 (2017). [Google Scholar]
- 9. Gwirtz JA and Garcia‐Casal MN, Processing maize flour and corn meal food products. Ann N Y Acad Sci 1312:66–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pillay K, Derera J, Siwela M and Veldman FJ, Consumer acceptance of yellow, provitamin A‐biofortified maize in KwaZulu‐Natal: original research. S Afr J Clin Nutr 24:186–191 (2011). [Google Scholar]
- 11. Rosales A, Agama‐Acevedo E, Arturo Bello‐Pérez L, Gutiérrez‐Dorado R and Palacios‐Rojas N, Effect of traditional and extrusion Nixtamalization on carotenoid retention in tortillas made from Provitamin a biofortified maize (Zea mays L.). J Agric Food Chem 64:8289–8295 (2016). [DOI] [PubMed] [Google Scholar]
- 12. Muzhingi T, Palacios‐Rojas N, Miranda A, Cabrera ML, Yeum KJ and Tang G, Genetic variation of carotenoids, vitamin E and phenolic compounds in Provitamin a biofortified maize. J Sci Food Agric 97:793–801 (2017). [DOI] [PubMed] [Google Scholar]
- 13. Mugode L, Ha B, Kaunda A, Sikombe T, Phiri S, Mutale R et al., Carotenoid retention of biofortified provitamin a maize (Zea mays L.) after Zambian traditional methods of milling, cooking and storage. J Agric Food Chem 62:6317–6325 (2014). [DOI] [PubMed] [Google Scholar]
- 14. Ortiz D, Rocheford T and Ferruzzi MG, Influence of temperature and humidity on the stability of carotenoids in biofortified maize (Zea mays L.) genotypes during controlled postharvest storage. J Agric Food Chem 64:2727–2736 (2016). [DOI] [PubMed] [Google Scholar]
- 15. Cabrera‐Soto L, Pixley KV, Rosales‐Nolasco A, Galicia‐Flores LA and Palacios‐Rojas N, Carotenoid and tocochromanol profiles during kernel development make consumption of biofortified “fresh” maize an option to improve micronutrient nutrition. J Agric Food Chem 66:9391–9398 (2018). [DOI] [PubMed] [Google Scholar]
- 16. Bechoff A, Dhuique‐Mayer C, Dornier M, Tomlins KI, Boulanger R, Dufour D et al., Relationship between the kinetics of β‐carotene degradation and formation of norisoprenoids in the storage of dried sweet potato chips. Food Chem 121:348–357 (2010). [Google Scholar]
- 17. Otten JJ, Hellwig JP and Meyers LD, Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, D.C: National Academies Press; (2006). [Google Scholar]
- 18. Li S, Nugroho A, Rocheford T and White WS, Vitamin a equivalence of the β‐carotene in β‐carotene–biofortified maize porridge consumed by women. Am J Clin Nutr 92:1105–1112 (2010). [DOI] [PubMed] [Google Scholar]
- 19. Muzhingi T, Gadaga TH, Siwela AH, Grusak MA, Russell RM and Tang G, Yellow maize with high β‐carotene is an effective source of vitamin a in healthy Zimbabwean men. Am J Clin Nutr 94:510–519 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saltzman A, Birol E, Bouis HE, Boy E, De Moura FF, Islam Y et al., Biofortification: progress toward a more nourishing future. Glob Food Sec 2:9–17 (2013). [Google Scholar]
- 21. Pillay K, Siwela M, Derera J and Veldman FJ, Provitamin a carotenoids in biofortified maize and their retention during processing and preparation of south African maize foods. J Food Sci Technol 51:634–644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chavez A, Sanchez T, Ceballos H, Rodriguez‐Amaya D, Nestel P, Tohme J et al., Retention of carotenoids in cassava roots submitted to different processing methods. J Sci Food Agric 87:388–393 (2007). [Google Scholar]
- 23. Tefera T, Kanampiu F, De Groote H, Hellin J, Mugo S, Kimenju S et al., The metal silo: an effective grain storage technology for reducing post‐harvest insect and pathogen losses in maize while improving smallholder farmers' food security in developing countries. Crop Prot 30:240–245 (2011). [Google Scholar]
- 24. Buttery RG, Stern DJ and Ling LC, Studies on flavor volatiles of some sweet corn products. J Agric Food Chem 42:791–795 (1994). [Google Scholar]
- 25. Gonçalves JL, Figueira JA, Rodrigues FP, Ornelas LP, Branco RN, Silva CL et al., A powerful methodological approach combining headspace solid phase microextraction, mass spectrometry and multivariate analysis for profiling the volatile metabolomic pattern of beer starting raw materials. Food Chem 160:266–280 (2014). [DOI] [PubMed] [Google Scholar]
- 26. Cho S, Nuijten E, Shewfelt RL and Kays SJ, Aroma chemistry of African Oryza glaberrima and Oryza sativa rice and their interspecific hybrids. J Sci Food Agric 94:727–735 (2014). [DOI] [PubMed] [Google Scholar]
- 27. Annan NT, Poll L, Plahar WA and Jakobsen M, Aroma characteristics of spontaneously fermented Ghanaian maize dough for kenkey. Eur Food Res Technol 217:53–60 (2003). [Google Scholar]
- 28. Bredie WL, Mottram DS and Guy RC, Aroma volatiles generated during extrusion cooking of maize flour. J Agric Food Chem 46:1479–1487 (1998). [Google Scholar]
- 29. Sayaslan A, Chung OK, Seib PA and Seitz LM, Volatile compounds in five starches. Cereal Chem 77:248–253 (2000). [Google Scholar]
- 30. Goicoechea E and Guillén MD, Volatile compounds generated in corn oil stored at room temperature. Presence of toxic compounds. Eur J Lipid Sci Technol 116:395–406 (2014). [Google Scholar]
- 31. Pico J, Martínez MM, Bernal J and Gómez M, Evolution of volatile compounds in gluten‐free bread: from dough to crumb. Food Chem 227:179–186 (2017). [DOI] [PubMed] [Google Scholar]
- 32. Heiniö RL, Lehtinen P, Oksman‐Caldentey KM and Poutanen K, Differences between sensory profiles and development of rancidity during long‐term storage of native and processed oat. Cereal Chem 79:367–375 (2002). [Google Scholar]
- 33. Sjövall O, Virtalaine T, Lapveteläinen A and Kallio H, Development of rancidity in wheat germ analyzed by headspace gas chromatography and sensory analysis. J Agric Food Chem 48:3522–3527 (2000). [DOI] [PubMed] [Google Scholar]
- 34. Shahidi F and Wanasundara UN, Methods for measuring oxidative rancidity in fats and oils. Food Lipids Chem Nutr Biotechnol 17:387–403 (2002). [Google Scholar]
- 35. Lampi A‐M, Damerau A, Li J, Moisio T, Partanen R, Forssell P et al., Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J Cereal Sci 62:102–109 (2015). [Google Scholar]
- 36. Suzuki Y, Ise K, Li C, Honda I, Iwai Y and Matsukura U, Volatile components in stored rice [Oryza sativa (L.)] of varieties with and without lipoxygenase‐3 in seeds. J Agric Food Chem 47:1119–1124 (1999). [DOI] [PubMed] [Google Scholar]
- 37. Mahajan S, Goddik L and Qian M, Aroma compounds in sweet whey powder. J Dairy Sci 87:4057–4063 (2004). [DOI] [PubMed] [Google Scholar]
- 38. Janeš D, Kantar D, Kreft S and Prosen H, Identification of buckwheat (Fagopyrum esculentum Moench) aroma compounds with GC–MS. Food Chem 112:120–124 (2009). [Google Scholar]
- 39. Champagne ET, Rice aroma and flavor: a literature review. Cereal Chem 85:445–454 (2008). [Google Scholar]
- 40. Mottram D and Maarse H, Volatile Compounds in Foods and Beverages. Marcel Dekker, New York, pp. 107–177 (1991). [Google Scholar]
- 41. Stevens MA, Relationship between polyene‐carotene content and volatile compound composition of tomatoes. J Am Soc Hortic Sci 95:461–464 (1970). [Google Scholar]
- 42. Mbau SN, Zero Plastic Waste for a Healthier Ecosystem: An Assessment of Sustainable Waste Management in Runda Estate. University of Nairobi, Nairobi: (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effect of storage method on carotenoid retention in maize flour after 180 days at 4 °C
Table S2. Effect of storage method on carotenoid retention in maize flour after 180 days at 37 °C


