Editor,
Atopic dermatitis (AD) is a relapsing and remitting disease characterized by intense pruritus that can lead to scratching and eczematous lesions that vary in extent and severity. 1 Over 60% of AD cases are mild, characterized by slight erythema, induration and lichenification. 2 , 3 Chronic pruritus, pruritus lasting more than 6 weeks, has been reported by 91% of AD patients. 4 , 5 The pathophysiology of AD is driven by a combination of skin barrier dysfunction, neuroinflammation and immune system dysregulation. 6 , 7
Elevated substance P (SP) is found in both serum and lesional skin of patients with AD. 5 , 8 , 9 SP, a neuropeptide released from the activation of sensory neurons, preferentially binds to the neurokinin‐1 (NK‐1) receptor and is a known itch mediator. 5 Tradipitant (VLY‐686), a novel NK‐1 receptor antagonist, has the potential to reduce itch related to AD through inhibition of SP‐mediated itch signalling. We examined the efficacy and safety of tradipitant, in reduction of chronic pruritus in adults with mild to severe AD.
EPIONE was a phase 3, randomized, placebo‐controlled, double‐blind clinical trial conducted at 74 US centres. Altogether 375 patients [mean (SD): age, 41.8 (15.0) years; sex, 243 (64.8%) female] were randomly assigned to tradipitant (n = 188) or placebo (n = 187). Although there was a numerical benefit in the tradipitant group over placebo, EPIONE did not meet its primary endpoint of reduction in pruritus [Least Squares (LS) Mean difference (95% CI), −0.2 (−0.8 to 0.4), P = 0.567]. However, robust antipruritic effect was observed in patients with mild lesion severity [rated 1 or 2 by the validated Investigator Global Assessment for Atopic Dermatitis at baseline −1.6 (−2.9 to −0.3), P = 0.015; Figs 1 and 2a]. This result was confirmed by daily diary [−2.09 (−3.31 to −0.87), P = 0.001] and observed after one full day of treatment [Fig. 2b, −0.61 (−1.21 to −0.01), P = 0.0457]. Improvement in nighttime sleep was also observed in mild AD [−1.46 (−2.60 to −0.32) P = 0.013]. The most frequent treatment‐emergent adverse events (TEAEs) were mild to moderate. There were no common TEAEs identified in the treatment arm, defined by >5% incidence.
Figure 1.
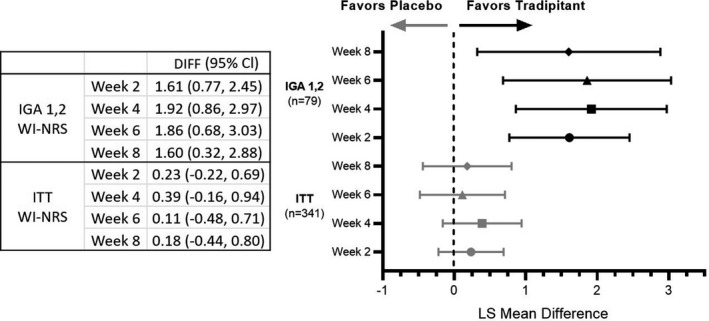
Worst Itch‐Numeric Rating Scale (WI‐NRS) change by week. Mild atopic dermatitis (AD) patients have greater improvement in worst itch after tradipitant treatment. Forest plots of the analysis of intent‐to‐treat and IGA 1,2 WI‐NRS change by week. Plotted as least squares mean difference and 95% CI after tradipitant or placebo treatment.
Figure 2.
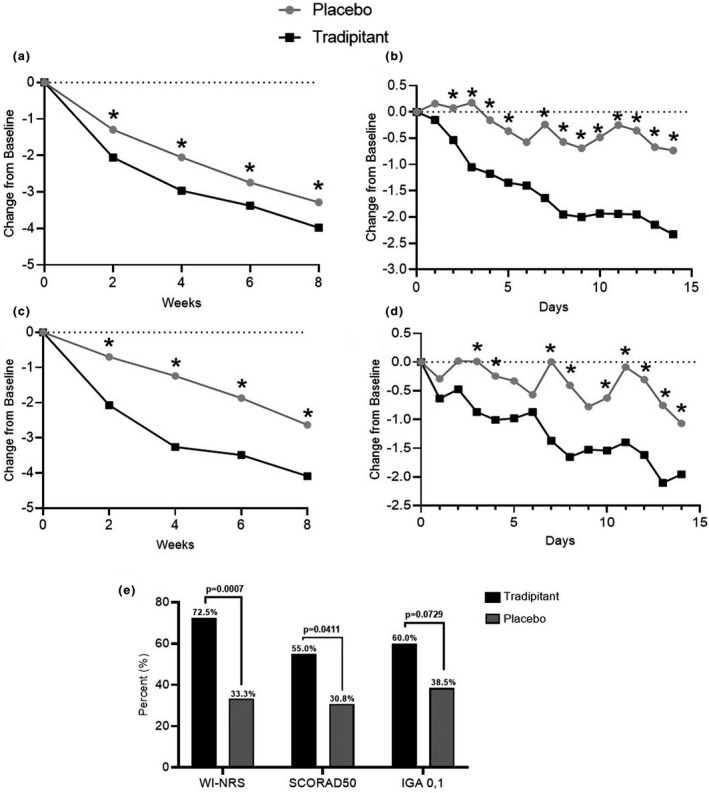
Tradipitant treatment improves itch and sleep in mild atopic dermatitis. (a) Tradipitant treatment improved worst itch in mild atopic dermatitis (AD). (b) Improvement in itch was observed after one full day of tradipitant treatment. (c) Tradipitant treatment improved sleep disturbance in mild AD. (d) Improvement in sleep was seen after two full days of tradipitant treatment. (e) A greater proportion of mild AD patients achieved success of four points or greater on Worst Itch‐Numeric Rating Scale (WI‐NRS) and at least a 50% improvement on SCORing atopic dermatitis. (a–d) P values are from Mixed Model Repeated Measures analysis. (e) P values are from Fisher's exact test. *P < 0.05.
Tradipitant treatment resulted in a clinically meaningful reduction in patient‐reported worst itch and sleep disturbance in the mild AD study population. Statistically significant improvement was seen after one day of treatment for itch and two days for sleep.
It is possible that the immediate and robust improvements observed in the mild AD subgroup were seen not only because of different levels of cutaneous inflammation between AD severities but also because of distinct AD endotypes including different clinical manifestations, molecular levels and genetic associations. 10 The concept of different endotypes in AD underlines the need for targeted therapeutics in different AD disease types. 11
Itch related to nerve hypersensitivity causes increased scratching which worsens eczema and induces excoriations. Suppression of itch in mild AD can thus prevent worsening of atopic dermatitis. In our study, we saw no significant Eczema Area and Severity Index or SCORing Atopic Dermatitis (SCORAD) index improvement in early weeks; however, we did not expect improvement of acute lesions but rather of excoriations in the long term. Improvement in excoriations of mild patients was largest at Week 2 (P = 0.0374). We observed numerical improvement across all time‐points studied in terms of lesion severity (excoriations).
Tradipitant may represent a new oral systemic option for mild AD patients based on the well‐tolerated safety profile and immediate robust improvement in itch and sleep. Future studies are needed to confirm these efficacy results and refine treatment recommendations for AD patients who despite having mild lesions, experience significant pruritus.
Conflicts of interest
SEW is a former employee of Vanda and a stockholder. CX is an employee of Vanda and a stockholder. ARK is an employee of Vanda and a stockholder. JLB is an employee of Vanda and a stockholder. MAM is an employee of Vanda and a stockholder. JW is an employee of Vanda and a stockholder. SPS is an employee of Vanda and a stockholder. BP is an employee of Vanda and a stockholder. SS is an investigator for Dermasence, Galderma, Kiniksa Pharmaceuticals, Menlo Therapeutics, Trevi Therapeutics, Novartis, Sanofi, and Vanda Pharmaceuticals Inc.; and is a consultant and/or member of the advisory board for Almirall, Bayer, Beiersdorf, Bellus Health, Bionorica, Cara Therapeutics, Celgene, Clexio Biosciences, DS Biopharma, Galderma, Menlo Therapeutics, Novartis, Perrigo, and Trevi Therapeutics. CP is an employee of Vanda and a stockholder. GB is an employee of Vanda and a stockholder. MHP is Chief Executive Officer of Vanda.
Funding source
Vanda Pharmaceuticals Inc.
Role of funding source
The sponsor designed the study. All authors, including those representing the sponsor, contributed to data interpretation and writing of the report. All authors had final responsibility for the decision to submit for publication.
Data sharing statement
Vanda shares data related to this trial in peer‐reviewed journals, medical conferences and Clinicaltrials.gov. Individual patient data are not publically available.
Trial Registration: Clinicaltrials.gov: NCT03568331, https://clinicaltrials.gov/ct2/show/NCT03568331
IRB Approval Status: Reviewed and approved by Advarra IRB; Pro00025510.
This manuscript has not been published and is not under consideration for publication elsewhere.
References
- 1. Barbarot S, Auziere S, Gadkari A et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy Eur J Allergy Clin Immunol 2018; 6: 1–10. [DOI] [PubMed] [Google Scholar]
- 2. Chiesa Fuxench ZC, Block J, Boguniewicz M et al. Atopic Dermatitis in America Study: a cross‐sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol 2019; 139: 583–590. [DOI] [PubMed] [Google Scholar]
- 3. Correale CE, Walker C, Murphy L, Craig TJ. Atopic dermatitis: a review of diagnosis and treatment. Am Fam Physician 1999; 60: 1110–1191. [PubMed] [Google Scholar]
- 4. Dawn A, Papoiu ADP, Chan YH, Rapp SR, Rassette N, Yosipovitch G. Itch characteristics in atopic dermatitis: results of a web‐based questionnaire. Br J Dermatol 2009; 160: 642–644. [DOI] [PubMed] [Google Scholar]
- 5. Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol 2016; 51: 263–292. [DOI] [PubMed] [Google Scholar]
- 6. Cabanillas B, Brehler A‐C, Novak N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol 2017; 17: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy 2013; 68: 974–982. [DOI] [PubMed] [Google Scholar]
- 8. Teresiak‐Mikołajczak E, Czarnecka‐Operacz M, Jenerowicz D, Silny W. Neurogenic markers of the inflammatory process in atopic dermatitis: relation to the severity and pruritus. Postepy Dermatol Alergol 2013; 30: 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nattkemper LA, Tey HL, Valdes‐Rodriguez R et al. The genetics of chronic itch: gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J Invest Dermatol 2018; 138: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 10. Smieszek SP, Przychodzen B, Welsh SE et al. Genomic and phenotypic characterization of Investigator Global Assessment (IGA) scale based endotypes in atopic dermatitis. J Am Assoc Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 11. Czarnowicki T, He H, Krueger JG, Guttman‐Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019; 143: 1–11. [DOI] [PubMed] [Google Scholar]


