Abstract
Introduction
The EMBRACE study (Clinical Trials No. NCT02462759) evaluated nusinersen in infants/children with infantile‐ or later‐onset spinal muscular atrophy (SMA) who were ineligible for the ENDEAR and CHERISH studies.
Methods
Participants were randomized to intrathecal nusinersen (12‐mg scaled equivalent dose; n = 14) or sham procedure (n = 7) in part 1 (~14 months) and subsequently received open‐label nusinersen for ~24 months in part 2 of the study.
Results
Part 1 was stopped early after the demonstration of motor function benefit with nusinersen in ENDEAR. There were no nusinersen‐related adverse events (AEs) and no study discontinuations due to nusinersen‐related AEs. The most common AEs included pyrexia, cough, pneumonia, and upper respiratory tract infections. Motor milestone responder rates were higher in those receiving nusinersen at last available assessment (93%) than in those receiving sham procedure in part 1 (29%) or transitioned from sham to nusinersen in part 2 (83%). This functional improvement was observed despite the small sample size and shortened part 1 trial duration that undermined the power of the study to demonstrate such treatment effects at a significant level.
Discussion
Nusinersen demonstrated a favorable long‐term benefit‐risk profile in this broad population of individuals with infantile‐ or later‐onset SMA.
Keywords: clinical tria, nusinersen, safety, spinal muscular atrophy, therapeutic use
Abbreviations
- AE
adverse event
- CSF
cerebrospinal fluid
- CGI‐C
Clinical Global Impression of Change
- HINE‐2
Hammersmith Infant Neurological Evaluation Section 2
- pNF‐H
phosphorylated neurofilament heavy chain
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
1. INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disease characterized by progressive weakness and atrophy of the skeletal muscles. 1 , 2 , 3 It is caused by bi‐allelic mutation, most often deletion, of the survival motor neuron (SMN) 1 gene, with consequent loss of SMN protein. 1 , 2 , 4 , 5 The paralogous SMN2 gene in humans provides partial rescue; exon 7 is excluded from all but 5% to 10% of the mature SMN2 transcript and thus expresses only a limited amount of functional full‐length protein. 2 The resulting insufficient SMN protein is responsible for the SMA phenotype. The number of SMN2 gene copies varies between individuals and is an important determinant of disease severity. 6
Nusinersen is a synthetic antisense oligonucleotide approved for treating SMA in Europe, 7 the United States, 8 and elsewhere. Nusinersen modifies splicing of SMN2 pre–messenger RNA by binding to a repressive splicing site within intron 7, resulting in exon 7 inclusion into the pre–messenger RNA transcript and consequent increased production of functional full‐length SMN protein. 9 , 10 , 11 , 12
Two pivotal phase 3 trials with intrathecal nusinersen in symptomatic individuals 13 , 14 demonstrated significant and clinically meaningful improvements in motor function and a favorable benefit‐risk profile. ENDEAR enrolled infants predicted to have SMA type I based on age of symptom onset and two SMN2 gene copies. CHERISH enrolled children predicted to have SMA type II or III based on older age at symptom onset. NURTURE is an additional, ongoing, open‐label study; interim data have shown substantial clinical benefits associated with nusinersen initiation before symptom onset. 15
Inclusion into ENDEAR or CHERISH depended on a specific combination of onset age and SMN2 copy number associated with a well‐characterized prognosis, enabling the most efficient trial design possible. Not all children with SMA fit into these groups, however. 4 , 16 EMBRACE was designed as a phase 2 study of nusinersen treatment of symptomatic infants and children with SMA who did not meet the eligibility characteristics of those primary trials. Assessing nusinersen in individuals with less well‐defined prognosis expands experience to a broader range of infants and children with SMA. The primary aim of EMBRACE was to determine safety, tolerability, and pharmacokinetics of nusinersen in this broader group, but EMBRACE also offered an opportunity to explore additional efficacy endpoints, including attainment of threshold changes on the Hammersmith Infant Neurological Examination (HINE) motor milestones scale.
2. METHODS
2.1. Study design and participants
EMBRACE (Clinical Trials No. NCT02462759) was a randomized, double‐blind, sham procedure–controlled, 14‐month study of intrathecal nusinersen treatment in children with genetically confirmed 5q SMA due to recessive disabling mutation of the SMN1 gene. The ENDEAR trial was halted because of demonstrable efficacy at a defined interim analysis. 13 As a consequence, the blinded portion of EMBRACE was also halted (data cutoff March 30, 2017), and eligible participants transferred to an amended open‐label protocol (termed part 2; see Appendix S1) for ~28 months (24 months of nusinersen and final follow‐up ~4 months after last dose; Figure S1). Participants could enter part 2 if they had completed the final part 1 evaluation and showed no significant change in clinical status that would make them unsuitable for trial participation, as determined by the investigator.
The EMBRACE inclusion criteria targeted three groups of infants and young children with SMA who were excluded from the pivotal ENDEAR and CHERISH trials: (1) three SMN2 gene copies with onset of clinical signs and symptoms consistent with SMA at 6 months of age or younger; (2) two SMN2 gene copies with onset of clinical signs and symptoms consistent with SMA at 6 months of age or younger, but greater than 7 months of age at screening; and (3) two or three SMN2 gene copies and onset of clinical signs and symptoms consistent with SMA at greater than 6 months of age, but 18 months of age or younger at screening. Full inclusion/exclusion criteria are given in the Appendix S1. Genetic testing was performed by Athena Diagnostics (Marlborough, MA), the central laboratory used to confirm the genetic diagnosis after enrollment.
The study was approved by institutional review boards at participating centers in accordance with the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. Written informed parental consent was obtained for all participants. An independent data safety monitoring board monitored the study (see Appendix S2).
2.2. Randomization and masking (part 1)
Participants were randomly assigned (2:1) to intrathecally administered nusinersen or sham procedure (control group). Randomization was stratified according to age at onset of clinical signs and symptoms consistent with SMA: 6 months or younger (infantile‐onset) vs older than 6 months of age (later‐onset). Sham procedure comprised conventional preparation for lumbar puncture with a skin‐depth single‐needle puncture at the site, without injection or intraspinal needle advancement, and subsequent bandage application. To maintain blinding, procedures were performed by a team unblinded to treatment.
Upon part 1 termination, key personnel were unblinded to facilitate evaluation assessment completion so that the sham procedure group could receive nusinersen as soon as possible in part 2, while the nusinersen group continued treatment.
2.3. Procedures
In part 1, participants received sham procedure or 12 mg nusinersen (scaled by age‐equivalent dose based on cerebrospinal fluid [CSF] volume scaling 17 ) by intrathecal lumbar puncture in four loading doses on days 1, 15, 29, and 64, followed by maintenance doses on days 183 and 302 (every 4 months).
In part 2, participants treated with nusinersen in part 1 continued to receive nusinersen every 4 months. For this group, day 1 of part 2 occurred ~4 months after the last nusinersen injection in part 1. Those randomized to sham procedure in part 1 initiated nusinersen in part 2 and received four loading doses on days 1, 15, 29, and 64, then maintenance doses every 4 months. For this group, day 1 of part 2 occurred as soon as possible after the final part 1 evaluation. Participants enrolled in part 2 had a final follow‐up evaluation of ~4 months after the last open‐label nusinersen dose.
Further details on administration and collecting samples for assessing nusinersen concentration and anti‐nusinersen antibodies are given in the Appendix S1.
2.4. Outcomes
Safety and tolerability of nusinersen vs sham procedure was the EMBRACE primary outcome. Assessments included the incidence of adverse events (AEs) and serious AEs, as well as change from baseline in clinical laboratory parameters (serum chemistry, hematology, and urinalysis), 12‐lead electrocardiograms, vital signs (resting systolic and diastolic blood pressure, pulse rate, respiratory rate, temperature, pulse oximetry, and transcutaneous carbon dioxide), and neurological examination outcomes (assessment of mental status, level of consciousness, sensory motor function, cranial nerve function, and reflexes). AEs were coded using the Medical Dictionary for Regulatory Activities version 20.0.
Nusinersen concentration in plasma and CSF were secondary endpoints, determined using an electrochemiluminescence method (PPD, Richmond, VA). An exploratory safety endpoint was development of plasma antibodies to nusinersen. Plasma phosphorylated neurofilament heavy‐chain (pNF‐H) levels in available samples (post hoc analyses) were measured as described elsewhere. 18 Exploratory efficacy endpoints were change from baseline in ventilator use (including bilevel positive airway pressure, tracheostomy, and endotracheal tube), measured as percentage of time on ventilatory support; attainment of motor milestones assessed by HINE Section 2 (HINE‐2) (developmental milestones) 19 , 20 , 21 ; change from baseline in growth parameters 22 ; and Clinical Global Impression of Change (CGI‐C) score.
The HINE‐2 documents motor function development with eight milestone items, and scores achievement on a range from 0 to 26, with higher scores indicating better motor function for which age norms are available. 13 , 20 , 21 The definition of motor milestone responders in EMBRACE was based on the HINE‐2 categories of kicking, head control, rolling, sitting, crawling, standing, and walking; the eighth item, voluntary grasp, was excluded because the task is difficult to scale in the setting of weakness. The prespecified response criteria were considered met if there was: (1) improvement in at least one category (ie, an increase in the score for head control, rolling, sitting, crawling, standing, or walking of ≥1 point; an increase in the score for kicking of ≥2 points; or achievement of the maximal score for kicking); and (2) more categories with improvement than categories with worsening (ie, a decrease in the score for head control, rolling, sitting, crawling, standing, or walking of ≥1 point or a decrease in the score for kicking of ≥2 points). Participants who died or withdrew from the study were counted as nonresponders. Measured growth parameters included body weight and length.
The CGI‐C is scored on a 7‐point ordinal scale from 1 (very much improved) to 7 (very much worse), and was completed independently by the investigator and caregiver. 23 Responders were defined (per S.A.P.) as much improved (score ≤2), any improvement (score ≤3), or no worsening (score ≤4).
2.5. Statistical analysis
EMBRACE aimed to enroll up to 21 participants. All individuals treated with at least one dose of nusinersen or sham procedure were included in the intention‐to‐treat and safety populations. The efficacy population was defined as the subset of participants in the safety set who had the opportunity to be assessed at each visit; in part 2, all participants in the safety and efficacy sets were assessed at the same visit, and hence these sets were identical. All participants who had at least one evaluable post‐nusinersen or post–sham‐procedure sample were included in the pharmacokinetic and immunogenicity populations.
Summary statistics for continuous and categorical endpoints were calculated with SAS version 9.4 or higher (SAS Institute, Inc, Cary, NC). The proportion of individuals who had a HINE‐2 motor milestone response was analyzed using the Wilson score method with continuity correction.
3. RESULTS
Between August 19, 2015 and December 20, 2016, 21 infants or children with SMA in seven centers were screened, randomized (14 to nusinersen, 7 to sham procedure), and underwent the assigned procedure in part 1 (Figure 1). When part 1 was unblinded and terminated, six participants (all in the nusinersen group) had completed the day 422 final follow‐up evaluation. Participants in the sham procedure group were asked to return as soon as possible; individual end‐of‐study visits for part 1 were completed and open‐label nusinersen treatment initiated in part 2. One individual with infantile‐onset SMA assigned to the sham procedure group died on day 289. In total, 20 participants (all 14 randomized to nusinersen, and 6 randomized to sham procedure) were enrolled into part 2.
FIGURE 1.
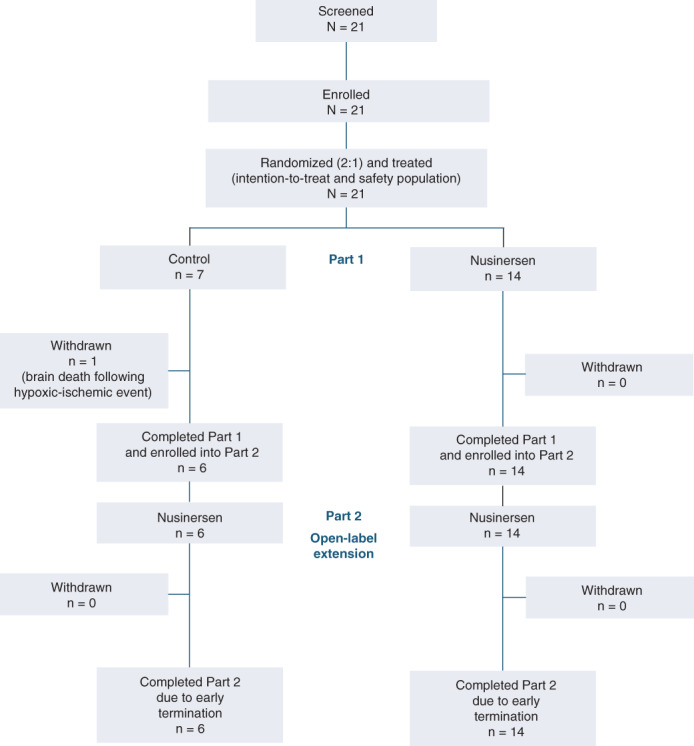
Participant disposition. Part 2 was terminated early to allow participants to transition to the SHINE study [Color figure can be viewed at wileyonlinelibrary.com]
Baseline demographics and disease‐related characteristics are summarized in Table 1. For the group that initiated nusinersen in part 2, baseline data are at initiation of nusinersen. Median age at first dose, sex, ethnicity, and SMN2 copy number varied between groups (Table 1), likely a consequence of the small sample size. All participants were unable to stand or walk at baseline.
TABLE 1.
Baseline demographics and clinical characteristics
| Characteristic | Initially randomized to sham procedure | Randomized to nusinersen (n = 14) | |
|---|---|---|---|
| Randomized to sham procedure in part 1 (n = 7) | Surviving, received nusinersen in part 2 (n = 6) | ||
| Infantile‐onset SMA, n (%) | 4 (57) | 3 (50) | 9 (64) |
| Later onset SMA, n (%) | 3 (43) | 3 (50) | 5 (36) |
| Female sex, n (%) | 5 (71) | 4 (67) | 5 (36) |
| Race, n (%) | |||
| White | 2 (29) | 2 (33) | 7 (50) |
| Asian | 3 (43) | 2 (33) | 2 (14) |
| Other | 1 (14) | 1 (17) | 1 (7) |
| Not reported | 1 (14) | 1 (17) | 4 (29) |
| Median (range) age at symptom onset, months | 5.1 (1.8‐11.0) | 6.0 (1.8‐11.0) | 5.5 (2.0‐11.0) |
| Median (range) age at SMA diagnosis, months | 12.0 (5.5‐14.0) | 12.5 (6.0‐14.0) | 10.0 (6.9‐15.0) |
| Median (range) age at first dose, months | 18.5 (15.3‐53.3) | 28.7 (24.5‐65.3) | 16.7 (7.3‐48.6) |
| SMN2 gene copy number, n (%) | |||
| 2 | 4 (57) | 3 (50) | 3 (21) |
| 3 a | 3 (43) | 3 (50) | 11 (79) |
| Median (range) weight, kg | 10.6 (7.6‐15.5) | 12.8 (10.8‐16.5) | 9.3 (7.2‐11.9) |
| Median (range) length, cm | 80.5 (76.1‐103.0) | 92.9 (85.0‐109.7) | 78.2 (71.0‐90.7) |
| Mean (SD) total HINE‐2 score | 5.9 ± 4.5 | 6.7 ± 5.0 | 7.6 ± 5.4 |
| Motor milestones ever achieved, n (%) b | |||
| Ability to sit without support | 3 (43) | — | 8 (57) |
| Ability to stand without support | 0 | — | 0 |
For one individual in the nusinersen group (age of onset 6 months or younger), central laboratory results were not clearly categorized, and records indicated that the individual had three SMN2 gene copies. For all analyses, this individual was considered to have three SMN2 gene copies.
These data do not reflect the maximal milestone achieved.
Abbreviations: HINE‐2, Hammersmith Infant Neurological Examination Section 2; SD, standard deviation; SMA, spinal muscular atrophy; SMN2, survival motor neuron 2.
Median (range) number of nusinersen doses administered overall was 10 (8‐12). Participants originally randomized to nusinersen received 10 to 12 doses in total, and those randomized to sham procedure who completed both parts of the study received 8 doses (Table S1). For the group initially randomized to nusinersen, median (interquartile range) total time (parts 1 and 2 combined) in the study was 995 (range, 890‐1010) days. For those randomized to sham procedure, the median (interquartile range) time in part 1 was 302 (230‐366) days, followed by nusinersen treatment in part 2 for 656 (653‐659) days.
3.1. Safety and tolerability
There were no AEs considered by investigators as related to study treatment and no study discontinuations due to nusinersen‐related AEs (Table 2). The most common AEs were consistent with conditions associated with childhood in general and SMA specifically (pyrexia, cough, pneumonia, upper respiratory tract infection; Table S2). Incidences of moderate or severe, severe, and serious AEs are shown in Table 2. In addition, the most common AE occurring within 72 hours after lumbar puncture was vomiting (noted in 4 of 14 [29%] of the participants treated with nusinersen in parts 1 and 2 vs 1 of 7 [14%] of participants in part 1 who were treated with sham procedure; vomiting was not observed in any of the participants initially receiving sham procedure who received nusinersen in part 2).
TABLE 2.
Summary of adverse events
| Randomized participants | Total nusinersen a (n = 20) | |||
|---|---|---|---|---|
| Randomized to sham procedure | Randomized to nusinersen (n = 14) | |||
| Randomized to sham procedure in part 1 (n = 7) | Surviving, received nusinersen in part 2 (n = 6) | |||
| Any AE b | 6 (86) | 6 (100) | 14 (100) | 20 (100) |
| Moderate or severe AE | 5 (71) | 5 (83) | 14 (100) | 19 (95) |
| Severe AE | 3 (43) | 2 (33) | 8 (57) | 10 (50) |
| AE related to study treatment | 0 | 0 | 0 | 0 |
| AE possibly related to study treatment | 0 | 0 | 2 (14) c | 2 (10) |
| Serious AE | 3 (43) | 4 (67) | 9 (64) | 13 (65) |
| AE leading to discontinuation | 1 (14) d | 0 | 0 | 0 |
| AE leading to withdrawal | 1 (14) d | 0 | 0 | 0 |
| Shift in ECG results e | ||||
| Shift to abnormal, not clinically significant f | 3/7 (43) | 1/5 (20) | 2/12 (17) | 3/17 (18) |
| Shift to abnormal, clinically significantf | 0/7 | 0/6 | 0/14 | 0/20 |
Note: Data expressed as number of AEs (%).
Nusinersen for the first time in part 2 and continuously in parts 1 and 2.
Number of individuals with an event.
Viral upper respiratory tract infection, n = 1; weight increased, n = 1.
One participant died due to brain death after a cardiopulmonary arrest.
Presented as number with a shift/number at risk. Number at risk is the number of individuals whose baseline value was not abnormal and who had ≥1 postbaseline value.
Shift to “abnormal, not clinically significant” included “unknown” or “normal” to “abnormal, not clinically significant.” Shift to “abnormal, clinically significant” included “unknown” or “normal” to “abnormal, not clinically significant” to “abnormal, clinically significant.” Participants could be counted twice in these rows.
Abbreviations: AE, adverse event; ECG, electrocardiogram.
There were no clinically significant nusinersen‐related changes or patterns in electrocardiograms, vital signs, clinical laboratory testing results, neurological examination, or physical examination. No participants developed hydrocephalus or liver failure, clinically meaningful thrombocytopenia or urinalysis results, or detectable antibodies against nusinersen during the study.
3.2. Nusinersen concentrations in CSF and plasma
In participants treated with nusinersen in both study parts, mean predose CSF concentration ranged from 3.9 ng/mL at day 15 to 11.32 ng/mL at day 898. In those originally randomized to sham procedure who received nusinersen during part 2, the mean range was 4.8 ng/mL at day 29 to 8.4 ng/mL at day 540 (Figure 2A). The predose CSF concentrations and concentration‐time profiles in participants with infantile‐ and later‐onset SMA were consistent with each other.
FIGURE 2.
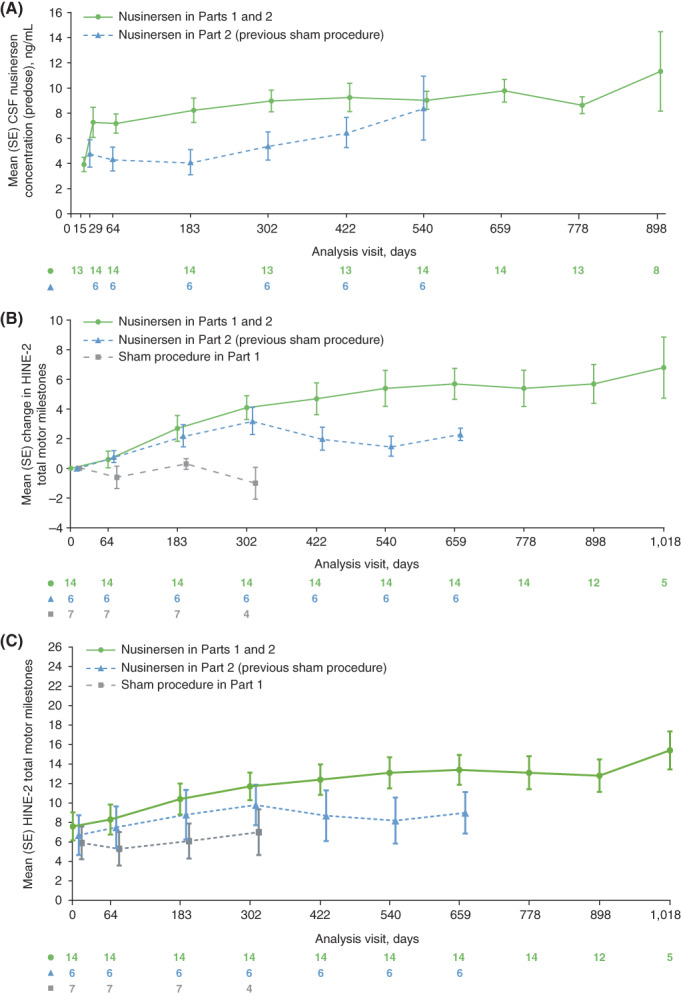
Mean (standard error) data for: (A) predose CSF nusinersen concentrations (ng/mL; CSF‐evaluable population), (B) change from baseline in HINE‐2 total milestones, and (C) HINE‐2 total milestones. In A, values considered as outliers were excluded from the analyses and included observed concentrations in day 1 predose samples. Only visits with five or more participants are included. In B and C, only visits with four or more participants are included. For the purposes of comparison, the group that initiated nusinersen in part 2 is graphed from day 0 (first nusinersen dose), and in B the initial baseline HINE‐2 score reset to 0. In all graphs, data at each timepoint are offset to allow visualization of all participant groups. CSF, cerebrospinal fluid; HINE‐2, Hammersmith Infant Neurological Examination Section 2; SE, standard error [Color figure can be viewed at wileyonlinelibrary.com]
Predose mean plasma nusinersen concentrations were low during multiple dosing (Figure S2). Plasma concentrations in participants with infantile‐onset and later‐onset SMA were similar.
3.3. Efficacy
In the group initially randomized to nusinersen 3 of 14 participants (21%) were on ventilation at baseline, compared with 4 of the 7 (57%) randomized to sham procedure. Overall mean percentage time on study on ventilator support (including bilevel positive airway pressure, intubation, tracheostomy, and endotracheal tube) was lower in participants treated with nusinersen in parts 1 and 2 (11.3%) than in those in the sham procedure group in part 1 (29.8%) or who initiated nusinersen in part 2 after initial assignment to the sham group (28.6%). However, overall percentage time on ventilator in the nusinersen group was also lower at baseline. No participants required a tracheostomy. The mean percentage of time on ventilator support was lower for individuals with later‐onset SMA (mean 0% for those treated with nusinersen in parts 1 and 2 [n = 5]; 11.1% for sham procedure in part 1 [n = 3]; 8.5% for nusinersen initiated in part 2 [n = 3]) compared with infantile‐onset SMA (mean 17.6% for those treated with nusinersen in parts 1 and 2 [n = 9]; 43.8% for sham procedure in part 1 [n = 4]; 48.6% for nusinersen initiated in part 2 [n = 3]).
Mean HINE‐2 total milestone scores over time are shown in Figure 2B,C, and details of HINE‐2 motor milestone responders stratified by age of SMA onset during part 1 are shown in Table 3. Of participants originally randomized to sham procedure two of seven (29%) were classified as HINE‐2 milestone responders at last available assessment in part 1, and five of six (83%) were classified as responders at last available assessment in part 2 where they received nusinersen. Of participants receiving nusinersen throughout the study, 13 of 14 (93%) were classified as responders (last available assessment). In the subgroup with infantile‐onset SMA, zero of four participants randomized to sham procedure in part 1, two of three (67%) who started nusinersen in part 2, and eight of nine (89%) treated with nusinersen in parts 1 and 2 were classified as responders (last available assessment). Among those with later‐onset SMA, two of three (67%) participants treated with sham procedure in part 1, three of three (100%) who started nusinersen in part 2, and five of five treated with nusinersen in parts 1 and 2 were classified as responders (last available assessment).
TABLE 3.
HINE‐2 motor milestone responders stratified by age of SMA onset a (part 1 only)
| Status | Age of SMA onset | |||||
|---|---|---|---|---|---|---|
| 6 months or younger | Over 6 months | All participants | ||||
| Sham procedure (n = 4) | Nusinersen (n = 9) | Sham procedure (n = 3) | Nusinersen (n = 5) | Sham procedure (n = 7) | Nusinersen (n = 14) | |
|
Achieved the following motor milestone improvements from baseline b | ||||||
| Ability to kick | ||||||
| ≥2‐point increase | 0 | 1 (11) | 0 | 1 (20) | 0 | 2 (14) |
| Toe touching c | 0 | 1 (11) | 0 | 1 (20) | 0 | 2 (14) |
| Head control (≥1‐point increase) | 0 | 4 (44) | 0 | 1 (20) | 0 | 5 (36) |
| Rolling (≥1‐point increase) | 0 | 6 (67) | 0 | 3 (60) | 0 | 9 (64) |
| Sitting (≥1‐point increase) | 0 | 5 (56) | 1 (33) | 4 (80) | 1 (14) | 9 (64) |
| Crawling (≥1‐point increase) | 0 | 0 | 1 (33) | 3 (60) | 1 (14) | 3 (21) |
| Standing (≥1‐point increase) | 0 | 0 | 2 (67) | 2 (40) | 2 (29) | 2 (14) |
| Walking (≥1‐point increase) | 0 | 0 | 0 | 1 (20) | 0 | 1 (7) |
| Demonstrated improvement in more motor milestone categories than worsening | 0 | 7 (78) | 2 (67) | 4 (80) | 2 (29) | 11 (79) |
| HINE‐2 motor milestone responder | 0 | 7 | 2 | 4 | 2 | 11 |
| Proportion (95% CI) d | 0 (0.00‐0.60) | 0.78 (0.45‐0.94) | 0.67 (0.21‐0.94) | 0.80 (0.38‐0.96) | 0.29 (0.08‐0.64) | 0.79 (0.52‐0.92) |
Note: Data expressed as number (%) unless otherwise noted.
Abbreviations: CI, confidence interval; HINE‐2, Hammersmith Infant Neurological Examination Section 2; SMA, spinal muscular atrophy.
Individuals with the opportunity for at least a 6‐month (day 183) assessment were included.
Individuals with a 6‐month (day 183), 10‐month (day 302), or 14‐month (day 422) assessment. The last available assessment was used for this analysis.
Toe touching is the highest milestone in the category “Ability to kick.”
Wilson score CI with continuity correction.
Mean weight, body length, head circumference, and chest circumference increased over time for participants in all groups. Weight‐for‐age percentile, length‐for‐age percentile, and head‐to‐chest circumference ratios were similar between treatment groups and appeared to show decreased growth rates at later timepoints. Mean (standard deviation) changes from baseline to day 183 in body length and weight were 4.5 (4.9) cm and 0.6 (0.6) kg for the group treated with sham procedure in part 1 (n = 7), from baseline to day 659 were 10.8 (4.12) cm and 3.1 (1.4) kg for nusinersen in part 2 (n = 6), and from baseline to day 898 were 17.2 (5.6) cm and 3.9 (2.9) kg for nusinersen in parts 1 and 2 (n = 12). Results of CGI‐C investigator and caregiver assessments at the last observed visit indicated that a higher proportion of participants initially treated with nusinersen were rated as having improved, compared with those initially treated with sham procedure (Figure S3).
3.4. Biomarker analyses (part 1 data)
In participants treated with nusinersen, plasma pNF‐H levels declined rapidly during the loading dose period, then stabilized. A general and less rapid decline in pNF‐H levels was observed in participants treated with sham procedure (Figure 3). This pattern was followed by the majority of participants, independent of age of SMA onset (Figure S4A). The decrease in plasma pNF‐H levels was accompanied by an increase in change in HINE‐2 total score (Figure S4B).
FIGURE 3.
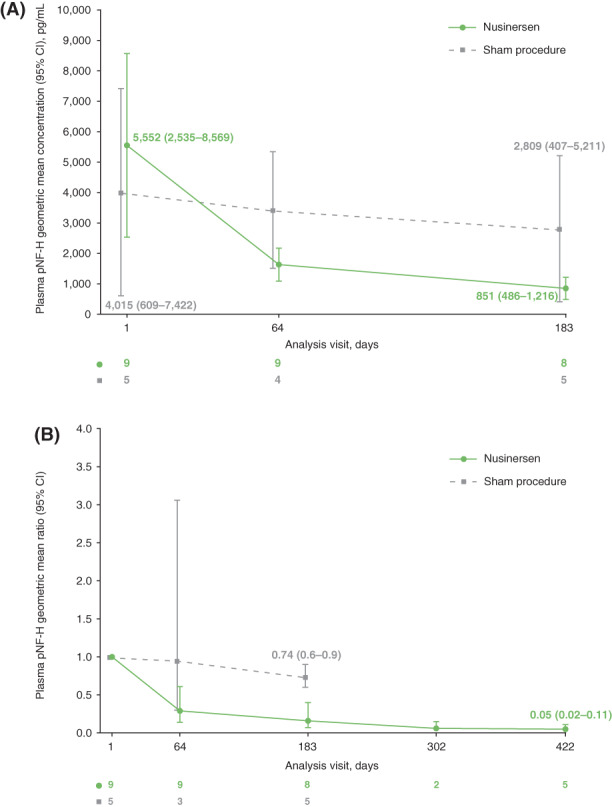
Plasma pNF‐H levels over time in part 1 of the study: (A) geometric mean (95% CI) and (B) geometric mean ratio (95% CI). One participant in the sham procedure group had a pNF‐H level below the limit of quantification at day 64 that was not included in the graph. CI, confidence interval; pNF‐H, phosphorylated neurofilament heavy chain [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Nusinersen demonstrated a favorable benefit‐risk profile in the unique population of symptomatic infants and children who were ineligible for participation in ENDEAR 13 and CHERISH. 14 No new nusinersen‐related safety concerns were identified over a mean 2.4 years. Exploratory efficacy outcomes demonstrated functional improvement, despite the small sample size and shortened part 1 trial duration that undermined the power of the study to demonstrate such treatment effects at a significant level. These findings parallel the positive findings from ENDEAR and CHERISH 13 , 14 and support extending the recommended use of nusinersen for all infants/children with SMA, not just those fulfilling the ENDEAR and CHERISH enrollment criteria.
The most common AEs during nusinersen treatment included infections and respiratory events, consistent with events typically observed in children with SMA. 12 The incidence of vomiting, a known complication of lumbar puncture and sedation, 24 was higher in the total nusinersen group than in the sham procedure group, in which participants did not undergo lumbar puncture (Table S2), but was not seen in those initiating unblinded treatment in part 2 after initial randomization to the sham group. The incidence of back pain and headache, two other lumbar puncture complications, 24 , 25 , 26 was low (<20% of the total group), although ascertainment may be concealed by the young age of the participants. A higher incidence of pyrexia was observed in nusinersen‐treated participants overall, compared with those treated with sham procedure; this increase was not observed in the larger ENDEAR study (56% with nusinersen vs 59% for sham procedure). 13
There was also no evidence to warrant concern regarding changes in platelet counts or renal AEs, both of which have been suggested in earlier small studies of 2′‐O‐methoxyethyl–modified antisense oligonucleotides 27 , 28 directed to other targets.
All efficacy analyses in this study were positive for participants receiving nusinersen, and were consistent with results from previous nusinersen studies in individuals treated after symptom onset. 12 , 13 , 14 Overall, participants treated with nusinersen throughout the study required less ventilator support than those in the sham procedure group and those treated with nusinersen in part 2, although it should be noted that the group randomized to nusinersen required less ventilator use at baseline. All participants achieved and maintained HINE‐2 motor milestones throughout the study and at last available assessment, all but one participant treated with nusinersen in both parts of the study met HINE‐2 motor milestone response criteria, independent of age at SMA onset. These results are consistent with previous studies reporting progressive improvement in motor function over time with nusinersen treatment, as measured by HINE‐2, and a significant change compared with baseline. 12 , 13
Measurement of predose concentrations in CSF showed a different profile of increasing CSF nusinersen concentrations between groups: the concentration in those initially randomized to nusinersen increased to day 29 and remained stable thereafter; in those who initiated nusinersen in part 2, concentrations increased to day 540. This may be because of the small number of participants, especially in the group initiating nusinersen in part 2 after initial assignment to the sham group, which makes it difficult to draw definite conclusions. The 4‐monthly dosing regimen used in this study is currently approved for all patients with SMA in most locations, 7 , 8 and differs from the less frequent dosing regimen used in CHERISH in children with later‐onset SMA. 14 This 4‐monthly nusinersen regimen was found to have an acceptable risk‐benefit profile in participants with both infantile‐ and later‐onset SMA, as shown by meaningful efficacy without concern for compromised safety or tolerability.
In this broad population of individuals with infantile‐ and later‐onset SMA, plasma pNF‐H levels declined during the loading phase of nusinersen treatment and then appeared to stabilize. This finding is consistent with previous studies in participants with infantile‐onset SMA and those who were presymptomatic. 15 , 18
In conclusion, the EMBRACE study findings support the use of nusinersen administered as maintenance doses every 4 months among a broad population of infants and children with SMA. Of the 20 participants who completed EMBRACE, all have enrolled in the comprehensive SHINE protocol (NCT02594124), which combines participants from earlier nusinersen clinical trials for long‐term follow‐up.
CONFLICT OF INTEREST
G.A. has participated in advisory boards for AveXis/Novartis, Biogen, Genentech, and Sarepta; and has participated in consultation and speaker engagements for Biogen. T.O.C. has been an advisor/consultant for AveXis/Novartis, Biogen, Catalyst, Cure SMA, Cytokinetics, Marathon, Novartis, Roche, Sarepta, and the SMA Foundation. W.M.‐F. has participated in advisory boards for AveXis/Novartis, Biogen, PTC, Roche, and Sarepta; has received honoraria from AveXis/Novartis, Biogen, and PTC; has received research funding from BMBF; and has clinical trial research contracts with Biogen, Santhera, and Sarepta. P.B.S. has participated in advisory boards for Biogen and, outside of the submitted work, has participated in advisory boards for AveXis/Novartis, PTC, and Sarepta. R.R. has participated in advisory boards for AveXis/Novartis and Biogen, and has clinical trial research contracts with Biogen. N.N. has received research funding from Biogen for execution of clinical trial projects and National Institutes of Health funding for site principal investigator work on the HEAL‐EEG study. D.C. has participated in advisory board for AveXis/Novartis, Biogen, PTC, and Sarepta; has received research funding from AveXis/Novartis, Biogen, FibroGen, PTC, ReveraGen, and Sarepta; and has participated in medical advisory boards for GBS‐CIDP Foundation, Myasthenia Gravis Foundation, and the Cure SMA Care Center Network. D.R.‐S., G.G., P.S., and W.F. are employees of and hold stock/stock options in Biogen.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
APPENDIX S1 Supplemental methods.
APPENDIX S2 Supporting information.
FIGURE S1 EMBRACE study design. aRandomization was stratified by age at symptom onset: >6 months vs ≤6 months. bParticipants randomized to sham procedure in part 1 received four loading doses of nusinersen in part 2 on days 1, 15, 29, and 64, then maintenance doses every 4 months. cIndividuals eligible for ENDEAR (symptom onset at age ≤6 months, age ≤7 months at screening, 2 SMN2 copies) or CHERISH (symptom onset at age >6 months, age 2‐12 years at screening) were not eligible for EMBRACE. D, day; SMA, spinal muscular atrophy; SMN2, survival motor neuron 2.
FIGURE S2 Mean (SE) predose plasma nusinersen concentration (ng/mL; pharmacokinetic analysis population). Values considered as outliers were excluded from the analyses and included observed concentrations in day 1 predose samples. Only visits with five or more participants are included. Data at each timepoint are offset to allow visualization of all participant groups. SE, standard error
FIGURE S3 Summary of investigator and caregiver CGI‐C at last observed visit. Responder definitions were based on a 7‐point ordinal scale: much improved, score ≤2; any improvement, score ≤3; and no worsening, score ≤4. Hence, a “much improved” responder was also counted as having any improvement and no worsening (categories overlap). The reference point was baseline (start of part 1) for the sham procedure in part 1 group; baseline (start of part 1) for the nusinersen in parts 1 and 2 group; and the start of part 2 for the nusinersen in part 2 (previous sham procedure) group. CGI‐C, Clinical Global Impression of Change.
FIGURE S4 (A) Plasma pNF‐H levels over time in part 1 of the study by treatment, and (B) correlation between plasma pNF‐H levels and HINE‐2 total score. One participant in the sham procedure group had a pNF‐H level below the limit of quantification at day 64 that was not included in the graphs. HINE‐2, Hammersmith Infant Neurological Examination Section 2; pNF‐H, phosphorylated neurofilament heavy chain; SMN2, survival motor neuron 2.
TABLE S1 Number of doses received and time on study in the safety‐evaluable population
TABLE S2 Common adverse events (≥20% in the total group)a
ACKNOWLEDGMENTS
Information about the study oversight is provided in the Appendix S2. Ionis Pharmaceuticals, Inc (Carlsbad, CA), was the study sponsor in the United States and Biogen was the sponsor in the rest of the world. Biogen provided funding for medical writing support in the development of this article. Becky Ayles PhD (Excel Scientific Solutions) wrote the first draft of the manuscript based on input from the authors, and Nathaniel Hoover (Excel Scientific Solutions) copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the manuscript to the authors. The authors had full editorial control of the manuscript and provided their final approval of all content.
Acsadi G, Crawford TO, Müller‐Felber W, et al. Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. Muscle & Nerve. 2021;63:668–677. 10.1002/mus.27187
Funding informationBiogen (Cambridge, MA); Ionis Pharmaceuticals, Inc (Carlsbad, CA).
DATA AVAILABILITY STATEMENT
Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com).
REFERENCES
- 1. Crawford TO , Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996;3:97‐110. [DOI] [PubMed] [Google Scholar]
- 2. Markowitz JA, Singh P, Darras BT. Spinal muscular atrophy: a clinical and research update. Pediatr Neurol. 2012;46:1‐12. [DOI] [PubMed] [Google Scholar]
- 3. Finkel R, Bertini E, Muntoni F, Mercuri E, Workshop Study ENMCSMA. Group. 209th ENMC international workshop: outcome measures and clinical trial readiness in spinal muscular atrophy 7‐9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord. 2015;25:593‐602. [DOI] [PubMed] [Google Scholar]
- 4. Russman BS. Spinal muscular atrophy: clinical classification and disease heterogeneity. J Child Neurol. 2007;22:946‐951. [DOI] [PubMed] [Google Scholar]
- 5. Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16:265‐269. [DOI] [PubMed] [Google Scholar]
- 6. Feldkötter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real‐time LightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70:358‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Medicines Agency . Spinraza 12 mg solution for injection. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004312/WC500229704.pdf. Accessed June 30, 2020.
- 8.Biogen. Spinraza (nusinersen) injection, for intrathecal use. 2020. https://www.spinraza.com/content/dam/commercial/specialty/spinraza/caregiver/en_us/pdf/spinraza-prescribing-information.pdf. Accessed June 30, 2020.
- 9. Hua Y, Sahashi K, Hung G, et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010;24:1634‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Passini MA, Bu J, Richards AM, et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med. 2011;3:72ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a phase 1 study of nusinersen (ISIS‐SMNRx) in children with spinal muscular atrophy. Neurology. 2016;86:890‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile‐onset spinal muscular atrophy with nusinersen: a phase 2, open‐label, dose‐escalation study. Lancet. 2016;388:3017‐3026. [DOI] [PubMed] [Google Scholar]
- 13. Finkel RS, Mercuri E, Darras BT, et al. ENDEAR Study Group. Nusinersen versus sham control in infantile‐onset spinal muscular atrophy. N Engl J Med. 2017;377:1723‐1732. [DOI] [PubMed] [Google Scholar]
- 14. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later‐onset spinal muscular atrophy. N Engl J Med. 2018;378:625‐635. [DOI] [PubMed] [Google Scholar]
- 15. De Vivo DC, Bertini E, Swoboda KJ, et al. NURTURE Study Group. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019;29:842‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harada Y, Sutomo R, Sadewa AH, et al. Correlation between SMN2 copy number and clinical phenotype of spinal muscular atrophy: three SMN2 copies fail to rescue some patients from the disease severity. J Neurol. 2002;249:1211‐1219. [DOI] [PubMed] [Google Scholar]
- 17. Matsuzawa J, Matsui M, Konishi T, et al. Age‐related volumetric changes of brain gray and white matter in healthy infants and children. Cereb Cortex. 2001;11:335‐342. [DOI] [PubMed] [Google Scholar]
- 18. Darras BT, Crawford TO , Finkel RS, et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann Clin Transl Neurol. 2019;6:932‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frisone MF, Mercuri E, Laroche S, et al. Prognostic value of the neurologic optimality score at 9 and 18 months in preterm infants born before 31 weeks' gestation. J Pediatr. 2002;140:57‐60. [DOI] [PubMed] [Google Scholar]
- 20. Bishop KM, Montes J, Finkel RS. Motor milestone assessment of infants with spinal muscular atrophy using the Hammersmith Infant Neurological Exam—part 2: experience from a nusinersen clinical study. Muscle Nerve. 2018;57:142‐146. [DOI] [PubMed] [Google Scholar]
- 21. Haataja L, Mercuri E, Regev R, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135:153‐161. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . WHO child growth standards. 2006. https://www.who.int/childgrowth/standards/Technical_report.pdf. Accessed June 29, 2020.
- 23. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28‐37. [PMC free article] [PubMed] [Google Scholar]
- 24. Haché M, Swoboda KJ, Sethna N, et al. Intrathecal injections in children with spinal muscular atrophy: nusinersen clinical trial experience. J Child Neurol. 2016;31:899‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ebinger F, Kosel C, Pietz J, Rating D. Headache and backache after lumbar puncture in children and adolescents: a prospective study. Pediatrics. 2004;113:1588‐1592. [DOI] [PubMed] [Google Scholar]
- 26. Morgenlander JC. Lumbar puncture and CSF examination. Answers to three commonly asked questions. Postgrad Med. 1994;95:125‐128. [PubMed] [Google Scholar]
- 27. Crooke ST, Baker BF, Witztum JL, et al. The effects of 2'‐O‐methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther. 2017;27:121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crooke ST, Baker BF, Pham NC, et al. The effects of 2'‐O‐methoxyethyl oligonucleotides on renal function in humans. Nucleic Acid Ther. 2018;28:10‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supplemental methods.
APPENDIX S2 Supporting information.
FIGURE S1 EMBRACE study design. aRandomization was stratified by age at symptom onset: >6 months vs ≤6 months. bParticipants randomized to sham procedure in part 1 received four loading doses of nusinersen in part 2 on days 1, 15, 29, and 64, then maintenance doses every 4 months. cIndividuals eligible for ENDEAR (symptom onset at age ≤6 months, age ≤7 months at screening, 2 SMN2 copies) or CHERISH (symptom onset at age >6 months, age 2‐12 years at screening) were not eligible for EMBRACE. D, day; SMA, spinal muscular atrophy; SMN2, survival motor neuron 2.
FIGURE S2 Mean (SE) predose plasma nusinersen concentration (ng/mL; pharmacokinetic analysis population). Values considered as outliers were excluded from the analyses and included observed concentrations in day 1 predose samples. Only visits with five or more participants are included. Data at each timepoint are offset to allow visualization of all participant groups. SE, standard error
FIGURE S3 Summary of investigator and caregiver CGI‐C at last observed visit. Responder definitions were based on a 7‐point ordinal scale: much improved, score ≤2; any improvement, score ≤3; and no worsening, score ≤4. Hence, a “much improved” responder was also counted as having any improvement and no worsening (categories overlap). The reference point was baseline (start of part 1) for the sham procedure in part 1 group; baseline (start of part 1) for the nusinersen in parts 1 and 2 group; and the start of part 2 for the nusinersen in part 2 (previous sham procedure) group. CGI‐C, Clinical Global Impression of Change.
FIGURE S4 (A) Plasma pNF‐H levels over time in part 1 of the study by treatment, and (B) correlation between plasma pNF‐H levels and HINE‐2 total score. One participant in the sham procedure group had a pNF‐H level below the limit of quantification at day 64 that was not included in the graphs. HINE‐2, Hammersmith Infant Neurological Examination Section 2; pNF‐H, phosphorylated neurofilament heavy chain; SMN2, survival motor neuron 2.
TABLE S1 Number of doses received and time on study in the safety‐evaluable population
TABLE S2 Common adverse events (≥20% in the total group)a
Data Availability Statement
Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com).


