Abstract
Emergent biomarkers for Alzheimer's disease (AD) are expected to provide earlier and more precise diagnoses. However, even if biomarkers live up to these expectations, it cannot be taken for granted that patients actually would value an earlier and more precise AD diagnosis. Based on an interview study, we aim to give more insight into the value of an AD diagnosis for patients, in existing as well as future practices, by describing how a diagnosis enables or may enable knowing, foreseeing, and acting in relation to one's illness. Our findings show that how people with AD value a diagnosis is not only characterised by great variety, as previous studies have shown, but also by profound ambivalence for the individual. With lack of treatment and poor prognostics as the status quo, this ambivalence and the way people deal with it are particularly linked to the far‐from‐straightforward capacity of an AD diagnosis to support anticipation of the future. We argue that in otherwise unchanged practices the envisioned future biomarker‐based diagnostics are unlikely to reduce the ambivalence about receiving an AD diagnosis and, in particular, the challenges of anticipation that it entails. Rather, biomarker‐based innovations may even reinforce some of the main issues involved.
Keywords: Alzheimer's disease, ambivalence, biomarkers, diagnosis, value
INTRODUCTION
Research into AD biomarkers has abounded in the last decades. Such biomarkers have typically been put forward as promising tools for establishing early diagnosis—with early diagnosis often presented as a crucial condition to procure a drug treatment for AD (Boenink, 2016; Lock, 2013b; Whitehouse, 2016). Along the way, the functions ascribed to AD biomarkers have multiplied, ranging from prediction, early detection, increased diagnostic precision, monitoring disease progression, prognostics, therapy selection to monitoring of effectiveness. At the same time, the notion “early diagnosis” has become rather ambiguous, being applied to both pre‐symptomatic prediction and diagnosis of symptomatic individuals, partly due to the introduction of the label “pre‐clinical AD”, which can be assigned on the basis of biomarker test results alone, even though this label was intended for research settings only (Boenink, 2018). Biomarker tests for pre‐symptomatic prediction have been announced repeatedly, but never really touched ground (Lock, 2013a; Whitehouse, 2016). Such announcements tend to invite both enthusiasm and concerns among journalists, patients and caregivers, and have triggered social science and ethical research on the desirability of pre‐symptomatic prediction (e.g., Boenink et al., 2016; Bunnik et al., 2018; Schicktanz et al., 2014). In comparison, biomarker promises to diagnose individuals with complaints earlier or more precisely (e.g., Blennow & Zetterberg, 2018; Okamura et al., 2018; Palmqvist et al., 2020; Sun et al., 2018) look much less dramatic. They hardly ever make it to the media headlines—or only do so by being associated or confused with pre‐symptomatic prediction. Likewise, social sciences and humanities research focusing on AD biomarkers for earlier and more precise diagnosis is lacking.
This paper aims to address this gap. After all, even though potential biomarkers for earlier and/or more precise diagnosis of symptomatic individuals may seem less controversial than biomarkers for prediction of future disease, their added value for patients should not be taken for granted (Porteri et al., 2017; Vanderschaeghe et al., 2018). As in pre‐symptomatic testing, the presumed desirability of an earlier and/or more precise diagnosis typically rests on a presumed link between knowing and being able to act. Yet, in the absence of effective drug treatment it is not clear whether, and how, an earlier and/or more precise diagnosis would enable actions different from the ones allowed by a diagnosis in current practices. This raises the basic questions: what work (Bowker & Star, 2000; Jutel, 2009) would an earlier and/or more precise diagnosis do? And would this work be valuable for those receiving the diagnosis?
We have explored these questions by inviting people newly diagnosed with AD to reflect on current diagnostic practices, as well as on the potential value of an earlier and/or more precise diagnosis. In doing so, we built on previous studies of how people with dementia experience receiving a diagnosis that together highlight the great diversity in how patients value current diagnostic practices (see the following section). However, exploring how patients might value changes that could be brought about by emerging diagnostic tools, we had to go beyond collecting current experiences, and facilitate reflections on future scenarios, thus combining a mapping with an anticipatory approach. So far, only few anticipatory studies with AD patients have been done and, to our knowledge, only in relation to emergent pre‐symptomatic diagnostics (van der Burg et al., 2019; Wikler et al., 2013).
Our study originated in the context of a biomedical research project investigating a particular technology: tau‐PET imaging. While initially mainly heralded for its potential for research into disease mechanisms, this technology is also surrounded by promises of clinical utility: in the first instance, to enable earlier and more precise AD diagnostics (Villemagne et al., 2015), even if primarily in highly specialised settings. Whether tau‐PET imaging and other emergent markers will live up to such promises is still an open question. However, even if the answer turns out to be affirmative we should realise that clinical utility of AD biomarkers is often established using narrowly framed outcome measures (e.g., as diagnostic accuracy or as increase in clinician confidence). As a result, clinical utility of novel tests definitely does not suffice to guarantee patient benefit. Taking into account patients’ concerns and valuations of diagnosis is necessary to establish such benefits.
FIRST‐PERSON PERSPECTIVES ON AD DIAGNOSTICS
While long a blind spot in science, policymaking and media alike (Moore & Hollett, 2003), the perspectives of people with AD (and, more broadly, dementia) have increasingly been brought to the fore (Bethell et al., 2018; Hillman et al., 2018; Pickett & Murray, 2018). Continuing the heritage of scholars such as Kitwood and Bredin (1992) who already in the late 1980s developed more inclusive accounts of people with dementia, applied studies on dementia care services and interventions have been forerunners (e.g., Bamford & Bruce, 2000), whereas qualitative studies of dementia diagnostics, regrettably, have tended to treat patient perspectives more indirectly. Many studies focus on disclosure and testing practices (for an overview, see Werner et al., 2013) and often rely on observational methods (e.g., Gjødsbøl & Svendsen, 2018; Xanthopoulou et al., 2019), surveys or interviews with clinicians (e.g., Bailey et al., 2019; Karnieli‐Miller et al., 2007), and/or interviews with caregivers (e.g., Connell et al., 2004; Laakkonen et al., 2008). Studies of patients’ firsthand accounts of receiving a dementia diagnosis have been less common (for an earlier overview, see Bunn et al., 2012; Robinson et al., 2011).
Recently, however, important insights have been produced into the wide range of reactions that people have to a dementia diagnosis, looking either at patients’ experience of testing and disclosure practices (e.g., Karnieli‐Miller et al., 2012; van der Laan, 2016; Visser et al., 2019) or more broadly at the emotional impact of being diagnosed with dementia (e.g., Aminzadeh et al., 2007; Beard, 2016; Greenwood & Smith, 2016; MacRae, 2008; Portacolone et al., 2018; Pratt & Wilkinson, 2003; Robinson, Clare & Evans, 2005; Vernooij‐Dassen et al., 2006). This body of literature charts experiences in terms of a diagnosis’ “effects”, “implications”, or “impact”. It also indicates how patients evaluate these impacts, for example listing relief, clarity, explanation and access to care as benefits, and distress, limitation/stigma and feeling powerless as disadvantages of diagnosis. However, this literature usually does not analyse which values might lead to such evaluations. Van der Laan (2016) takes a first step, though, by specifically analysing the “goods” pursued in diagnostic practices (by patients, caregivers and clinicians) and distinguishing five “values” of diagnosis (causal explanation, describing functionality, prognosis, control and living with).
In addition, there is a growing body of literature on the ethics and potential (clinical) value of emergent pre‐symptomatic AD diagnostics. This literature has mainly discussed clinicians’, researchers’ and ethicists’ perspectives, with patient concerns only indirectly represented (e.g., Boenink et al., 2016; Bunnik et al., 2018; Schermer & Richard, 2019; Schicktanz et al., 2014; Schweda et al., 2018; Swallow, 2017). However, a few studies have directly involved (future) patients. By means of a survey, Wikler et al. (2013) map public attitudes towards early (pre‐symptomatic) testing for AD, showing a generally positive attitude among these possible future patients (see also Caselli et al., 2014). Van der Burg et al. (2019) explore the potential value of pre‐symptomatic AD diagnostics in more depth, from the perspective of a wider public, as well as current patients and caregivers. They find that while (future) patients and caregivers hold a broad range of sentiments towards pre‐symptomatic testing, they all base their valuation on whether a diagnosis would enable one to care—for oneself, another, and society. It is as yet unclear, however, whether patients have similar considerations regarding diagnostics targeting those already experiencing symptoms.
Building on and moving forward from this body of literature, we therefore set out to explore how patients value an AD diagnosis in current and in future practices that, specifically, would involve earlier and/or more precise diagnoses.
METHODS
Our approach to involving patients in the assessment of emergent diagnostics is constructive and practice‐based (see also Nielsen & Boenink, 2020). We start from the assumption that values and voices are not just “there” for researchers to collect, but are (re‐)constructed in relation to specific situations and contexts and this is even more the case when the object of reflection is a technology that is only emerging (Kiran et al., 2015; Swierstra et al., 2009). Secondly, we view values as “the evolving results of valuing processes” (Boenink & Kudina, 2020). We therefore did not ask which values patients hold, but rather how they value diagnosis. As an unintended benefit, such an approach overcomes the challenge of identifying the “real values and preferences” of people with dementia. Whether people with dementia can be said to “still be themselves” and, thus, hold their “authentic” values, is contested. Even if the answer is positive, they may have difficulty recollecting and reporting these values. If, in contrast, we understand values as evolving and focus on the process of valuation, the question becomes whether people with dementia still have the ability to value. Jaworska (1999) has made a strong argument that this ability remains—even if the voices of people with dementia are rather “subtle” (Nielsen & Boenink, 2020).
Setting
The setting of our study defined the specific practices, current and envisioned, which we invited participants to value. The participants were all diagnosed in a Dutch academic hospital. In this setting, an AD diagnosis is often preceded by years of experiencing symptoms and seeking help and ultimately based on a wide range of information and tests. Notwithstanding these complex diagnostics, however, the AD diagnosis provided is still not certain but probable, has low specificity, and does not allow for prognostication.
In contrast, biomarker visions such as those of the tau‐PET project we engaged with sketch future diagnostic practices that, first of all, enable the diagnosis to be made earlier, upon the very first symptoms. This suggests that the diagnostic trajectory would be shorter than the one most participants experienced. Moreover, whereas biomarkers are currently just one piece of the diagnostic puzzle, emergent markers such as tau‐PET are expected to enable a certain AD diagnosis in vivo. Secondly, tau‐PET in particular is envisioned to enable more precise diagnoses by bringing about new disease (sub‐)categories. In addition, tau‐PET is thought to, down‐the‐line, facilitate prognostication on the level of sub groups (Sun et al., 2018).
Interviews
Following explorative fieldwork, we conducted either one or two interviews with 15 patients (22 interviews in total). First, we explored the participants’ experiences of current diagnostic practices by means of a semi‐structured interview guide. Subsequently, either in the last part of the interview or in a subsequent interview, we used conversational cards (Figure 1) to stimulate participants to consider and value current and potential future practices. The cards were developed to guide participants’ imagination of potential futures in an open way by inviting responses to promises and concerns from different perspectives (for further descriptions of this ethical deliberation method, see Boenink et al., 2018; Felt et al., 2014; Nielsen & Boenink, 2020). The cards were, moreover, particularly useful to the specific participant group, suffering cognitive impairment, helping them maintain focus and relate to abstract issues. Because our methods evolved during the study, a few participants (n = 5) were only subjected to classic interviewing, not the card method. However, all participants were stimulated to value future diagnostic practices. The interviews took place in the patients’ homes (n = 16) or in the clinic (n = 6). A majority of the participants (n = 10) were accompanied by an informal caregiver, typically a spouse.
FIGURE 1.
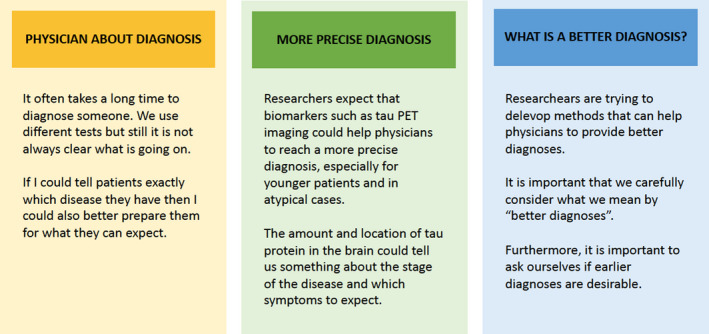
Example conversational cards, translated from Dutch into English
All participants had received an AD diagnosis (n = 12) or the diagnosis “MCI due to AD” (n = 3) within two years before the interview. Due to the specific patient population at the clinic of recruitment, most participants were suffering relatively early‐onset dementia, with their age at the time of interview ranging from 57 to 77. Men and women were almost equally represented (8/7). Often, informal caregivers would play a significant role in the interviews. They not only assisted patients with recollecting facts and formulating answers, but also joined in reflection and valuation. In the analysis underlying this paper, however, we focused on patients’ perspectives, while bearing in mind that a complete separation of patient and caregiver perspectives is not possible. Elsewhere, we reflect on this issue (Nielsen & Boenink, 2020).
Involving people with dementia in qualitative research, and interviews in particular, requires a very sensitive and dynamic approach (e.g., Cridland et al., 2016; Hellström et al., 2007). Among other things, we strived to continuously seek consent/assent; balance the voices of patients and caregivers, respectively; and pay close attention to the impact of the interview in the process. Ethical approval was obtained prior to the study from the participating institutions (a university and an academic hospital).
Analysis
With all interviews completed, we did a rough coding of the interviews in which we identified the passages of particular relevance for the question guiding this paper: how people with dementia value diagnosis in current and envisioned practices. We then analysed the passages, coding these inductively. Supported by memos written right after each interview, we could then start mapping and synthesising a multitude of perspectives. For further organising and exploring these, we found the three overall categories of value proposed by van der Laan (2016) useful. The epistemic value concerns how a diagnosis allows one to “know”, that is how it answers the question “what is the matter?”. The predictive value concerns how a diagnosis allows one to foresee “what will happen?”. The directive value concerns how a diagnosis allows one to act and, thus, answer the question “what to do?”. We then did another round of coding in which we linked the many topics that had already emerged to these value categories and, for each of them, identified contrasts between perspectives on current and potential future practices. Our analytical process, thus, combined an inductive with a deductive approach, resulting in a comprehensive “map” of how participants valued diagnosis in current and future practices (Table 1). During the process of mapping and (re‐)reading key passages, one common theme emerged. In the following sections, we take the reader through this map by presenting the three overall types of value one by one and, for each, demonstrating the central topics and tensions expressed by the participants in relation to diagnosis, firstly, in current practices and, subsequently, in potential future practices. Gradually, we carve out the common denominator in the participants’ valuations of diagnosis.
TABLE 1.
The value of a diagnosis in current and envisioned future practices
| Current practices | Earlier diagnosis | More precise diagnosis | ||||
|---|---|---|---|---|---|---|
| + | − | + | − | + | − | |
| Epistemic value | Clarity that hurts | Clarity, and then what? | Clarity of what? | |||
| Informative aspect | Gives certainty | Provides no real news | Gives certainty earlier | Provides harsh news | Could provide “good” news | Could provide “bad” news |
| Emotional aspect | Gives relief | Causes distress | (no relief) | Causes distress earlier | Gives relief | Causes distress |
| Social aspect | Provides an explanation | Causes stigma | Provides some explanation | Causes stigma earlier | Provides more explanation | Causes stigma |
| Predictive value | Filling in the blanks | More blanks to fill? | Less blanks to fill? | |||
| Informative aspect, formal | Provides basic prediction of decline | No personal prognosis | Provides basic prediction of decline | No personal prognosis | More precise prediction? | In principle, no personal prognosis |
| Informative aspect, informal | Allows one to look for “good” examples | Allows room to identify with “bad” examples | Allows one to look for “good” examples | Opens for earlier identification with “bad” examples | Allows one to look for the “right” examples | Could leave one with the “bad” examples |
| Emotional aspect | Provides room for hope | Causes anxiety/fear | More room for hope | More room for fear | Less room for fear | Less room for hope |
| Directive value | Limited power to act | (Un)timely action? | More power to act? | |||
| Anticipation aspect | Allows some anticipatory action | Makes the future unmanageable | Allows some anticipatory action earlier/timely | Causes premature anticipation | Allows more/better anticipatory action? | Still makes the future unmanageable |
| Management aspect | Empowers one to adapt | Makes one feel powerless | Empowers one to adapt earlier/timely | Causes premature adaptation | Empowers one to better adapt | Still makes one feel powerless |
| Social aspect | Gives certain rights/access | Entails loss of or “unfit” rights/access | Gives certain rights/access earlier/timely | Causes earlier/premature loss of or “unfit” rights/care | Gives better fitted rights/access | Entails loss of rights/access |
THE EPISTEMIC VALUE OF A DIAGNOSIS
Across the interviews, we repeatedly heard a story of mixed feelings upon receiving a diagnosis. This often related to the limited directive value of a diagnosis. However, mixed feelings also surrounded the value of knowing “in itself”.
Current diagnostic practices
At a certain point we went to [the hospital], after all, because we thought, yes, we have to know a bit about where this is going. […] What is going on. […] After all, you do want to know that for yourself. […] [The diagnosis] took uncertainty away about what is going on. That does not make it better, of course. (P14)
It does hurt, the diagnosis. But [laughs ironically], at least you know what is going on and what you might be able to do about it. […] There is nothing as nasty as uncertainty. (P13)
These two quotes illustrate how knowing “what is going on” is not just instrumental for knowing “what to do”, but somehow valuable in itself. There is an informative and an emotional aspect to this that overlap and, furthermore, both entail a basic tension. A diagnosis may provide clarity, it might provide confirmation and even relief after uncertainty, suspicion and fear. However, for some patients the diagnosis does not provide any real news—they have already drawn the conclusion themselves. In any case, the actual diagnosis also causes great distress.
Moreover, there is a social aspect to the value of knowing. A diagnosis often provides a welcomed explanation for one's behaviour to the outside world, while at the same time it causes stigma. For some, the value of explanation clearly outweighs the risk of stigma, while for others not:
I was really happy with [being diagnosed]. Because I had already struggled for two years. […] Just the recognition. […] We came home afterwards and then we immediately (looked it up] on the internet. […] And in the evening, we sat together and read and everything made sense. Everything. And that gives… I don't know… for me, it was an enormous relief. Also, as in ‘it is not just me, I am not just a complainer or something’. And at my work, it just did not go well. That was clear, I just could not do it. […] When the diagnosis came then they [the colleagues] were ashamed. They really found it terrible, also just crying and tears as in ‘this is not okay, what have we done to you’. That did me good, felt good. I thought ‘now, look, […] I was not putting on a show’. (P16)
You know how people react, right. If they know and they notice […] then you will be left alone. So, you might as well skip that phase and not tell. (P10)
However, other participants did not express such clear preferences and strategies. For them, receiving an AD diagnosis implied continuously weighing these two effects against each other in different social contexts.
Earlier and/or more precise diagnosis
A number of the tensions around the “value of knowing” in current practices reoccurred, or were even reinforced, when participants reflected on diagnosis in the potential future practices.
An earlier diagnosis, understood as “upon the very first symptoms”, might provide some sort of clarity and explanation, but at the same time bring more questions than answers. Clearly, if one until recently has had no or little suspicion that something is wrong, a diagnosis is less likely to provide long‐wanted confirmation. The epistemic value would thus be limited. At the same time, one would still experience the distress that follows from what would be “real news” and, furthermore, one would risk stigmatisation at an even earlier stage. In short, as one patient summarised his sentiment towards an earlier diagnosis: “If you do not know, then you do not know, so then you might live more carefree”. (P12).
When asked about the potential value of a more precise diagnosis, participants’ reflections revolved around whether a more precise diagnosis would be relatively “good news”. First of all, a more precise diagnosis will introduce new labels—and the label matters. Robinson et al. (2011) found that for patients (and non‐patients) the label “Alzheimer's” often comes with more negative connotations than “dementia”. We found examples and counterexamples of this, demonstrating how a specific diagnosis may represent relatively “good news” to some, while for others it does the opposite:
Alzheimer's sounds really bad, I think. […] The word Alzheimer's sounds so threatening. Much more threatening that [for instance] memory complaints, mild memory complaints, as it is called. (P3)
To me, [Alzheimer's and dementia] are two different things. […] Dementia sounds so far already. Then I’d rather have Alzheimer's. Because then you are able to do something with it, for yourself. (P14)
Such sentiments are also related to the social connotations of a diagnosis. New (sub‐)diagnoses are likely to eventually also carry different connotations to different people and, thus, have different social effects—something that in itself is a point of concern to the one “carrying” the label (see also Beard, 2016; Hillman et al., 2018).
Moreover, the “good or bad news” condition was central to how participants valued the added clarity of a more precise diagnosis. In the following quote, a participant responds to the question if a more precise diagnosis based on brain imaging would be valuable by, for instance, reducing uncertainty:
Yes, unless, of course, if you hear that well, it is rather hopeless. […] Then of course you know what you are facing but of course you would rather not hear that. You are always a bit busy [thinking] ‘well, maybe it won't be so bad after all’. […] But if such a scan provides 100 percent certainty that it will go this way [...], I think that will make you really depressed. (P14)
Whether “clarity” is valuable, thus, for some depends on which scenario becomes clear. “Hoping for good news” can be seen as a problematic bias in attitudes towards diagnostic testing. Leaving the notion of bias aside, our study shows that the obvious preference for receiving good news at least causes ambivalence as to the “value of knowing”—and specifically, “the value of knowing more precisely”. Simply, clarity might hurt. Furthermore, what constitutes “good news” is strongly linked to the social and personal connotations of a diagnosis and, not least, the specific futures it tells about. This latter point leads us to the predictive value of a diagnosis.
THE PREDICTIVE VALUE OF A DIAGNOSIS
Indeed, for the participants in our study the epistemic value of a diagnosis seemed deeply intertwined with its predictive value. Since prognostication is hardly part of current practices, the “formal” predictive value of a diagnosis is very limited—and this may also be the case in the envisioned future practices. That being said, the knowledge that a diagnosis entails (“what is the matter”) is also, inevitably, somehow knowledge about the future (“what is the matter tomorrow, next week, and in a year”) and thus predictive in some very basic sense, causing frustration but also holding some benefits.
Current diagnostic practices
In the current “prognostic void”, people with AD have to develop alternative strategies of “knowing the future” – of filling in the blanks, so to speak. We encountered two overall strategies, which the participants would often mix. On the one hand, they tried to foresee the future using very general statistics, examples, and their own experience of decline on parameters of importance to them. On the other hand, they tried to avoid picturing the future in too much detail and instead focus on the present.
The lack of prognostic information and the subsequent alternative strategies of “filling in the blanks” have benefits and drawbacks. This reflected in how most of the participants shifted between the positions: “not really knowing the future is not so bad after all”, “even very little knowledge about the future can be useful/enough”, and “not knowing the future is a source of great distress”. The following sequence of quotes from the same participant illustrates this ambivalence:
I would not want to know what to expect. You can just fill it in yourself [based] on people you know who are demented. (P9)
For me, the word Alzheimer was enough – that word was actually enough. I just recognized that. […] My father was also demented, so I just know what, in the end, your destiny might be. (P9)
I don't want to look too far ahead ‐ that maybe, in the end, I’ll be sitting drooling in a chair or something like that. No. That will then be my family's problem, if they still want to come by and so. By then, I won't know anymore. (P9)
You just can't really predict it, right. […] It is all just […] guessing. […] Here, it [prediction] can't be part of [diagnostics], and it would be nice if it was possible. […] Then you can already think about what you want to do with your house and your money and so, also if you want to give it away and so. (P9)
This sequence of quotes also shows how examples come to play an important role, in this case in a discouraging way. Other examples may represent relatively good scenarios. In general, the lack of prognosis allows a lot of room for fear as well as hope, which those affected have to somehow manage. With a diagnosis, the future becomes less open and too open at the same time. Anticipation becomes a pressing, yet painful and challenging task.
Earlier and/or more precise diagnosis
An earlier diagnosis would, without further prognostic innovation, also give only very general predictive information, indicating a future with illness, but without saying much about the exact progression. Valuing diagnosis in this scenario, several participants highlighted that it would mean that anxieties about what to expect would also be introduced earlier:
[With an early diagnosis] you have a dark cloud hanging over your head. Look, if you know that there is drug that can help it to last longer… […]. But we are not that far yet. (P11)
Simply, there would be more blanks to fill—mainly for the worse, but also for the better: being confronted with the diagnosis earlier could, for instance, give more room for hope regarding therapeutic breakthroughs.
In contrast, participants imagined that a more precise diagnosis could improve the capacity to anticipate. However, this optimism was based on the assumption that a more precise diagnosis would entail a more precise prognosis:
Well, I think that if you have a clear picture of everything, then you can also more or less say ‘there is a prognosis and for you, with these deviations and things like that, we see that this and this will occur within a year or two’. [I: And would that be valuable to you?] Yes, to see ‘okay now I really need to take action. […] That you have to make sure that you can go somewhere. That you cannot live on your own anymore. That is, of course, the most important. (P10)
The value of a presumed more precise prognosis is then, as in the quote, typically linked to the opportunity for acting. Whether looking at current or future practices, the predictive value of a diagnosis is, indeed, ultimately closely linked to its directive value, as an instrument or even premise for action. Reversely, there is a strong future orientation in the directive value: acting is to a large extent about acting on the future, as we will show below. Yet, a more precise diagnosis could also have predictive value without being linked to action: by simply narrowing down potential future scenarios, a more precise diagnosis also narrows down the room for hope—and fear. There would be less blanks to fill, for better and for worse.
Note on the role of euthanasia
When exploring the predictive value of an AD diagnosis within a Dutch context, one topic keeps occurring: euthanasia. In the Netherlands, this is a legal and for many people desirable option when suffering from a severe, uncurable disease. However, in relation to AD a euthanasia request is highly complex, since the clinical manifestation of AD (cognitive decline) makes it challenging to satisfy the legal criteria (Mangino et al., 2020). For people with AD considering euthanasia, timing is crucial: one should not be too late—nor too early. The lack of prognosis becomes especially problematic in this context. In our study, some participants would address the role of euthanasia very directly. Others would only subtly refer to it when talking about the future:
I think that in a about three or four years then it is probably over, I think. […] If I can't operate technical equipment anymore, for instance. […] If you think ‘what am I supposed to do with this’. [Then] you are not capable of acting [“handelsbekwaam”] anymore. […] [It is about] that you can keep yourself busy. I photograph a lot so if I couldn't do that anymore… then it is quickly over and done with. (P12)
For this participant, for whom euthanasia was a desirable option, his prediction of when “it is probably over” had both a metaphorical and a very concrete meaning. At some point, the disease would have progressed to a point where he considered life not worth living anymore—and would want to end it. His own “prediction” would, thus, become self‐fulfilling, if his request for euthanasia would be accepted. This is just one example of how euthanasia played an role in the anticipatory reflections of participants and, thus, also for the predictive (and directive) value of an AD diagnosis.
THE DIRECTIVE VALUE OF A DIAGNOSIS
Ultimately, while valuing diagnosis the participants in our study kept emphasising the link between knowing and acting. However, in both current and imagined future diagnostic practices the possibilities for and benefits of action are not clear‐cut.
Current diagnostic practices
An AD diagnosis empowers and disempowers at the same time. First of all, a diagnosis makes it possible to anticipate to some extent, that is to foresee (as shown above) and act in relation to the future. Among other things, anticipation can involve making financial arrangements, setting up a life will and doing things “in time”. However, while our participants agreed that a diagnosis makes it possible to influence one's own future, they also indicated that a diagnosis makes the future highly unmanageable at the same time. Uncertainty about what to expect, how to deal with the unexpected, and what one's capacity to influence the future is challenges the directive value of a diagnosis.
Secondly, a diagnosis and the associated awareness of the specific disease manifestations may empower one to better manage symptoms and adapt in daily life. This also includes taking responsibility and caring for others (van der Burg et al., 2019):
I just surrender to it [the diagnosis] because you can't change it anyway. […] Okay, now this is how it is. Well, then [you have to] look at it differently, I always think. […] Well, just that you are not allowed to drive a car anymore, in any case, then I just have to take the bike everywhere. And I have to make sure to get a public transportation card. […] You know, that kind of things. […] Well, indeed, it had been nicer not to know, but then I might have caused an accident. (P9)
However, an AD diagnosis also makes one feel powerless: disease manifestations are irreversible and may not easily be managed. In current practices, an AD diagnosis simply does not entail availability of “hard” solutions: there is no effective therapy to diminish the symptoms or cure the disease.
Finally, an AD diagnosis often entails a loss of rights and/or access (e.g., to the labour market, to drive a car). On the other hand, it may give certain rights and access to care. However, such access is not always valuable:
She [the case manager] then comes and asks how it is going. Then I think: gosh, when will she say ‘well, you say that it all goes well, but it does not go well. I will now start arranging things’. Well, I would not like to see her doing that. […] I’m a bit alert, ‘what is she doing here?’. […] Because she also talks about ‘well, we have an Alzheimer café..’. No way that I would consider going there. I have no idea why I would need such an Alzheimer café. […] Well, we do let her [the case manager] in [laughs]. […] She is of course a nice person who does her job. But I still at times think ‘you are not needed here yet’, but that is difficult to tell her. (P14)
As clear from this quote, an AD diagnosis may trigger access to what some see as unfit or untimely care—care that simply does not “hit the target”, increases the experience of stigma or catapults one into a feared future.
Earlier and/or more precise diagnosis
In general, participants primarily linked the possible benefits of an earlier diagnosis to being able to act, thus highlighting its added directive, rather than epistemic and predictive value. However, the precise possibilities for acting seemed hard to coin, with participants often keeping it rather abstract when imagining the benefits of earlier diagnosis:
Well, that you can keep control of yourself, or of your life. (P10)
Look, if you break too late, well, then you drive into the pole. You have to break a bit before. (P13)
Meanwhile, most participants also recognised that the disadvantages of being diagnosed would still be present and even be introduced earlier. It would, for example, introduce dilemmas around the proper (re)action in relation to one's job, not least from oneself:
Well, I don't know actually, what difference that [knowing earlier] would have made. […] If it is really a form of dementia then it is a process of decline, so the moment comes where you have to stop [working]. And if I had known that a month before, then I would have been less.. […] You look critically at yourself daily: ‘Am I still doing it all well enough?’, to be able to manage. But then you also ask too much of yourself. […] You approach the world with a lot of self‐criticism. That also does not bring [anything good]. (P11)
Overall, an earlier diagnosis still both empowers and disempowers one to act, while timing action becomes even more complicated. An earlier diagnosis might spark one to prematurely adapt and/or gain and loose rights. Actions which in current practices might be enabled too late, such as arranging one's future care, might be undertaken too early with an earlier diagnosis. In the Dutch context, this also applies to considering euthanasia where, obviously, a lot is at stake with regard to timing. In some biomarker visions, earlier diagnosis is rephrased as timely diagnosis (Dubois et al., 2016; Whitehouse, 2016). This shift seems to take the issue of timing seriously, yet does not solve the challenge of defining “timely”.
In contrast, participants indicated that a more precise diagnosis might shift the balance between empowerment and disempowerment. However, this would be conditioned on whether a more precise diagnosis would, first, entail better prognostics, as also described above, and, second, be matched by more tailored responses, such as more suitable (non‐stigmatising) care and social responses. However, this clearly hinges on the exact new diagnoses and on the corresponding care new diagnostic tools would bring along. In any case, participants indicated that no matter the specificity of the diagnosis, it would still inevitably make them feel fundamentally powerless.
CONCLUDING DISCUSSION
An AD diagnosis has a Janus face: it confers benefits, as well as drawbacks, regardless of whether it provides knowledge, predicts or directs action. New biomarker tools enabling an earlier and/or more precise diagnosis may slightly change these benefits and drawbacks, but it seems unlikely that they will resolve the fundamental tensions arising from an AD diagnosis. Table 1 provides an overview of the insights gained. Here, we reflect on how they enrich discussions about the desirability of novel diagnostic technologies, also pointing to some limitations and perspectives for further research.
Ambivalence and anticipation: implications for biomarkers
Biomarker visions pursuing earlier and/or more precise diagnosis typically presume a straightforward link between knowing and acting. Ethicists pointing out that clinical utility is a precondition for personal utility (Bunnik et al., 2018; Vanderschaeghe et al., 2018), actually affirm the importance of the knowing–acting nexus. Our study confirms earlier findings (e.g., van der Burg et al., 2019; van der Laan, 2016) that AD patients do indeed strongly link the value of “knowing what is the matter” and “knowing what will come” to possibilities for action. However, our study underscores that this link is far from straightforward—neither in present, nor in envisioned diagnostic practices.
On the one hand, the actions enabled by knowing at present are limited to adaptation, obtaining care and certain rights, and, not least, anticipation. This last ability, to somehow manage the future in light of an untreatable, progressive disease, is complicated, however, by the lack of formal prognostics. On the other hand, “knowing” is not always a prerequisite for action. Moreover, non‐medical ways of knowing hold benefits, such as allowing more room for hope. Overall, these findings show that patients’ responses to an AD diagnosis not only vary widely and depend on time and context (as put forward by Aminzadeh et al., 2007; Bunn et al., 2012; Portacolone et al., 2018; Pratt & Wilkinson, 2003), but also indicate a profound ambivalence towards the value of diagnosis (as also suggested by Beard, 2016). Connecting this finding with the expectations raised by emergent biomarkers, our study furthermore shows that earlier and/or more precise diagnoses on their own are unlikely to reduce this ambivalence.
The variety of and ambivalence in patient perspectives have different implications for the potential introduction of biomarker tools in AD diagnostics. Variety of perspectives implies that good biomarker‐based diagnostics should accommodate patients with different preferences. Ambivalence, however, indicates that safeguarding choice is not sufficient. The main challenge is that patients struggle to develop clear preferences, due to an acute sense of the drawbacks of diagnosis. Moreover, their ambivalence will not vanish once they made a choice, because the drawbacks are real. Therefore, supporting the process leading to a choice, as well as the process of living with a choice, is key. Even if biomarker tests for earlier and/or more precise diagnosis become sufficiently reliable, they will benefit patients only if the testing and care infrastructures are also attended to.
Limitations and perspectives for further research
The positive or negative impact of an earlier and/or more precise diagnosis can be evaluated only if it is clear how to conceptualise (and operationalise) benefits and disadvantages. Our research explored what patients conceive of as potential benefits and drawbacks of such emergent diagnostic practices. A potential concern about any research eliciting values regarding new and emerging technologies, however, is that it may actually bring such values—or valuations—about, rather than uncover something that is already there. Our use of cards during the interviews was an attempt to reflexively shape this inevitable impact, by presenting a diverse and balanced set of viewpoints and inviting feedback from patients as well as researchers in the process. Further research would be necessary to find out whether our approach led to neglect of, and/or an unjustified focus on particular issues.
Moreover, our study is based on interviews with a very specific group of patients, suffering from relatively early‐onset AD and recruited through a highly specialised clinic where current diagnostic practices involve elaborate testing and often lead to various kinds of research participation. While we believe that the insights gained have broader relevance, they should be qualified and supplemented by perspectives of other patient groups. Moreover, exploring how patients’ valuations of diagnosis evolve over time would be highly relevant.
A related, and final, issue is that we focused solely on the value of an AD diagnosis. This enabled us to explore how patients’ experience of an illness is mediated by the specific diagnostic label at stake. Such processes may change, however, when new diagnostic tools lead to new disease labels which in due time may acquire their own connotations. The views participants brought forward on this are necessarily somewhat speculative. In fact, the same could be said for participants’ views on emergent diagnostic practices. However, these views are informed by their past and current experiences with closely related practices. We are convinced, therefore, that patients and their caregivers are the best source available for anticipating the potential impacts of novel tools for diagnosing AD on patient lives and to critically reflect on what is or is not desirable.
AUTHOR CONTRIBUTIONS
Karen Dam Nielsen: Conceptualization (equal); Investigation (lead); Methodology (equal); Writing‐original draft (lead); Writing‐review & editing (lead). Marianne Boenink: Conceptualization (equal); Methodology (equal); Writing‐original draft (supporting); Writing‐review & editing (supporting).
ACKNOWLEDGEMENTS
We thank the patients and caregivers who took part in this study for their time, effort and trust. We also thank the researchers involved in the related biomarker project for facilitating inclusion and letting us follow their work. The study was funded by the ZonMW, Memorabel Programme 1, [733050203].
DATA AVAILABILITY STATEMENT
The data (interview recordings and transscriptions) underlying this study is not publicly available due to privacy and ethical restrictions. The data is securely stored under the conditions required by the hosting institutions and is available on request from the corresponding author.
REFERENCES
- Aminzadeh, F. , Byszewski, A. , Molnar, F.J. & Eisner, M. (2007) Emotional impact of dementia diagnosis: Exploring persons with dementia and caregivers’ perspectives. Aging & Mental Health, 11(3), 281–290. [DOI] [PubMed] [Google Scholar]
- Bailey, C. , Dooley, J. & McCabe, R. (2019) ‘How do they want to know?’ Doctors’ perspectives on making and communicating a diagnosis of dementia. Dementia, 18(7–8), 3004–3022. [DOI] [PubMed] [Google Scholar]
- Bamford, C. & Bruce, E. (2000) Defining the outcomes of community care: The perspectives of older people with dementia and their carers. Ageing & Society, 20(5), 543–570. [Google Scholar]
- Beard, R.L. (2016) Living with Alzheimer’s. Managing memory loss, identity, and illness. New York, NY: New York University Press. [Google Scholar]
- Bethell, J. , Commisso, E. , Rostad, H.M. , Puts, M. , Babineau, J. , Grinbergs‐Saull, A. et al. (2018) Patient engagement in research related to dementia: A scoping review. Dementia, 17(8), 944–975. [DOI] [PubMed] [Google Scholar]
- Blennow, K. & Zetterberg, H. (2018) Biomarkers for Alzheimer's disease: Current status and prospects for the future. Journal of Internal Medicine, 284(6), 643–663. [DOI] [PubMed] [Google Scholar]
- Boenink, M. (2016) Biomarkers for Alzheimer’s disease: Searching for the missing link between biology and clinic. In: Boenink, M. , van Lente, H. and Moors, E. (Eds.) Emerging technologies for diagnosing Alzheimer's disease: Innovating with care. London, UK: Palgrave Macmillan, pp. 1–17. [Google Scholar]
- Boenink, M. (2018) Gatekeeping and trailblazing: The role of biomarkers in novel guidelines for diagnosing Alzheimer’s disease. BioSocieties, 13(1), 213–231. [Google Scholar]
- Boenink, M. & Kudina, O. (2020) Values in responsible research and innovation: From entities to practices. Journal of Responsible Innovation, 7(3), 450–470. [Google Scholar]
- Boenink, M. , van der Scheer, L. , Garcia, E. & van der Burg, S. (2018) Giving voice to patients: developing a discussion method to involve patients in translational research. NanoEthics, 12(3), 181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenink, M. , van Lente, H. & Moors, E. (Eds.) (2016) Emerging technologies for diagnosing Alzheimer’s Disease: Innovating with care. London, UK: Palgrave Macmillan. [Google Scholar]
- Bowker, G.C. & Star, S.L. (2000) Sorting things out: Classification and its consequences. Cambridge: MIT press. [Google Scholar]
- Bunn, F. , Goodman, C. , Sworn, K. , Rait, G. , Brayne, C. , Robinson, L. et al. (2012) Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: A systematic review of qualitative studies. PLoS Medicine, 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnik, E.M. , Richard, E. , Milne, R. & Schermer, M.H. (2018) On the personal utility of Alzheimer’s disease‐related biomarker testing in the research context. Journal of Medical Ethics, 44(12), 830–834. [DOI] [PubMed] [Google Scholar]
- Caselli, R.J. , Langbaum, J. , Marchant, G.E. , Lindor, R.A. , Hunt, K.S. Henslin, B.R. et al. (2014) Public perceptions of presymptomatic testing for Alzheimer disease. Mayo Clinic Proceedings, 89(10), 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell, C.M. , Boise, L. , Stuckey, J.C. , Holmes, S.B. & Hudson, M.L. (2004) Attitudes toward the diagnosis and disclosure of dementia among family caregivers and primary care physicians. The Gerontologist, 44(4), 500–507. [DOI] [PubMed] [Google Scholar]
- Cridland, E.K. , Phillipson, L. , Brennan‐Horley, C. & Swaffer, K. (2016) Reflections and recommendations for conducting in‐depth interviews with people with dementia. Qualitative Health Research, 26(13), 1774–1786. [DOI] [PubMed] [Google Scholar]
- Dubois, B. , Padovani, A. , Scheltens, P. , Rossi, A. & Dell’Agnello, G. (2016) Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. Journal of Alzheimer's disease, 49(3), 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felt, U. , Schumann, S. , Schwarz, C.G. & Strassnig, M. (2014) Technology of imagination: A card‐based public engagement method for debating emerging technologies. Qualitative Research, 14(2), 233–251. [Google Scholar]
- Gjødsbøl, I.M. & Svendsen, M.N. (2018) Recognizing dementia: Constructing deconstruction in a Danish memory clinic. Medical Anthropology Quarterly, 32(1), 103–119. [DOI] [PubMed] [Google Scholar]
- Greenwood, N. & Smith, R. (2016) The experiences of people with young‐onset dementia: A meta‐ethnographic review of the qualitative literature. Maturitas, 92, 102–109. [DOI] [PubMed] [Google Scholar]
- Hellström, I. , Nolan, M. , Nordenfelt, L. & Lundh, U. (2007) Ethical and methodological issues in interviewing persons with dementia. Nursing Ethics, 14(5), 608–619. [DOI] [PubMed] [Google Scholar]
- Hillman, A. , Jones, I.R. , Quinn, C. , Nelis, S.M. & Clare, L. (2018) Dualities of dementia illness narratives and their role in a narrative economy. Sociology of Health and Illness, 40(5), 874–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska, A. (1999) Respecting the margins of agency: Alzheimer's patients and the capacity to value. Philosophy & Public Affairs, 28(2), 105–138. [DOI] [PubMed] [Google Scholar]
- Jutel, A. (2009) Sociology of diagnosis: A preliminary review. Sociology of Health & Illness, 31(2), 278–299. [DOI] [PubMed] [Google Scholar]
- Karnieli‐Miller, O. , Werner, P. , Aharon‐Peretz, J. & Eidelman, S. (2007) Dilemmas in the (un) veiling of the diagnosis of Alzheimer's disease: Walking an ethical and professional tight rope. Patient Education and Counseling, 67(3), 307–314. [DOI] [PubMed] [Google Scholar]
- Karnieli‐Miller, O. , Werner, P. , Aharon‐Peretz, J. , Sinoff, G. & Eidelman, S. (2012) Expectations, experiences, and tensions in the memory clinic: The process of diagnosis disclosure of dementia within a triad. International Psychogeriatrics, 24(11), 1756–1770. [DOI] [PubMed] [Google Scholar]
- Kiran, A.H. , Oudshoorn, N. & Verbeek, P.P. (2015) Beyond checklists: Toward an ethical‐constructive technology assessment. Journal of Responsible Innovation, 2(1), 5–19. [Google Scholar]
- Kitwood, T. & Bredin, K. (1992) Towards a theory of dementia care: Personhood and well‐being. Ageing & Society, 12(3), 269–287. [DOI] [PubMed] [Google Scholar]
- Laakkonen, M.L. , Raivio, M.M. , Eloniemi‐Sulkava, U. , Saarenheimo, M. , Pietilä, M. , Tilvis, R.S. et al. (2008) How do elderly spouse care givers of people with Alzheimer disease experience the disclosure of dementia diagnosis and subsequent care? Journal of Medical Ethics, 34(6), 427–430. [DOI] [PubMed] [Google Scholar]
- Lock, M. (2013a) The Alzheimer conundrum. Entanglements of dementia and aging. Princeton, NJ: Princeton University Press. [Google Scholar]
- Lock, M. (2013b) Detecting amyloid biomarkers: Embodied risk and Alzheimer prevention. BioSocieties, 8(2), 107–123. [Google Scholar]
- MacRae, H. (2008) “Making the best you can of it”: Living with early‐stage Alzheimer’s disease. Sociology of Health and Illness, 30(3), 396–412. [DOI] [PubMed] [Google Scholar]
- Mangino, D.R. , Nicolini, M.E. , De Vries, R.G. & Kim, S.Y. (2020) Euthanasia and assisted suicide of persons with dementia in the Netherlands. The American Journal of Geriatric Psychiatry, 28(4), 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, T.F. & Hollett, J. (2003) Giving voice to persons living with dementia: The researcher’s opportunities and challenges. Nursing Science Quarterly, 16(2), 163–167. [DOI] [PubMed] [Google Scholar]
- Nielsen, K.D. & Boenink, M. (2020) Subtle voices, distant futures: a critical look at conditions for patient involvement in Alzheimer’s biomarker research and beyond. Journal of Responsible Innovation, 7(2), 170–192. [Google Scholar]
- Okamura, N. , Harada, R. , Ishiki, A. , Kikuchi, A. , Nakamura, T. & Kudo, Y. (2018) The development and validation of tau PET tracers: current status and future directions. Clinical and Translational Imaging, 6(4), 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist, S. , Janelidze, S. , Quiroz, Y.T. , Zetterberg, H. , Lopera, F. , Stomrud, E. et al. (2020) Discriminative accuracy of plasma Phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA, 324(8), 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett, J. & Murray, M. (2018) Editorial: Patient and public involvement in dementia research: Setting new standards. Dementia, 17(8), 939–943. [DOI] [PubMed] [Google Scholar]
- Portacolone, E. , Johnson, J.K. , Covinsky, K.E. & Halpern, J. (2018) The effects and meanings of receiving a diagnosis of mild cognitive impairment or Alzheimer’s disease when one lives alone. Journal of Alzheimer’s Disease, 61, 1517–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteri, C. , Albanese, E. , Scerri, C. , Carrillo, M.C. , Snyder, H.M. , Martensson, B. et al. (2017) The biomarker‐based diagnosis of Alzheimer's disease. Ethical and societal issues. Neurobiology of Aging, 52, 132–140. [DOI] [PubMed] [Google Scholar]
- Pratt, R. & Wilkinson, H. (2003) A psychosocial model of understanding the experience of receiving a diagnosis of dementia. Dementia, 2(2), 181–199. [Google Scholar]
- Robinson, L. , Clare, L. & Evans, K. (2005) Making sense of dementia and adjusting to loss: Psychological reactions to a diagnosis of dementia in couples. Aging & Mental Health, 9(4), 337–347. [DOI] [PubMed] [Google Scholar]
- Robinson, L. , Gemski, A. , Abley, C. , Bond, J. , Keady, J. , Campbell, S. et al. (2011) The transition to dementia–individual and family experiences of receiving a diagnosis: A review. International Psychogeriatrics, 23(7), 1026–1043. [DOI] [PubMed] [Google Scholar]
- Schermer, M.H. & Richard, E. (2019) On the reconceptualization of Alzheimer’s disease. Bioethics, 33(1), 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicktanz, S. , Schweda, M. , Ballenger, J.F. , Fox, P.J. , Halpern, J. , Kramer, J.H. et al. (2014) Before it is too late: Professional responsibilities in late‐onset Alzheimer’s research and pre‐symptomatic prediction. Frontiers in Human Neuroscience, 8, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweda, M. , Kögel, A. , Bartels, C. , Wiltfang, J. , Schneider, A. & Schicktanz, S. (2018) Prediction and early detection of Alzheimer’s dementia: Professional disclosure practices and ethical attitudes. Journal of Alzheimer's Disease, 62(1), 145–155. [DOI] [PubMed] [Google Scholar]
- Sun, B.L. , Li, W.W. , Zhu, C. , Jin, W.S. , Zeng, F. , Liu, Y.H. et al. (2018) Clinical research on Alzheimer’s disease: Progress and perspectives. Neuroscience Bulletin, 34(6), 1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow, J. (2017) Expectant futures and an early diagnosis of Alzheimer's disease: Knowing and its consequences. Social Science & Medicine, 184, 57–64. [DOI] [PubMed] [Google Scholar]
- Swierstra, T. , Boenink, M. & Stemerding, D. (2009) Exploring techno‐moral change: The case of the obesity pill. In Sollie, P. & Duwell, M. (Eds.), Evaluating new technologies. Methodological problems for the ethical assessment of technology developments. Dordrecht, the Netherlands: Springer, pp. 119–138. [Google Scholar]
- van der Burg, S. , Schreuder, F.H. , Klijn, C.J. & Verbeek, M.M. (2019) Valuing biomarker diagnostics for dementia care: Enhancing the reflection of patients, their care‐givers and members of the wider public. Medicine, Health Care and Philosophy, 22(3), 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan, A.L. (2016) Grey matters: Emergent biomarkers and good Alzheimer’s diagnostics. In: Boenink, M. , van Lente, H. and Moors, E. (Eds.) Emerging technologies for diagnosing Alzheimer's disease: Innovating with care. London, UK: Palgrave Macmillan, pp. 103–122. [Google Scholar]
- Vanderschaeghe, G. , Dierickx, K. & Vandenberghe, R. (2018) Review of the ethical issues of a biomarker‐based diagnoses in the early stage of Alzheimer’s disease. Journal of Bioethical Inquiry, 15(2), 219–230. [DOI] [PubMed] [Google Scholar]
- Vernooij‐Dassen, M. , Derksen, E. , Scheltens, P. & Moniz‐Cook, E. (2006) Receiving a diagnosis of dementia: The experience over time. Dementia, 5(3), 397–410. [Google Scholar]
- Villemagne, V.L. , Fodero‐Tavoletti, M.T. , Masters, C.L. & Rowe, C.C. (2015) Tau imaging: Early progress and future directions. The Lancet Neurology, 14(1), 114–124. [DOI] [PubMed] [Google Scholar]
- Visser, L.N. , Kunneman, M. , Murugesu, L. , van Maurik, I. , Zwan, M. , Bouwman, F.H. et al. (2019) Clinician‐patient communication during the diagnostic workup: The ABIDE project. Alzheimer's & Dementia, 11, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, P. , Karnieli‐Miller, O. & Eidelman, S. (2013) Current knowledge and future directions about the disclosure of dementia: A systematic review of the first decade of the 21st century. Alzheimer’s and Dementia, 9(2), e74–e88. [DOI] [PubMed] [Google Scholar]
- Whitehouse, P.J. (2016) The diagnosis and treatment of Alzheimer’s: Are we being (Ir) responsible? In: Boenink, M. , van Lente, H. and Moors, E. (Eds.) Emerging technologies for diagnosing Alzheimer's disease: Innovating with care. London, UK: Palgrave Macmillan, pp. 21–40. [Google Scholar]
- Wikler, E.M. , Blendon, R.J. & Benson, J.M. (2013) Would you want to know? Public attitudes on early diagnostic testing for Alzheimer’s disease. Alzheimers Res Ther, 5(5), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthopoulou, P. , Dooley, J. , Meo, I. , Bass, N. & Mccabe, R. (2019) Patient and companion concerns when receiving a dementia diagnosis: An observational study of dementia diagnosis feedback meetings. Ageing & Society, 39(8), 1782–1805. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data (interview recordings and transscriptions) underlying this study is not publicly available due to privacy and ethical restrictions. The data is securely stored under the conditions required by the hosting institutions and is available on request from the corresponding author.


