Abstract
Seizure clusters must be treated quickly and effectively to prevent progression to prolonged seizures and status epilepticus. Rescue therapy for seizure clusters has focused on the use of benzodiazepines. Although intravenous benzodiazepine administration is the primary route in hospitals and emergency departments, seizure clusters typically occur in out‐of‐hospital settings, where a more portable product that can be easily administered by nonmedical caregivers is needed. Thus, other methods of administration have been examined, including rectal, intranasal, intramuscular, and buccal routes. Following US Food and Drug Administration (FDA) approval in 1997, rectal diazepam became the mainstay of out‐of‐hospital treatment for seizure clusters in the United States. However, social acceptability and consistent bioavailability present limitations. Intranasal formulations have potential advantages for rescue therapies, including ease of administration and faster onset of action. A midazolam nasal spray was approved by the FDA in 2019 for patients aged 12 years or older. In early 2020, the FDA approved a diazepam nasal spray for patients aged 6 years or older, which has a different formulation than the midazolam nasal product and enhances aspects of bioavailability. Benzodiazepines, including diazepam, present significant challenges in developing a suitable intranasal formulation. Diazepam nasal spray contains dodecyl maltoside (DDM) as an absorption enhancer and vitamin E to increase solubility in an easy‐to‐use portable device. In a Phase 1 study, absolute bioavailability of the diazepam nasal spray was 97% compared with intravenous diazepam. Subsequently, the nasal spray demonstrated less variability in bioavailability than rectal gel (percentage of geometric coefficient of variation of area under the curve = 42%–66% for diazepam nasal spray compared with 87%–172% for rectal gel). The diazepam nasal spray safety profile is consistent with that expected for rectal diazepam, with low rates of nasal discomfort (≤6%). To further improve the efficacy of rescue therapy, investigation of novel intranasal benzodiazepine formulations is underway.
Keywords: absorption, acute repetitive seizures, benzodiazepine intranasal formulations, rescue medication, seizure clusters
Key Points.
Seizure clusters mostly occur outside hospitals; thus, rescue therapy should be portable and easily administered by nonmedical persons
Advantages of intranasal rescue therapies compared with rectal treatment include greater social acceptability and ease of administration
Benzodiazepines present challenges to nasal formulations, such as issues with absorption and solubility, requiring mitigating components
Diazepam nasal spray includes Intravail A3 as an absorption enhancer and vitamin E to assist with solubility
Additional intranasal diazepam formulations are being investigated, including a water‐soluble prodrug and enzyme combination
1. INTRODUCTION
Seizure clusters, also known as acute repetitive seizures, may be defined as intermittent increases of seizure activity experienced while on stable regimens of antiseizure drugs. 1 , 2 This type of seizure emergency must be treated quickly and effectively to interrupt the cluster and prevent the progression to prolonged seizures and/or status epilepticus (SE). 3 , 4 Most seizure clusters occur outside the hospital environment, so patients need readily available, easily administered, nonintravenous rescue therapies. 5 , 6 Benzodiazepines, such as diazepam, lorazepam, and midazolam, administered by various routes, both on and off label, have for several decades been used as the preferred first‐line treatment for seizure clusters because of their rapid onset of action, high level of efficacy, and good tolerability. 6 , 7 , 8
Intravenous (IV) administration of benzodiazepines is effective when treating seizure emergencies in medical facilities or when administered by emergency medical personnel, but it is not practical for nonmedical caregivers. However, use of selected non‐IV routes (e.g., buccal, intramuscular [IM], and intranasal [IN]) can permit quicker drug delivery than IV administration, largely owing to the time required to set up IV equipment and the several minutes recommended for drug infusions. 9 , 10 , 11 In a double‐blind, randomized study of IM midazolam versus IV lorazepam in children and adults in SE who were treated by paramedics, the IM route had a significantly shorter median time to administration of active treatment (1.2 min) than the IV route (4.8 min) in patients who had stopped seizing following drug administration before arriving at the emergency department. 12 Likewise, in a prospective, randomized study of IN midazolam versus IV diazepam in children with prolonged febrile seizures, the IN route had a significantly shorter mean time from hospital arrival to treatment initiation (3.5 min) than the IV route (5.5 min). 13 These results are supported by a systematic review of 75 controlled trials that found that benzodiazepines were administered more quickly via non‐IV routes than via IV injection and that time to seizure termination, adverse events, and seizure recurrence rates were generally comparable between IV and non‐IV treatments. 14 In a meta‐analysis, certain non‐IV routes (i.e., buccal, IM, IN, and rectal) were shown to have a faster time to dosing and faster seizure control than IV delivery (mean difference = 3.41 min, 95% confidence interval [CI] = 1.69–5.13 min), similar rates of respiratory complications (risk difference = .00, 95% CI = −.02 to .01), and fewer treatment failures in adults (odds ratio = .72, 95% CI = .56–.92). 15
The need for alternatives to IV benzodiazepine for seizure emergencies triggered the development of new therapies using other routes of administration, which recently culminated in US Food and Drug Administration (FDA) approval of IN benzodiazepine formulations for management of seizure clusters. The steps toward the introduction of these products were not straightforward and presented developers with a number of challenges. This review focuses on the physiological, chemical, pharmacological, and pharmaceutical challenges encountered in the development of an IN diazepam formulation for management of seizure clusters and the approaches used to overcome those challenges.
1.1. Features of the ideal rescue therapy
Out‐of‐hospital benzodiazepine rescue therapy should allow for quick and easy administration by the patient or a family member, a caregiver/care partner, school nurses, or emergency medical personnel with minimal discomfort for the patient. The route and formulation should offer rapid absorption, consistent and high bioavailability, good potency at small dose volumes, reliable and early onset of action, a wide therapeutic index, sufficient duration of action (i.e., hours), a good safety profile, low interpatient variability, dosing guidelines for adults and children, and a long shelf‐life. With these features in mind, several benzodiazepines prepared as different formulations and administered via various routes, including rectal, IN, IM, buccal, subcutaneous, and intrapulmonary, have been studied. 5 , 7
1.2. Alternative routes of drug administration for rescue therapies
Rectal diazepam was approved by the FDA in 1997 and became the mainstay of out‐of‐hospital treatment for seizure clusters in the United States. 16 Despite the efficacy of rectal formulations, many adults and older children find this route of administration socially embarrassing, which leads to underutilization. Adults with epilepsy (n = 80 with at least partially completed questionnaires) reported being equally embarrassed about having a seizure (48%; 13/27) and having to use rectal medications (52%; 14/27). Most (62%; 34/55) preferred to use rectal therapy in a private setting. 17 Because of these concerns, the rectal formulation is primarily used for younger children rather than older children or adults. 18 Institutional barriers to the use of the rectal formulation have also been reported. In a small survey (N = 43), 19% of parents reported that their child’s school refused to administer rectal diazepam gel, citing purported legal restrictions, the child’s privacy, and ignorance about dosing. 19
As an alternative route of administration, development of IN diazepam and midazolam formulations began soon after the approval of diazepam rectal gel. 20 , 21 Because of the challenges of developing such formulations, more than 2 decades elapsed before 2 IN products for the acute treatment of seizure clusters were approved. A midazolam nasal spray (Nayzilam) for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity in patients with epilepsy aged 12 years and older was approved by the FDA in 2019. 10 Diazepam nasal spray (Valtoco, discussed below), which has a similar indication, but includes patients aged 6 years and older, was approved in early 2020. 9
IM benzodiazepines for seizure emergencies also have been studied. As noted above, a controlled trial in SE found that IM midazolam was superior to IV lorazepam and could be delivered more quickly. 12 It is now approved for use by trained health care professionals for adults with SE. 12 , 22 A buccal midazolam solution, which is approved in Europe for seizure emergencies in children younger than 18 years, appears to be as effective as rectal diazepam based on open‐label trials. 23 , 24
As shown in Table 1, other formulations are in development. One example is a diazepam buccal film; however, this route of administration has several limitations (e.g., clenched jaw, excessive drooling, spitting out dose, altered pharmacokinetics when swallowed). 6 , 7 , 25 Another approach is intrapulmonary alprazolam, involving aerosolized drug particles for inhalation. 26 Some level of active patient participation is required for this route, which may be challenging during a seizure.
TABLE 1.
| Route | Drug(s) | Advantages | Disadvantages |
|---|---|---|---|
| Approved by the US Food and Drug Administration | |||
| Rectal |
Diazepam
|
|
|
| Intranasal |
Diazepam
Midazolam
|
|
|
| Intramuscular | Midazolam |
|
|
| Investigational therapies | |||
| Intranasal | Diazepam prodrug |
|
|
| Intramuscular |
Diazepam Diazepam prodrug Lorazepam |
|
|
| Buccal |
Diazepam Midazolam |
|
|
| Sublingual |
Lorazepam Diazepam |
|
|
| Intrapulmonary | Alprazolam |
|
|
Abbreviations: AE, adverse event; CNS, central nervous system; IM, intramuscular; IV, intravenous; SC, subcutaneous; TEAE, treatment‐emergent adverse event.
Table 1 lists some advantages and disadvantages of several non‐IV benzodiazepine formulations approved or in development, including formulations for intranasal administration. 2 , 5 , 6 , 7 , 26 , 27 Among the available routes of administration, nasal drug delivery has the potential to fulfill most of the requirements for an ideal rescue therapy, which can be given during a seizure, while bypassing the gastrointestinal tract and potential issues with variable gastric absorption or vomiting. However, the development of such products must account for the limitations imposed by the anatomy and physiology of the nose and the physicochemical and pharmacokinetic properties of benzodiazepines.
2. CHALLENGES OF THE NASAL ANATOMY AND PHYSIOLOGY
IN administration has a number of theoretical advantages that make it an attractive option for rescue therapies. The IN route is noninvasive and easy to access, may allow rapid onset of effect, avoids first‐pass metabolism, does not require patient cooperation, and is socially acceptable. 7 , 27 , 28
The nasal cavity effectively captures medicating droplets produced by aerosol drug‐delivery devices. Nasal tissue possesses microvilli and a rich vasculature that facilitates drug absorption. Furthermore, nasal cavity innervation by the olfactory nerve and the ophthalmic and maxillary branches of the trigeminal nerve may potentially permit direct nose‐to‐brain delivery (Figure 1). 7 , 29 , 30 Factors that may limit the rate and extent of absorption are the total surface area (about 160 cm2) and total volume (about 15 ml) of the nasal cavity, 30 renewal of the mucus layer, mucociliary clearance, and posterior and anterior drainage. The posterior drainage may result in an objectionable taste. Seasonal allergies, which might be a limitation, do not appear to have a clinical impact on nasal drug absorption. 31 , 32 Absorption, efficacy, and safety of nasal formulations have been similar in subjects without and with seasonal allergies despite the presence of mucosal inflammation. 33 , 34
FIGURE 1.
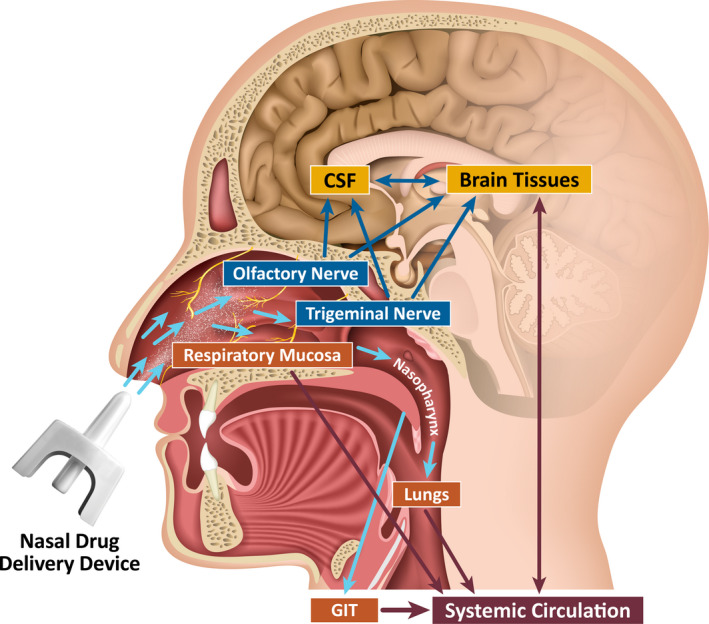
Pathways of intranasal drug delivery: direct to the brain or following absorption into the systemic circulation. CSF, cerebrospinal fluid; GIT, gastrointestinal tract. Reproduced with permission from Kapoor et al. 7
3. CHALLENGES IN DEVELOPING INTRANASAL BENZODIAZEPINE FORMULATIONS
The selection of a specific benzodiazepine and formulation must take into consideration the limitations imposed by the anatomy and physiology of the nasal cavity. For seizure clusters, a liquid formulation is required to enable rapid absorption, and the drug must be highly potent so as to deliver a therapeutic dose in a small volume. Because of the nasal cavity's small surface area, 30 drug delivery is limited to 200 μl (optimally, 100 μl) to avoid leakage or swallowing. 7 , 35 Hence, the solubility of the drug must be high enough to provide a therapeutic dose in a limited volume. This is a challenge for benzodiazepines as a class, in that they are hydrophobic molecules with poor solubility in water. 7 Several strategies have been employed to overcome this problem for IN formulations, such as manipulation of pH, use of surfactants, solubilizing agents such as cyclodextrins or organic solvents, microemulsions, particulate formulations (e.g., nanoparticles), and supersaturated solutions. 7 , 36 Nasal irritation is common with many of these strategies, whether the formulation incorporates organic solvents or lowering of pH. Poor tolerability related to nasal discomfort may reduce a patient's use of product. 37 , 38
Table 2 lists the characteristics of solvents tested for enhancing the solubility of benzodiazepines for IN administration. 39 For several of these solvents, greater fold increase in brain delivery often comes at the expense of increased nasal mucosal toxicity and discomfort.
TABLE 2.
Characteristics of absorption‐enhancing agents suitable for intranasal administration 39
| Absorption enhancer | Concentration | Mean fold increase in brain delivery a | Variability of mean fold increase, range | Nasal mucosal toxicity |
|---|---|---|---|---|
| Chitosan | .5% wt/vol | 6.1 | 3–11 fold | No |
| Decyl maltoside | .5% wt/vol | 4.3 | ND | No |
| n‐Dodecyl beta‐D‐maltoside (Intravail A3) | .25% wt/vol | 7.6 | 6–9 fold | No |
| Propylene glycol | 10%–20% | 4.5 | 4–10 fold | No |
| Heptakis‐(3‐O‐methyl‐2,6‐di‐O‐pentyl)‐β‐cyclodextrin | 5% wt/vol | 3.1 | ND | Yes |
| 1,2 Didecanoyl‐glycero‐3‐phosphocholine | 2% wt/vol (as oil emulsion) | 9.6 | ND | Yes |
| Glycocholate | 1% wt/vol | 17.6 | ND | Yes |
| Taurocholate | 1% wt/vol | 14 | ND | Yes |
| Tauroursodeoxycholate | 1% wt/vol | 4 | ND | Yes |
Source: US Patent No. 8883728 B2: Intranasal Administration of Active Agents to the Central Nervous System.
Abbreviations: ND, not done; wt/vol, weight/volume concentration.
Represents mean fold increase in brain delivery efficiency of nasally administered Homo sapiens‐derived antibody Fc (hFc) fragment relative to control formulation (phosphate‐buffered saline; pH 7.4 and 36 mg/ml hFc).
The rate and extent of absorption, and thus the onset and magnitude of effect, are largely determined by a drug’s physicochemical and pharmacokinetic properties. The greater a drug’s lipid solubility, the faster diffusion occurs across a biological membrane. Among the benzodiazepines used for managing seizure clusters, the lipid solubilities of diazepam and midazolam are approximately four and six times greater, respectively, than lorazepam. 40 , 41 This factor largely determines the rate or absorption as expressed by time to maximum concentration (t max). The duration of effect is largely determined by a drug’s residence time in the brain. 8
4. DEVELOPMENT OF INTRANASALLY ADMINISTERED MIDAZOLAM
An early study of IN midazolam utilized the commercial injectable solution (pH ≈ 3). 37 Although the pharmacokinetic profile was good, the administered volume was large, 1 ml, and the formulation was poorly tolerated. Subsequently, midazolam was investigated for intranasal administration using a formulation that included a solvent or absorption enhancer that allowed for a small therapeutic volume but was associated with some nasal adverse events. 42 Another development effort involved a highly concentrated, aqueous midazolam formulation (Nayzilam) for IN administration. This formulation utilizes several organic solvents, including ethanol, PEG‐6 methyl ether, polyethylene glycol 400, and propylene glycol, to increase the solubility of midazolam while maintaining the pH in the range of 5–9. 10 The product was approved by the FDA in 2019 for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy aged 12 years or older. 10
In a Phase 1, open‐label study of this formulation in healthy adults, all doses of the midazolam nasal spray exhibited variable absorption (62%–73% absolute bioavailability). 43 Prescribing information lists the mean absolute bioavailability of intranasal midazolam as approximately 44%. 10
A Phase 3, randomized, double‐blind study of midazolam 5 mg nasal spray in seizure clusters included an in‐clinic, open‐label test‐dose phase (two doses, 10 min apart) conducted to assess patient safety under observation (N = 292; age = 12–65 years, with 6.2% <18 years old), followed by a double‐blind, placebo‐controlled comparative phase (n = 201). 44 For the primary endpoint (seizure termination in <10 min with no recurrence 10 min to 6 h afterward), the treatment success rate was 53.7% versus 34.3% for IN midazolam and placebo (p = .011). A second, open‐label dose was required by 31.3% of midazolam‐treated patients by 6 h. In the midazolam and placebo groups, 27.6% and 22.4% of patients, respectively, experienced one or more treatment‐emergent adverse event (TEAE). Sixteen midazolam patients (5.5%) discontinued because of a TEAE, all during the test‐dose phase, 13 owing to a TEAE considered treatment related (62% sedation‐type, including sedation, somnolence, and hypersomnia). 44
In an open‐label extension trial, 161 patients received intranasal midazolam and had 1998 seizure cluster episodes. 45 After a median of 16.8 months, TEAEs within 2 days of treatment were reported by 40.4% of patients, primarily nasal discomfort (12.4%) and somnolence (9.3%). Two patients discontinued due to nonserious treatment‐related TEAEs (one owing to somnolence, one to treatment‐related nasal discomfort). Overall, 57.1% of patients experienced a TEAE during the study, most commonly nasal discomfort (12.4%). Treatment success with a single dose was similar to the double‐blind trial (55.5%) and was 80.2% after a second dose; overall, 39.9% of seizure clusters required a second dose. 45
A recent randomized, double‐blind, placebo‐controlled Phase 3 study of patients admitted to an epilepsy monitoring unit evaluated the efficacy and safety of IN midazolam in patients with epilepsy who had two or more seizures in the 6 h preceding the trial (31 patients each in the midazolam and placebo groups). 46 The proportion of patients without seizures in the 6 h posttreatment was higher in the midazolam group (54.8% vs. 38.7%), but the difference was not statistically significant (p = .1972) compared with placebo. Safety for midazolam was generally comparable to placebo. 46
5. DEVELOPMENT OF INTRANASALLY ADMINISTERED DIAZEPAM
Initial investigations explored the potential of diazepam as an IN therapy with the aim of optimizing the rate and extent of absorption and characterizing pharmacodynamics. A small open‐label crossover study in healthy volunteers of IN diazepam in a polyethylene and glycofurol‐based formulation versus IV diazepam showed intermediate bioavailability and mild central nervous system effects. 20 In another healthy volunteer study (n = 7), the investigators used polyethylene glycol 300 as a vehicle to solubilize diazepam for IN formulations (4 and 7 mg). Central nervous system effects of diazepam were measured using the P300–N100 amplitude difference in the electroencephalogram. For mean changes in P300–N100 amplitude before and after drug administration, significant differences were found versus placebo (placebo, −.9; 4 mg IN, −6.4; 7 mg IN, −8.6; p < .05), indicating a significant drug effect. 29 For both doses of IN diazepam, this effect became notable within the first 0–2 min and was evident throughout the 12‐min study period. 29
Pilot studies of IN diazepam in healthy volunteers using a glycofurol‐based formulation demonstrated comparable maximum concentration (C max) and t max, with absolute bioavailability ranging from 70% to 90% as compared to IV diazepam. However, subjects reported appreciable transient nasal discomfort. 37 , 38 Taken together, these studies suggested that IN delivery of diazepam for seizure clusters could be a viable alternative to rectal diazepam if improved tolerability could be achieved without sacrificing rapid, high, and consistent bioavailability.
Subsequently, several companies pursued development of an IN diazepam spray. One approach employed a glycol‐based organic solvent, with bioavailability initially reported to be comparable to rectal diazepam. 47 In a subsequent study, the formulation attained therapeutic diazepam concentrations in adults during the ictal or postictal phase of seizures. 48 However, development was discontinued after a study in patients found suboptimal nasal absorption compared with rectal diazepam. 49 Adverse events that may have been related to the use of the organic solvent included dysgeusia (25.8%), nasal discomfort (22.6%), increased lacrimation (16.1%), nausea (16.1%), rhinorrhea (16.1%), somnolence (16.1%), oropharyngeal pain (12.9%), paranasal sinus hypersecretion (12.9%), and tongue injury (12.9%). 48
Taken together, these formulation issues highlight the critical interplay among volume, bioavailability, and safety and tolerability to achieve a fine balance of all three factors to yield a viable therapeutic candidate (Figure 2).
FIGURE 2.
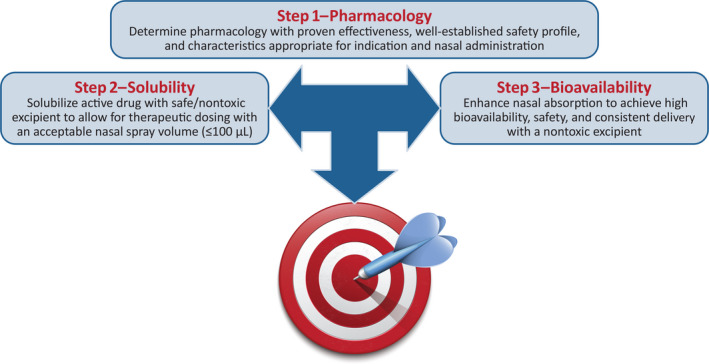
Diazepam intranasal formulation challenges
5.1. Diazepam nasal spray (Valtoco)
The developers of diazepam nasal spray (Valtoco) employed a different approach to overcome the limitations and problems noted with other IN benzodiazepine formulations. The design of this formulation was intended to exhibit a rate and extent of absorption that was comparable to or better than rectal diazepam gel, with less variability in bioavailability and better tolerability in terms of nasal discomfort and taste. The formulation includes DDM (Intravail A3; .25% weight/volume concentration), an alkylsaccharide, and vitamin E. 50 , 51 DDM enhances nasal absorption by transiently and reversibly loosening tight junctions between cells. In a crossover study in healthy adults, use of DDM increased the area under the plasma concentration–time curve (AUC) over the first 2 h postdose of nasally administered sumatriptan by a factor of two. 52 DDM also has been shown to be nonirritating in the rabbit eye model. 53 Vitamin E is used as a nonaqueous, nontoxic, nonirritating solvent for diazepam delivery. 9 In addition to its solubilizing properties, vitamin E protects against phospholipid‐mediated inflammation and damage to the sinonasal mucosa. 54 , 55
A randomized Phase 1 crossover study compared an IN diazepam (10 mg) solution and an IN suspension versus IV diazepam in 24 healthy, adult volunteers. Both formulations contained DDM and vitamin E. Absolute bioavailability was greater with the solution than with the suspension (97% vs. 67%), and it was well tolerated. 18 The solution exhibited pharmacokinetic parameters that compared favorably with a comparable rectal dose, including C max of 272 ng/ml, t max of 1–1.5 h, and low variability of absorption. 18
Subsequently, several open‐label, randomized Phase 1 studies using the solution formulation characterized diazepam nasal spray pharmacokinetics in comparison with rectal and oral diazepam. A dose‐ranging crossover study evaluated the pharmacokinetics of single doses (5, 10, and 20 mg) and a two‐dose regimen (2 × 10 mg, 4 h apart) of diazepam nasal spray in 33 healthy adults. 56 Single‐dose median t max was 1.4–1.5 h. Mean exposure appeared to be dose dependent, with C max of 85.6, 133.6, and 235.3 ng/ml, respectively, for the 5‐, 10‐, and 20‐mg doses. C max and AUC were comparable for the 10‐mg single‐dose and two‐dose regimens. Somnolence was the most common TEAE (range = 36%–50% for single doses, 61% for the two‐dose regimen).
A separate study compared the bioavailability and safety of diazepam nasal spray versus diazepam rectal gel and oral diazepam as the safety reference formulation in 48 healthy volunteers. 57 The nasal spray had a comparable t max, but less variability in C max and AUC than rectal diazepam, with the percentage of geometric coefficient of variation of AUC for diazepam nasal spray being 42%–66% compared with 87%–172% for rectal gel. TEAE rates in the three groups were 61%, 94%, and 85%, respectively, with more than 90% of TEAEs being mild (primarily somnolence). No serious TEAEs or discontinuations were recorded.
In a separate study, Hogan et al. 58 conducted an open‐label study in 57 patients (30% were ≤16 years of age) with epilepsy in which diazepam nasal spray was administered during an ictal/peri‐ictal period and an interictal period. The pharmacokinetic profiles from the two periods were similar. Seventeen patients (29.8%) reported TEAEs, and eight (14%) had treatment‐related TEAEs. Treatment‐related TEAEs reported in two or more patients were dysgeusia (n = 3, 5.3%) and nasal discomfort (n = 2, 3.5%). The investigators concluded that seizure conditions (ictal/peri‐ictal, interictal) had minimal impact on diazepam nasal spray pharmacokinetics.
The long‐term safety of the diazepam nasal spray in patients with seizure clusters is being assessed in a Phase 3, open‐label, repeat‐dose study over 12 months with dose based on body weight. 59 An interim analysis of this study included 132 patients, aged 6–65 years, with a total of 2274 treated seizure episodes. Interim results (cutoff, February 8, 2019) showed that the most common TEAEs were seizures (13%), nasopharyngitis (6%), influenza (5%), upper respiratory tract infection (5%), and nasal discomfort (5%; similar to the 6% rate listed on the label 9 ). Dysgeusia was reported in one patient (.8%). Nasal adverse events were mild and transient. 60 TEAEs were numerically higher with frequent use versus moderate use (76.1% vs. 61.5%). 59 Retention was high, with 85.6% of patients remaining in the trial at the interim cutoff, with no treatment‐related discontinuations due to TEAEs. A second dose was required in 8.4% of seizure episodes (191/2274) measured within the same calendar day. 60 Patients using clobazam 61 and patients with seasonal allergies 62 demonstrated similar safety and efficacy as other patients.
Historically, IN drug delivery systems have used nonmetered formulations in squeeze bottles or droppers that did not provide a specified dose each time and could result in leakage. 63 The type of IN device used for diazepam nasal spray (Unidose system, Aptar Pharma) provides a specified metered dose that provides an even dispersion of droplets to enhance absorption. 63 This type of device is used in a variety of conditions (e.g., diabetes, migraine, pain management, vaccines), 63 in part due to its intuitiveness, as shown by human factors studies, 64 ease of use in one hand, and portability, which allow patients who can participate in their own treatment to self‐administer the drug.
Diazepam nasal spray has been successfully used by patients to self‐dose for seizure clusters, with the youngest person doing so in a clinical trial aged 11 years. 65 Patients who self‐administered diazepam nasal spray (n = 27) were able to take steps to self‐control their treatment and reported that treatment was easy to administer, convenient to carry, and preferable to use in a social setting compared with rectal diazepam. Among these patients, 994 doses of diazepam nasal spray were administered (10 mg, 61 doses; 15 mg, 180 doses; 20 mg, 753 doses); the duration of exposure was ≥12 months for 96.3% and 6–<12 months for 3.7%. 65 Diazepam nasal spray has demonstrated a low rate of dosing error (31/2498, 1.2%) when used by nonmedical caregivers in children as young as 6 years 65 ; there were also no reports of leakage. These observations suggest that administration of diazepam nasal spray is likely to be effective and satisfactory to the patient.
5.2. Investigational IN diazepam formulations
The availability of an IN diazepam product provides patients and caregivers with an improvement in managing seizure clusters. Nonetheless, even faster onset of effect and greater consistency in absorption are desirable. One approach is to use a water‐soluble benzodiazepine prodrug combined with a converting enzyme. 36 , 66 Such a compound is avizafone, a highly water‐soluble prodrug of diazepam, which, when combined with an exogenous converting enzyme, results in rapid biotransformation to diazepam. 36 , 66 In vitro studies showed that avizafone given with a converting protease enzyme, but not organic solvents, attained a high level of supersaturation, enabling faster transport across the membrane than a conventional diazepam solution. 66
In a rat model, single IN doses of avizafone were given with human aminopeptidase B converting enzyme. 36 IN avizafone, administered in single doses equivalent to diazepam at .500, 1.00, and 1.50 mg/kg, resulted in rapid diazepam absorption, with a t max in brain tissue of 8 min and high and consistent systemic bioavailability of 77.8% ± 6.0%, 112% ± 10%, and 114% ± 7%, respectively. The prodrug + enzyme combination appeared to be well tolerated, as there were no inflammatory responses observed and few tissue changes (all minimal or mild and consistent with mechanical trauma related to dose administration), and there was no dose effect noted. Further exploration of the ideal ratio of enzyme to prodrug is warranted, as is development of a device to combine the components at time of use. 36 These early promising results suggest that a water‐soluble prodrug with a converting enzyme holds promise for even faster termination of seizure cluster episodes.
6. CONCLUSIONS
Beginning with the introduction of diazepam rectal gel, rescue medications have played an increasingly important role in the management of seizure emergencies. In the search for alternatives to rectal routes of administration, IN formulations have been extensively studied. The IN route offers opportunities in terms of patient acceptance, ease of administration by nonmedical caregivers, early treatment, and rapid onset of response. At the same time, this route poses special challenges that have an impact on the choice of the benzodiazepine and formulation. Midazolam and diazepam IN formulations have recently been approved for the treatment of seizure clusters. There are certain differences between the products, including composition of the formulations, bioavailability, pediatric indication, and available dosage strengths; however, no head‐to‐head studies have yet been done to assess what effect, if any, the differences have on safety and efficacy.
In this review, we have focused on the diazepam nasal spray. Given the challenges associated with nasal delivery of benzodiazepines, this formulation incorporated an absorption enhancer to improve the rate and extent of absorption and a solvent with low potential for tissue irritation. This formulation and route of administration offer the prospect of easy administration, high bioavailability, a good safety profile, and social acceptability. 18 Diazepam nasal spray achieves comparable exposure when compared with rectal diazepam, which has proven efficacy in clinical trials. 57 , 67 Nasal administration of diazepam provides an important treatment option that allows nonmedical caregivers, such as family members or school personnel, to treat seizure clusters, 68 with the goal of reducing seizure cluster episodes and emergency department visits, while improving quality of life in people with drug‐resistant epilepsy.
CONFLICT OF INTEREST
J.C. has received compensation for consulting from Neurelis. S.H. has no conflict of interest to disclose. E.C. is an employee of and has received stock and stock options from Neurelis. E.C. serves on the Board of Directors of Hawaii‐Biotech and Marinus Pharmaceuticals and has received stock and stock options from both. A.L.R. is an employee of and has received stock options from Neurelis.
ACKNOWLEDGMENT
Medical writing support was provided at the direction of the authors by the Curry Rockefeller Group, which also provided additional editorial assistance, including formatting and proofreading.
Funding information
Development of this article was supported by Neurelis.
REFERENCES
- 1. Buelow JM, Shafer P, Shinnar R, Austin J, Dewar S, Long L, et al. Perspectives on seizure clusters: gaps in lexicon, awareness, and treatment. Epilepsy Behav. 2016;57(Pt A):16–22. [DOI] [PubMed] [Google Scholar]
- 2. Jafarpour S, Hirsch LJ, Gainza‐Lein M, Kellinghaus C, Detyniecki K. Seizure cluster: definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. [DOI] [PubMed] [Google Scholar]
- 3. Komaragiri A, Detyniecki K, Hirsch LJ. Seizure clusters: a common, understudied and undertreated phenomenon in refractory epilepsy. Epilepsy Behav. 2016;59:83–6. [DOI] [PubMed] [Google Scholar]
- 4. Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015;14(6):615–24. [DOI] [PubMed] [Google Scholar]
- 5. Maglalang PD, Rautiola D, Siegel RA, Fine JM, Hanson LR, Coles LD, et al. Rescue therapies for seizure emergencies: new modes of administration. Epilepsia. 2018;59(Suppl 2):207–15. [DOI] [PubMed] [Google Scholar]
- 6. Mula M. New non‐intravenous routes for benzodiazepines in epilepsy: a clinician perspective. CNS Drugs. 2017;31(1):11–7. [DOI] [PubMed] [Google Scholar]
- 7. Kapoor M, Cloyd JC, Siegel RA. A review of intranasal formulations for the treatment of seizure emergencies. J Control Release. 2016;237:147–59. [DOI] [PubMed] [Google Scholar]
- 8. Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118(2):69–86. [DOI] [PubMed] [Google Scholar]
- 9. Neurelis. VALTOCO® (diazepam nasal spray). Full prescribing information. San Diego, CA: Neurelis; 2020. [Google Scholar]
- 10. UCB . NAYZILAM® (midazolam nasal spray). Full prescribing information. Smyrna, GA: UCB; 2019. [Google Scholar]
- 11. West‐Ward Pharmaceuticals, a Hikma Company . ATIVAN® (lorazepam injection). Full prescribing information. Eatontown, NJ: West‐Ward Pharmaceuticals, a Hikma Company; 2017. [Google Scholar]
- 12. Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321(7253):83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haut SR, Seinfeld S, Pellock J. Benzodiazepine use in seizure emergencies: a systematic review. Epilepsy Behav. 2016;63:109–17. [DOI] [PubMed] [Google Scholar]
- 15. Alshehri A, Abulaban A, Bokhari R, Kojan S, Alsalamah M, Ferwana M, et al. Intravenous versus nonintravenous benzodiazepines for the cessation of seizures: a systematic review and meta‐analysis of randomized controlled trials. Acad Emerg Med. 2017;24(7):875–83. [DOI] [PubMed] [Google Scholar]
- 16. Dreifuss FE, Rosman NP, Cloyd JC, Pellock JM, Kuzniecky RI, Lo WD, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338(26):1869–75. [DOI] [PubMed] [Google Scholar]
- 17. Tatum WO. Adult patient perceptions of emergency rectal medications for refractory seizures. Epilepsy Behav. 2002;3(6):535–8. [DOI] [PubMed] [Google Scholar]
- 18. Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105(3):362–7. [DOI] [PubMed] [Google Scholar]
- 19. Terry D, Paolicchi J, Karn M. Acceptance of the use of diazepam rectal gel in school and day care settings. J Child Neurol. 2007;22(9):1135–8. [DOI] [PubMed] [Google Scholar]
- 20. Gizurarson S, Gudbrandsson FK, Jonsson H, Bechgaard E. Intranasal administration of diazepam aiming at the treatment of acute seizures: clinical trials in healthy volunteers. Biol Pharm Bull. 1999;22(4):425–7. [DOI] [PubMed] [Google Scholar]
- 21. Knoester PD, Jonker DM, Van Der Hoeven RTM, Vermeij TAC, Edelbroek PM, Brekelmans GJ, et al. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Br J Clin Pharmacol. 2002;53(5):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garnett WR, Barr WH, Edinboro LE, Karnes HT, Mesa M, Wannarka GL. Diazepam autoinjector intramuscular delivery system versus diazepam rectal gel: a pharmacokinetic comparison. Epilepsy Res. 2011;93(1):11–6. [DOI] [PubMed] [Google Scholar]
- 23. Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353(9153):623–6. [DOI] [PubMed] [Google Scholar]
- 24. McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(9481):205–10. [DOI] [PubMed] [Google Scholar]
- 25. Seinfeld S, Gelfand MA, Heller AH, Buan C, Slatko G. Safety and tolerability associated with chronic intermittent use of diazepam buccal film in adult, adolescent, and pediatric patients with epilepsy. Epilepsia. 2020;61(11):2426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. French JA, Wechsler R, Gelfand MA, Pollard JR, Vazquez B, Friedman D, et al. Inhaled alprazolam rapidly suppresses epileptic activity in photosensitive participants. Epilepsia. 2019;60(8):1602–9. [DOI] [PubMed] [Google Scholar]
- 27. Agarwal SK, Cloyd JC. Development of benzodiazepines for out‐of‐hospital management of seizure emergencies. Neurol Clin Pract. 2015;5(1):80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Z, Xiong G, Tsang WC, Schatzlein AG, Uchegbu IF. Nose‐to‐brain delivery. J Pharmacol Exp Ther. 2019;370(3):593–601. [DOI] [PubMed] [Google Scholar]
- 29. Lindhardt K, Gizurarson S, Stefansson SB, Olafsson DR, Bechgaard E. Electroencephalographic effects and serum concentrations after intranasal and intravenous administration of diazepam to healthy volunteers. Br J Clin Pharmacol. 2001;52(5):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gizurarson S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr Drug Deliv. 2012;9(6):566–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaila T, Suonpaa J, Grenman R, Iisalo E. Vasomotor rhinitis and the systemic absorption of ipratropium bromide. Rhinology. 1990;28(2):83–9. [PubMed] [Google Scholar]
- 32. Lunell E, Molander L, Andersson M. Relative bioavailability of nicotine from a nasal spray in infectious rhinitis and after use of a topical decongestant. Eur J Clin Pharmacol. 1995;48(1):71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Argenti D, Colligon I, Heald D, Ziemniak J. Nasal mucosal inflammation has no effect on the absorption of intranasal triamcinolone acetonide. J Clin Pharmacol. 1994;34(8):854–8. [DOI] [PubMed] [Google Scholar]
- 34. Perelman M, Fisher AN, Smith A, Knight A. Impact of allergic rhinitis and its treatment on the pharmacokinetics of nasally administered fentanyl. Int J Clin Pharmacol Ther. 2013;51(5):349–56. [DOI] [PubMed] [Google Scholar]
- 35. Pires A, Fortuna A, Alves G, Falcao A. Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci. 2009;12(3):288–311. [DOI] [PubMed] [Google Scholar]
- 36. Rautiola D, Maglalang PD, Cheryala N, Nelson KM, Georg GI, Fine JM, et al. Intranasal coadministration of a diazepam prodrug with a converting enzyme results in rapid absorption of diazepam in rats. J Pharmacol Exp Ther. 2019;370(3):796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ivaturi VD, Riss JR, Kriel RL, Cloyd JC. Pharmacokinetics and tolerability of intranasal diazepam and midazolam in healthy adult volunteers. Acta Neurol Scand. 2009;120(5):353–7. [DOI] [PubMed] [Google Scholar]
- 38. Ivaturi VD, Riss JR, Kriel RL, Siegel RA, Cloyd JC. Bioavailability and tolerability of intranasal diazepam in healthy adult volunteers. Epilepsy Res. 2009;84(2–3):120–6. [DOI] [PubMed] [Google Scholar]
- 39. Maggio ET. Intranasal administration of active agents to the central nervous system. https://www.lens.org/lens/patent/041‐718‐551‐271‐297/fulltext. Accessed July 30, 2020.
- 40. Arendt RM, Greenblatt DJ, deJong RH, Bonin JD, Abernethy DR, Ehrenberg BL, et al. In vitro correlates of benzodiazepine cerebrospinal fluid uptake, pharmacodynamic action and peripheral distribution. J Pharmacol Exp Ther. 1983;227(1):98–106. [PubMed] [Google Scholar]
- 41. Cloyd J. Pharmacologic considerations in the treatment of repetitive or prolonged seizures. J Child Neurol. 2007;22(5 Suppl):47S–52S. [DOI] [PubMed] [Google Scholar]
- 42. Schrier L, Zuiker R, Merkus FWHM, Klaassen ES, Guan Z, Tuk B, et al. Pharmacokinetics and pharmacodynamics of a new highly concentrated intranasal midazolam formulation for conscious sedation. Br J Clin Pharmacol. 2017;83(4):721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bancke LL, Dworak HA, Rodvold KA, Halvorsen MB, Gidal BE. Pharmacokinetics, pharmacodynamics, and safety of USL261, a midazolam formulation optimized for intranasal delivery, in a randomized study with healthy volunteers. Epilepsia. 2015;56(11):1723–31. [DOI] [PubMed] [Google Scholar]
- 44. Detyniecki K, Van Ess PJ, Sequeira DJ, Wheless JW, Meng TC, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters—a randomized, double‐blind, placebo‐controlled trial. Epilepsia. 2019;60(9):1797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wheless JW, Meng TC, Van Ess PJ, Detyniecki K, Sequeira DJ, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters: an open‐label extension trial. Epilepsia. 2019;60(9):1809–19. [DOI] [PubMed] [Google Scholar]
- 46. Spencer DC, Sinha SR, Choi EJ, Cleveland JM, King A, Meng T‐C, et al. Safety and efficacy of midazolam nasal spray for the treatment of intermittent bouts of increased seizure activity in the epilepsy monitoring unit: a double‐blind, randomized, placebo‐controlled trial. Epilepsia. 2020;61(11):2415–25. [DOI] [PubMed] [Google Scholar]
- 47. Henney HR 3rd, Sperling MR, Rabinowicz AL, Bream G, Carrazana EJ. Assessment of pharmacokinetics and tolerability of intranasal diazepam relative to rectal gel in healthy adults. Epilepsy Res. 2014;108(7):1204–11. [DOI] [PubMed] [Google Scholar]
- 48. Sperling MR, Haas KF, Krauss G, Seif Eddeine H, Henney HR, Rabinowicz AL, et al. Dosing feasibility and tolerability of intranasal diazepam in adults with epilepsy. Epilepsia. 2014;55(10):1544–50. [DOI] [PubMed] [Google Scholar]
- 49. Acorda Therapeutics . Acorda to discontinue development of PLUMIAZ for treatment of epilepsy seizure clusters. http://ir.acorda.com/investors/investor‐news/investor‐news‐details/2016/Acorda‐to‐Discontinue‐Development‐of‐PLUMIAZ‐for‐Treatment‐of‐Epilepsy‐Seizure‐Clusters/default.aspx. Accessed September 16, 2020.
- 50. Maggio ET, Pillion DJ. High efficiency intranasal drug delivery using Intravail® alkylsaccharide absorption enhancers. Drug Deliv Transl Res. 2013;3(1):16–25. [DOI] [PubMed] [Google Scholar]
- 51. Arnold JJ, Ahsan F, Meezan E, Pillion DJ. Correlation of tetradecylmaltoside induced increases in nasal peptide drug delivery with morphological changes in nasal epithelial cells. J Pharm Sci. 2004;93(9):2205–13. [DOI] [PubMed] [Google Scholar]
- 52. Munjal S, Gautam A, Offman E, Brand‐Schieber E, Allenby K, Fisher DM. A randomized trial comparing the pharmacokinetics, safety, and tolerability of DFN‐02, an intranasal sumatriptan spray containing a permeation enhancer, with intranasal and subcutaneous sumatriptan in healthy adults. Headache. 2016;56(9):1455–65. [DOI] [PubMed] [Google Scholar]
- 53. Maggio ET. Intravail: highly effective intranasal delivery of peptide and protein drugs. Expert Opin Drug Deliv. 2006;3(4):529–39. [DOI] [PubMed] [Google Scholar]
- 54. Feldman C, Anderson R, Theron AJ, Steel HC, van Rensburg CE, Cole PJ, et al. Vitamin E attenuates the injurious effects of bioactive phospholipids on human ciliated epithelium in vitro. Eur Respir J. 2001;18(1):122–9. [DOI] [PubMed] [Google Scholar]
- 55. Testa D, Marcuccio G, Panin G, Bianco A, Tafuri D, Thyrion FZ, et al. Nasal mucosa healing after endoscopic sinus surgery in chronic rhinosinusitis of elderly patients: role of topic alpha‐tocopherol acetate. Aging Clin Exp Res. 2017;29(Suppl 1):191–5. [DOI] [PubMed] [Google Scholar]
- 56. Tanimoto S, Pesco Koplowitz L, Lowenthal RE, Koplowitz B, Rabinowicz AL, Carrazana E. Evaluation of pharmacokinetics and dose proportionality of diazepam after intranasal administration of NRL‐1 to healthy volunteers. Clin Pharmacol Drug Dev. 2020;9(6):719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hogan RE, Gidal BE, Koplowitz B, Koplowitz LP, Lowenthal RE, Carrazana E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61(3):455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hogan RE, Tarquinio D, Sperling MR, Klein P, Miller I, Segal EB, et al. Pharmacokinetics and safety of VALTOCO (NRL‐1; diazepam nasal spray) in patients with epilepsy during seizure (ictal/peri‐ictal) and nonseizure (interictal) conditions: a phase 1, open‐label study. Epilepsia. 2020;61(5):935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miller I, Wheless JW, Hogan RE, Dlugos D, Biton V. Safety and tolerability of NRL‐1, an intranasal formulation of diazepam, in relationship to usage frequency in subjects with epilepsy: interim results from a phase 3, open‐label, repeat dose study. Presented at: 73rd Annual Meeting of the American Epilepsy Society; December 6–10, 2019; Baltimore, MD.
- 60. Wheless JW, Sperling MR, Liow KK, Vazquez B, Segal E, Miller I, et al. Safety of Valtoco (NRL‐1; diazepam nasal spray) in patients with epilepsy: interim results from a phase 3, open‐label, 12‐month repeat dose study. Presented at: 73rd American Epilepsy Society Annual Meeting; December 6–10, 2019; Baltimore, MD.
- 61. Carrazana E, Segal E, Tarquinio D, Miller I, Wheless J. Evaluation of NRL‐1, an intranasal formulation of diazepam, in patients with epilepsy concomitantly using clobazam: an interim subgroup analysis from a phase 3, long‐term, open‐label study. Presented at: 73rd Annual Meeting of the American Epilepsy Society; December 6–10, 2019; Baltimore, MD.
- 62. Vazquez B, Sperling MR, Wheless JW, Liow K, Segal E. Effectiveness and safety of Valtoco™ (NRL‐1; diazepam nasal spray) in patients with epilepsy and a history of seasonal allergies: interim results from a phase 3, open‐label, 12‐month repeat dose study. Presented at: 73rd Annual Meeting of the American Epilepsy Society; December 6–10, 2019; Baltimore, MD.
- 63. Williams G. Nosing around. Pharmaceutical Manufacturing Packing Sourcer 2015;Autumn:30–40.
- 64. Krieter P, Chiang N, Gyaw S, Skolnick P, Crystal R, Keegan F, et al. Pharmacokinetic properties and human use characteristics of an FDA‐approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243–53. [DOI] [PubMed] [Google Scholar]
- 65. Cook DF, Penovich P, Wheless JW, Hogan RE, Guerra C, Carrazana E, et al. Characteristics of patients who self‐administered diazepam nasal spray for seizure clusters: interim results from a phase 3, open‐label, repeat dose safety study. Presented at: American Epilepsy Society; December 4, 2020, 2020; virtual meeting.
- 66. Siegel RA, Kapoor M, Cheryala N, Georg GI, Cloyd JC. Water‐soluble benzodiazepine prodrug/enzyme combinations for intranasal rescue therapies. Epilepsy Behav. 2015;49:347–50. [DOI] [PubMed] [Google Scholar]
- 67. Gidal B, Klein P, Hirsch LJ. Seizure clusters, rescue treatments, seizure action plans: unmet needs and emerging formulations. Epilepsy Behav. 2020;112:107391. [DOI] [PubMed] [Google Scholar]
- 68. Shafer PO. New drug: Valtoco (diazepam nasal spray) approved as rescue therapy for seizures. Epilepsy Foundation. https://www.epilepsy.com/article/2020/1/new‐drug‐valtoco‐diazepam‐nasal‐spray‐approved‐rescue‐therapy‐seizures. Accessed September 25, 2020.
- 69. Valeant Pharmaceuticals . Diastat ® (diazepam rectal gel rectal delivery system). Full prescribing information. Bridgewater, NJ: Valeant Pharmaceuticals North America; 2016. [Google Scholar]


