Abstract
Objective
In people with low‐grade intrinsic brain tumors, an epileptic focus is often located close to the lesion. High‐frequency oscillations (HFOs) in electrocorticography (ECoG) might help to delineate this focus. We investigated the relationship between HFOs and low‐grade brain tumors and their potential value for tumor‐related epilepsy surgery.
Methods
We analyzed pre‐ and postresection intraoperative ECoG in 41 patients with refractory epilepsy and a low‐grade lesion. Electrodes were designated as overlying the tumor, adjacent resected tissue (peritumoral), or outside the resection bed using magnetic resonance imaging (MRI) and intraoperative photographs. We then used a semiautomated approach to detect HFOs as either ripples (80–250 Hz) or fast ripples (250–500 Hz).
Results
The rate of fast ripples was higher in electrodes covering tumor and peritumoral tissue than outside the resection (p = .04). Mesiotemporal tumors showed more ripples (p = .002), but not more fast ripples (p = .07), than superficial tumors. Rates of fast ripples were higher in glioma and extraventricular neurocytoma than in ganglioglioma or dysembryoplastic neuroepithelial tumor (DNET). The rate of ripples and fast ripples in postresection ECoG was not higher in patients with residual tumor tissue on MRI than those without. The rate of ripples in postresection ECoG was higher in patients with good than bad seizure outcome (p = .03). Fast ripples outside the resection and in post‐ECoG seem related to seizure recurrence.
Significance
Fast ripples in intraoperative ECoG can be used to help guide resection in tumor‐related epilepsy surgery. Preresection fast ripples occur predominantly in epileptogenic tumor and peritumoral tissue. Fast ripple rates are higher in glioma and extraventricular neurocytoma than in ganglioglioma and DNET.
Keywords: corticography, epilepsy surgery, high‐frequency oscillations, tumor‐related epilepsy
Key Points.
Preresection fast ripples occur predominantly in epileptogenic tumor and peritumoral tissue
Fast ripple rates are higher in glioma and extraventricular neurocytoma than in ganglioglioma and DNET
Fast ripples in intraoperative ECoG can be used to help guide resection in tumor‐related epilepsy surgery
1. INTRODUCTION
Epilepsy is the most common presenting symptom in patients with low‐grade brain tumors, with an incidence of approximately 75%–90%. 1 , 2 Patients with higher grade tumors may also present with epileptic seizures, although this is less common. 3 When seizures continue with pharmacological treatment in otherwise indolent low‐grade tumors, surgical removal of the tumor becomes a treatment option. Gross–total resection of the tumor is sufficient to achieve seizure freedom in 65%–83% of the cases. 4 , 5 , 6 , 7 , 8 , 9 Although some guidelines state that exploration with invasive electroencephalography (EEG) is only required when the tumor is close to eloquent cortex, 10 the seizure onset zone may be located centimeters away from the tumor. 11 , 12 Several studies have shown that seizure freedom is more often achieved in cases where electrocorticography (ECoG) is used. 13 , 14 , 15 , 16
Intraoperative ECoG identifies interictal epileptic discharges, particularly spikes, to delineate epileptic tissue outside of the visible or palpable lesion. Recently, high‐frequency oscillations (HFOs), divided into ripples (80–250 Hz) and fast ripples (250–500 Hz), have been shown to be more specific markers than spikes in ECoG to delineate the epileptogenic tissue. 17 , 18 , 19 HFOs are also present in brain tumor‐related epilepsy, and are found at a higher rate in patients with mutant Krebs cycle enzyme isocitrate dehydrogenase 1 (IDH1) than in those without. 20 Although postoperative seizure control in brain tumor‐related epilepsy is generally good, tailoring based on HFOs might improve the seizure freedom rate. This work retrospectively analyzed intraoperative ECoG findings in patients who underwent tumor‐related epilepsy surgery. We investigated the distribution of ripples and fast ripples over the tumor, tissue surrounding the tumor, and tissue outside the resection area, and compared different histopathological types of tumors. Our main research question was: can we use intraoperative HFOs to guide tumor‐related epilepsy surgery?
2. MATERIALS AND METHODS
2.1. Cohort
We consecutively selected patients with refractory epilepsy who underwent brain surgery with intraoperative ECoG at the University Medical Center Utrecht between 2008 and 2015 and had a low‐grade (≤ World Health Organization [WHO] Grade II) intrinsic brain tumor confirmed by pathology. Intraoperative ECoG was used in all patients in whom seizure control, rather than tumor resection, was the main reason for surgery. Patients were only included if ECoG was recorded both before and after the resection, with a minimum sample frequency of 2048 Hz, and if photographs of the electrode positions and pre‐ and postoperative magnetic resonance imaging (MRI) were available for anatomic coregistration. Patients who had prior brain surgery or chemotherapy were excluded. The surgical outcome was determined at the latest follow‐up, with a minimum of 1 year, and quantified according to the Engel scale. 21 Good outcome was defined as Engel IA. The presence of residual tumor tissue was determined from the postsurgical MRI radiology report of the first MRI after surgery. Tumors were divided into mesiotemporal and superficial tumors based on the MRI. Superficial tumors are directly exposed by the craniotomy and could be covered with ECoG grid electrodes, whereas mesiotemporal tumors were not directly visible to the surgeon and were covered with electrode strips that were slid around the temporal pole or under the base of the temporal lobe. The institutional ethical committee approved the study and waived the need for written informed consent because of the retrospective character of the study, provided that data were coded and handled anonymously.
2.2. Surgical procedure
The surgical procedure was performed under general anesthesia with propofol in all patients. The location of the craniotomy was determined by the neurosurgeon who exposed the tissue to be removed and several centimeters of surrounding tissue. Intraoperative ECoG was used to localize epileptogenic tissue based on epileptic spikes, and was used for functional cortical mapping when needed. ECoG was recorded with 4 × 8 electrode grids and 1 × 6 or 1 × 8 electrode strips (Ad‐Tech), which consisted of platinum electrodes with 4.2‐mm2 contact surface, embedded in silicone, with 1‐cm interelectrode distance. Signals were recorded with a 64‐channel EEG system (Micromed), at 2048‐Hz sample frequency, with a low‐pass filter of 538 Hz. ECoG was recorded before the first resection, and after subsequent resections (there are often several staged resections during the surgery, with ECoG following each one). The grid and strips could be placed in various positions, and ECoG was recorded in each position for several minutes. Propofol anesthesia was stopped before recording, until a continuous stable background pattern was present, before interpreting the ECoG.
2.3. Analysis of ECoG
The preresection ECoG (pre‐ECoG) before the first resection and the postresection ECoG (post‐ECoG) after the last resection of each surgery were analyzed. The last 1‐min epoch of each ECoG position was selected for analysis, to minimize the effect of anesthesia. Data were analyzed in a bipolar montage. We used an automated HFO detection algorithm, based on Burnos et al., 22 and adapted for our intraoperative ECoG data, with a high‐pass finite impulse response filter at 80 Hz for ripples and at 250 Hz for fast ripples. The detected HFOs were visually checked in Stellate Harmonie Reviewer (v7.0) by two of three reviewers (N.v.K./W.Z./M.Z.) to correct for artifacts, false positives, and missed HFOs. Bipolar channels were reviewed by using a split screen to simultaneously visualize ripples (>80 Hz, 5 µV/mm) and fast ripples (>250 Hz, 1 µV/mm).
2.4. Electrode localization
The tumor volume was segmented in a presurgical three‐dimensional (3D) fluid‐attenuated inversion recovery MRI (1 × 1 × 1 mm), and the resection volume was segmented in a postsurgical MRI (3D T1, .6 × .6 × .6 mm). A rendering of the cortical surface was made from a presurgical 3D T1 MRI with SPM12 and 3D Slicer (v4.5.0‐1). The location of the ECoG electrodes was determined by comparing the gyral pattern on photographs of the grids to the cortex rendering (Figure 1). Electrode positions were defined as being located on tumor, on tissue that was later resected and was not tumor (peritumoral), or on tissue outside of the resection. The electrodes covering mesiotemporal tumors were not visible to the surgeon or on photographs; therefore, we considered the first three electrodes of the mesiotemporal strip to be located on the tumor. These three electrodes were included in the resection in all mesiotemporal tumors.
FIGURE 1.
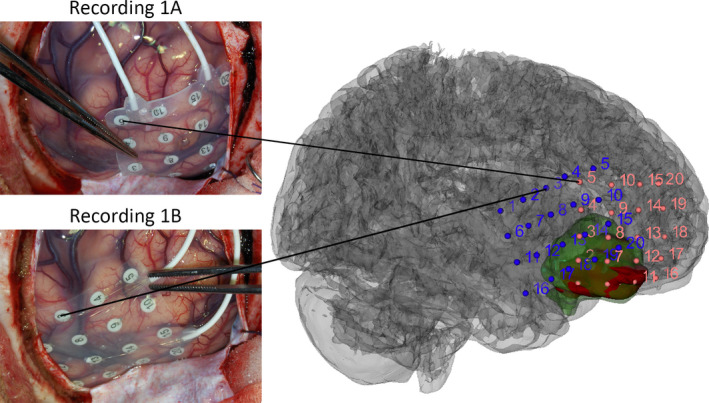
Example of electrode positions, tumor location (red), and resected area (green) in Patient 64, with a right fronto‐orbital tumor. The tumor and resected volumes were determined from the presurgical fluid‐attenuated inversion recovery magnetic resonance imaging (MRI) and the postsurgical T1 MRI, respectively. The electrode grid was positioned at Recording 1A first. After recording approximately 5 min of continuous background electrocorticogram, the grid was placed on Recording 1B for another 5 min of recording
2.5. Data analysis
The rate of events (ripples or fast ripples) was calculated as the number of events per minute, and determined per patient per electrode for all electrodes. We compared the event rates between electrodes on tumor tissue, on peritumoral tissue, and on tissue outside the resection (Friedman test), and between tumor types (Kruskal–Wallis test). The difference in rate between patients with a mesiotemporal or superficial tumor and temporal or extratemporal tumor was assessed with a Mann–Whitney U test.
We compared the event rates of both pre‐ECoG and post‐ECoG between patients with good or poor outcome and between patients with or without residual tumor tissue and with or without BRAF mutation (Mann–Whitney U test). We also tested for differences in outcome between patients with mesiotemporal and superficial tumors, and patients with and without residual tumor tissue on the first postoperative MRI (Fisher exact test).
All statistical tests were performed with IBM SPSS version 25. Probability values smaller than .05 were considered significant. We did not correct for multiple comparisons, because we considered reporting potential effects more relevant than strict reduction of type 1 errors.
3. RESULTS
3.1. Cohort
We evaluated 72 patients with low‐grade tumors and intraoperative ECoG. Twelve had to be excluded because not all photographs of the electrode positions were available. Eight patients were excluded because the sample frequency was too low, six were excluded because they had prior brain surgery, four had no post‐ECoG, and the quality of the data of one patient was too low for HFO analysis. Therefore, we included 41 patients (20 female) with a mean age of 16.6 years (range = 10 months–50 years) at surgery (Table 1). Eleven patients were also included in our previous retrospective study on HFOs. 19 , 23 Twenty‐one patients had a superficial tumor, and 20 had a mesiotemporal tumor. Twenty‐five patients had a ganglioglioma, seven had a dysembryoplastic neuroepithelial tumor (DNET), seven had a glioma, and two had an extraventricular neurocytoma. The gliomas included four diffuse astrocytomas, of which one was Grade II and IDH1 mutant, and the others were Grade I and IDH1 wild type. The other gliomas consisted of a pleomorphic xanthoastrocytoma (Grade II), a desmoplastic infantile astrocytoma (Grade I), and an oligodendroglioma (Grade II). All tumors were IDH1 wild type except for the one diffuse astrocytoma. Eighteen patients had a mutation in BRAF V600E, 19 patients had no BRAF mutation, and in four patients BRAF mutations were undetermined. There was no significant difference in the rate of pre‐ECoG ripples (p = .11), fast ripples (p = .63), post‐ECoG ripples (p = .12), or fast ripples (p = .07) between patients with and without BRAF mutation. Twenty‐nine patients had a good surgical outcome (Engel IA), of whom five were still using antiepileptic medication.
TABLE 1.
Patient characteristics
| Characteristic | Value |
| Patients, n | 41 |
| Male/female, n | 21/20 |
| Age, mean years (range) | 16.6 (10 months–50 years) |
| Tumor location, n | |
| Mesiotemporal | 20 |
| Left | 8 |
| Right | 12 |
| Superficial | 21 |
| Left | 8 |
| Right | 13 |
| Frontal | 6 |
| Parietal | 1 |
| Temporal | 14 |
| Pathology, n | |
| Ganglioglioma Grade I | 25 |
| DNET Grade I | 7 |
| Glioma (Grade I/Grade II) | 7 (4/3) |
| Extraventricular neurocytoma Grade II | 2 |
| Outcome, n (follow‐up) | |
| Engel IA | 29 (29 months) |
| >Engel IA | 12 (41 months) |
Abbreviation: DNET, dysembryoplastic neuroepithelial tumor.
3.2. ECoG analysis
We analyzed 1901 bipolar channels for pre‐ECoG (average = 46 per patient) and 1048 channels for post‐ECoG (average = 26 per patient). In pre‐ECoG, 388 channels were located on the tumor, 524 on peritumoral tissue, and 989 outside the resection. Three patients showed neither ripples nor fast ripples in pre‐ and post‐ECoG, and another 20 patients did not have fast ripples in pre‐ and post‐ECoG.
The rate of ripples in pre‐ECoG was not significantly different between electrodes overlying tumor, on resected peritumoral tissue, or on tissue outside the resection (Figure 2A). The rate of fast ripples was higher in electrodes on tumor and peritumoral tissue than outside the resection (mean .5/electrode/min in tumor, .3/electrode/min in peritumoral, .0/electrode/min outside, χ2[2] = 6.7, p = .035). Post hoc tests showed that the fast ripple rates in tumor and peritumoral tissue were not significantly different (Figure 2B).
FIGURE 2.
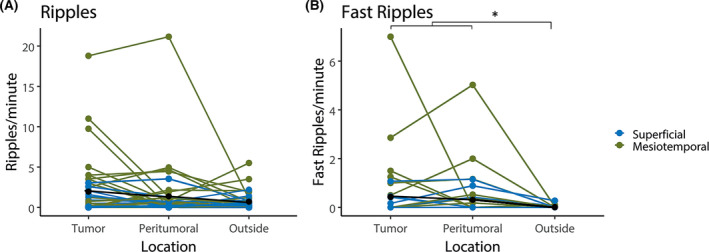
Rates of ripple (A) and fast ripples (B) per electrode per minute in preresection electrocorticography in channels located on tumor tissue, on peritumoral tissue, and outside the resection. Patients with a mesiotemporal tumor are shown in green, and those with a superficial tumor shown in blue; average is shown in black. Ripple rates are not significantly different on tumor tissue, on peritumoral tissue, or outside the resection. Fast ripple rates in tumor and peritumoral tissue are higher than outside the resection (*p = .035)
The rates of pre‐ECoG ripples and fast ripples on tumor were significantly different between the four pathology groups (χ2[3] = 9.47, p = .024 for ripples, χ2[3] = 17.75, p < .001 for fast ripples, Figure 3). Post hoc analysis showed significantly higher rates of fast ripples in extraventricular neurocytoma compared to ganglioglioma and DNET, and in glioma compared to ganglioglioma. The rates of ripples and fast ripples on peritumoral tissue and tissue outside the resection were not significantly different between the four pathology groups.
FIGURE 3.
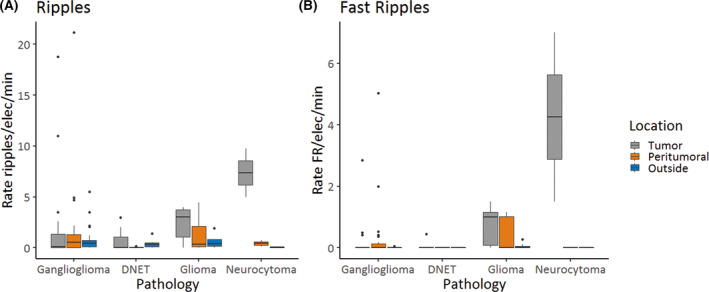
Boxplots showing rates of ripples (A) and fast ripples (FR; B) per electrode (elec) per minute in preresection electrocorticography in different pathologies. Each box shows the 25th to 75th percentiles, with a thick line at the median. Whiskers point to the highest and lowest values no further than 1.5*interquartile range. Outliers are indicated by dots. The rate of ripples and the rate of FR on tumor were significantly different between the four pathology groups (χ2[3] = 9.47, p = .024 for ripples, χ2[3] = 17.75, p < .001 for FR). Post hoc analysis showed significantly higher rates of FR in extraventricular neurocytoma compared to ganglioglioma and dysembryoplastic neuroepithelial tumor (DNET), and in glioma compared to ganglioglioma
Fast ripples were more often present in mesiotemporal tumors (12/20 patients) than in superficial tumors (6/21 patients). The rate of ripples in pre‐ECoG was significantly higher in patients with a mesiotemporal tumor than a superficial tumor (mean = 1.6/electrode/min vs. .5/electrode/min, U = 331, p = .002). The same positive trend was seen for fast ripple rates, although this was not significant (mean = .3/electrode/min vs. .1/electrode/min, U = 271, p = .07). Also, temporal tumors showed a trend toward higher rates of ripples and fast ripples compared to extratemporal tumors (temporal ripples: 1.2/electrode/min, extratemporal ripples: .5/electrode/min, p = .07; temporal fast ripples: .2/electrode/min, extratemporal fast ripples: .0/electrode/min, p = .05).
3.3. Pre‐ECoG events and outcome
The rate of ripples and fast ripples in pre‐ECoG was not different between patients with good or poor outcome (Engel IA vs. Engel IB and higher). When split out between channels on tumor, peritumoral, and outside the resection, only the rate of ripples outside the resection was significantly higher in patients with good outcome (U = 86.0, p = .011). Four patients showed fast ripples outside the resection, of whom three were not seizure‐free after surgery. These four patients also showed spikes outside the resection area, but it was decided not to extend the resection, because the spikes were far from the resection cavity, or because it was agreed the hippocampus would not be resected. One of them also showed fast ripples in post‐ECoG.
There was no difference in outcome between patients with mesiotemporal and superficial tumors (p = .734), or between patients with and without residual tumor tissue (p = .30).
3.4. Post‐ECoG events and outcome
The rate of ripples in post‐ECoG was higher in patients with good outcome than in patients with poor outcome (U = 99.5, p = .03), but did not differ for patients with a mesiotemporal or superficial tumor (U = 223.5, p = .72). The rate of ripples in post‐ECoG was not significantly different in patients with or without residual tumor tissue (U = 179.5, p = .68). In the 27 patients with post‐ECoG ripples, this rate was not significantly different from pre‐ECoG channels outside the resection (mean pre‐ECoG .9/min vs. post‐ECoG .6/min, Z = 140, p = .40).
Only three patients showed fast ripples in post‐ECoG. None showed residual tumor on postoperative MRI. Two of them had a recurrent seizure after discontinuation of antiepileptic drugs, but are seizure‐free since restarting these drugs for more than 6 years. The third patient with fast ripples in post‐ECoG is seizure‐free without antiepileptic drugs.
4. DISCUSSION
We explored the distribution of HFOs in intraoperative ECoG in various types of low‐grade brain tumors, and found that fast ripple rates were higher in electrodes on tumor and peritumoral tissue than outside the resection, indicating that they can be used to help guide the resection. Ripples did not show a difference between different locations. We found that fast ripple rates were higher in glioma and extraventricular neurocytoma than in ganglioglioma and DNET. The ripple rate outside the resection, both in pre‐ECoG and in post‐ECoG, was higher in patients with good outcome. Fast ripples outside the resection and in post‐ECoG seem to be related to seizure recurrence.
Fast ripple rates were higher on tumor and peritumoral tissue than outside the resection, confirming the epileptogenic nature of the tissue removed. The finding that rates did not differ between tumor and peritumoral tissue confirms what has been found with spikes before: it is not just the tumor itself that is epileptogenic; the peritumoral tissue is part of an extended epileptogenic network. 11 The outcome of tumor‐related epilepsy surgery is generally good, with 65%–83% seizure freedom. 4 , 5 , 6 , 7 , 13 In this study, 71% of the patients became completely seizure‐free (Engel IA), and 90% became free of disabling seizures (Engel I). All surgeries were tailored on epileptic spikes and spike patterns, which has been shown to increase seizure freedom rates before. 13 , 14 , 15 , 16 With this high seizure freedom rate, it is statistically difficult to retrospectively prove superiority of a biomarker over the clinically used method. Several studies have shown that the resection of especially fast ripples is related to good outcome. 19 , 24 Almost all channels with fast ripples in this study were resected, which may have contributed to the high number of seizure‐free patients. Only four patients had fast ripples outside the resection in pre‐ECoG, of whom three had recurrent seizures. Fast ripples in post‐ECoG were found in three patients, of whom two were not seizure‐free. The number of patients with poor outcome in this study was too small to draw conclusions about the relationship between fast ripples and outcome, but trends are concordant with our earlier work, demonstrating that postresection fast ripples predict poor outcome. 19 , 23 As none of the tested predictors (HFO rates, tumor location, tumor type) was significantly different between patients with good or poor outcome, we did not further elaborate in statistical models with multiple predictors.
Ripple rates were not different on tumor, peritumoral tissue, or tissue outside resection. Interestingly, we found a significantly higher rate of ripples in both pre‐ and post‐ECoG outside the resection in patients with good outcome. This is counterintuitive with the idea that ripples are biomarkers for epileptogenesis. We have seen this same trend in a heterogeneous group of pathologies before, 25 which appeared to be mainly ripples without spikes, which we hypothesized might be physiological ripples. Distinction between ripples with and without spikes might help to differentiate pathological from potentially physiological ripples. Our postresection ripples were a mixture of ripples on spikes and ripples without spike, but we did not mark all spikes for this study. In future studies, it would be interesting to differentiate pathological from potential physiological ripples to help the discussion of their predictive value. This study includes many patients with temporal lobe epilepsy, and it is known that mesiotemporal structures often produce physiological ripples. 26 Mesiotemporal tumors showed significantly higher rates of ripples and borderline significantly higher rates of fast ripples than superficial tumors. This is in line with our expectations, as we know that the hippocampus is easily involved in ictogenesis. The high HFO rates are probably a summation of physiological and pathological HFOs. 27
Glioma and extraventricular neurocytoma showed higher rates of HFOs than ganglioglioma and DNET. There seems to be no relationship between mesiotemporal or superficial location of these tumors. The higher rate might possibly be related to the higher grade of those tumors, which can be more infiltrative, compared with ganglioglioma and DNET, which were primarily WHO Grade I. One might hypothesize that more affected tissue will produce more pathological signals. One study found higher rates of HFOs in patients with IDH1 mutant tumors. 20 Our cohort only included one patient with an IDH1 mutant tumor, who showed both ripples and fast ripples but not at particularly high rates. Higher rates of HFOs are also found in patients with higher seizure frequency, 28 , 29 but seizure frequency data were not available for this cohort. These findings should be confirmed in a more equally distributed study population, as there were only two patients with extraventricular neurocytoma in this study.
All tumor tissue as identified on the intraoperative photographs and cortex rendering was removed, but the postoperative MRI identified some remaining tumor tissue in 14 patients. In all patients, this was just a rim of tumor tissue in the border of the resection cavity, macroscopically not identified on photographs. Therefore, it was not possible to assign electrodes to areas with residual tumor tissue to determine whether epileptic signals were still present in those specific areas. The presence of residual tumor did not correlate with seizure outcome in this study.
This study focused on low‐grade brain tumors, with the primary goal of achieving seizure freedom. The use of intraoperative ECoG for brain tumor‐related epilepsy is still under debate. ECoG is often used only when the tumor is close to eloquent cortex, which biases the results of systematic reviews on the predictive value for seizure outcome. 6 , 7 Studies including groups with and without the use of ECoG show better seizure outcome in cases where the lesion and surrounding epileptogenic tissue were resected. 13 , 14 , 15 , 16 We found fast ripples in similar rates on tumor and peritumoral tissue, indicating that tissue outside of what was identified by imaging or direct visualization/palpation may be undergoing a “subdetection” level of invasion by the tumor, and seizing in response to this invasion. One might hypothesize that HFOs reveal this damaged tissue beyond the visual tumor. It would be interesting to expand these findings to high‐grade tumors, as epilepsy is common in those patients as well. One large series of 648 patients showed preoperative seizures in 24% of the patients, and 77% seizure freedom after resection. 3 Although the primary goal in these surgeries is to stop tumor growth, tailoring these resections with adjuvant ECoG measurement might further improve seizure freedom rates, and therefore quality of life, in these patients, 30 particularly because the presence of epilepsy is a prognostic factor for longer survival in glioblastoma patients. 31 One study found HFOs in high‐grade brain tumor patients in similar rates as in low‐grade tumor patients. 20
To conclude, we found that fast ripple rates in intraoperative ECoG are higher on tumor tissue and on peritumoral resected tissue than on tissue outside the resection, indicating that fast ripples can be used to identify the epileptogenic region in tumor‐related epilepsy surgery. Ripples did not show such a relation. Tailoring tumor‐related epilepsy surgery based on ECoG‐detected spikes seems useful to identify the epileptogenic region, as almost all patients in this study became seizure‐free. Fast ripple rates were higher in glioma and extraventricular neurocytoma than in ganglioglioma and DNET. It would be interesting to investigate the added value of intraoperative ECoG in higher grade brain tumor resection as well.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ACKNOWLEDGMENTS
N.v.K. was supported by the Dutch Brain Foundation (2013‐139), W.Z. was supported by the UMC Utrecht Alexandre Suerman Stipendium 2015, K.J.M. was supported by the Van Wagenen Foundation, E.A. was supported by the AMC Foundation and Epilepsy Institutes of the Netherlands Foundation, and M.Z. was supported by ZonMw veni 91615149 and ERC Starting grant 803880. The authors thank L. Ietswaard and M. van ’t Klooster for help with the ECoG database.
REFERENCES
- 1. van Breemen MSM, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–30. [DOI] [PubMed] [Google Scholar]
- 2. Pallud J, Audureau E, Blonski M, Sanai N, Bauchet L, Fontaine D, et al. Epileptic seizures in diffuse low‐grade gliomas in adults. Brain. 2014;137(2):449–62. [DOI] [PubMed] [Google Scholar]
- 3. Chaichana KL, Parker SL, Olivi A, Quiñones‐Hinojosa A. Long‐term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas: clinical article. J Neurosurg. 2009;111(2):282–92. [DOI] [PubMed] [Google Scholar]
- 4. Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, et al. Seizure characteristics and control following resection in 332 patients with low‐grade gliomas. J Neurosurg. 2008;108(2):227–35. [DOI] [PubMed] [Google Scholar]
- 5. Minkin K, Klein O, Mancini J, Lena G. Surgical strategies and seizure control in pediatric patients with dysembryoplastic neuroepithelial tumors: a single‐institution experience. J Neurosurg Pediatr. 2008;1(3):206–10. [DOI] [PubMed] [Google Scholar]
- 6. Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low‐grade gliomas. A review. J Neurosurg. 2011;115(2):240–4. [DOI] [PubMed] [Google Scholar]
- 7. Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012;53(1):51–7. [DOI] [PubMed] [Google Scholar]
- 8. Lamberink HJ, Otte WM, Blümcke I, Braun KPJ, Aichholzer M, Amorim I, et al. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19(9):748–57. [DOI] [PubMed] [Google Scholar]
- 9. Faramand AM, Barnes N, Harrison S, Gunny R, Jacques T, Tahir MZ, et al. Seizure and cognitive outcomes after resection of glioneuronal tumors in children. Epilepsia. 2018;59(1):170–8. [DOI] [PubMed] [Google Scholar]
- 10. Rosenow F, Menzler K. Invasive EEG studies in tumor‐related epilepsy: when are they indicated and with what kind of electrodes? Epilepsia. 2013;54(Suppl 9):61–5. [DOI] [PubMed] [Google Scholar]
- 11. Mittal S, Barkmeier D, Hua J, Pai DS, Fuerst D, Basha M, et al. Intracranial EEG analysis in tumor‐related epilepsy: evidence of distant epileptic abnormalities. Clin Neurophysiol. 2016;127(1):238–44. [DOI] [PubMed] [Google Scholar]
- 12. Pallud J, Capelle L, Huberfeld G. Tumoral epileptogenicity: how does it happen? Epilepsia. 2013;54(Suppl 9):30–4. [DOI] [PubMed] [Google Scholar]
- 13. Sen YP, Zheng SF, Wang F, Kang DZ, Lin YX. Surgery guided with intraoperative electrocorticography in patients with low‐grade glioma and refractory seizures. J Neurosurg. 2018;128(3):840–5. [DOI] [PubMed] [Google Scholar]
- 14. Rassi‐Neto A, Ferraz FP, Campos CR, Braga FM, Paulo S, Jacurici R, et al. Patients with epileptic seizures and cerebral lesions who underwent lesionectomy restricted to or associated with the adjacent irritative area. Epilepsia. 1999;40(7):856–64. [DOI] [PubMed] [Google Scholar]
- 15. Sugano H, Shimizu H, Sunaga S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal‐lobe‐mass lesions. Seizure. 2007;16(2):120–7. [DOI] [PubMed] [Google Scholar]
- 16. Mikuni N, Ikeda A, Takahashi J, Nozaki K, Miyamoto S, Taki W, et al. A step‐by‐step resection guided by electrocorticography for nonmalignant brain tumors associated with long‐term intractable epilepsy. Epilepsy Behav. 2006;8(3):560–4. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs J, LeVan P, Châtillon C‐E, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132(Pt 4):1022–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zijlmans M, Jiruska P, Zelmann R, Leijten FSS, Jefferys JGR, Gotman J. High‐frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71(2):169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ‘van t Klooster MA , van Klink NEC, Zweiphenning WJEM, Leijten FSS, Zelmann R, Ferrier CH, et al. Tailoring epilepsy surgery with fast ripples in the intraoperative electrocorticogram. Ann Neurol. 2017;81(5):664–76. [DOI] [PubMed] [Google Scholar]
- 20. Feyissa AM, Worrell GA, Tatum WO, Mahato D, Brinkmann BH, Rosenfeld SS, et al. High‐frequency oscillations in awake patients undergoing brain tumor‐related epilepsy surgery. Neurology. 2018;90(13):e1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engel J, Van Ness P, Rasmussen T, Ojemann L. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical treatment of the epilepsies. 2nd ed. New York, NY: Raven Press; 1993. p. 609–21. [Google Scholar]
- 22. Burnos S, Hilfiker P, Sürücü O, Scholkmann F, Krayenbühl N, Grunwald T, et al. Human intracranial high frequency oscillations (HFOs) detected by automatic time‐frequency analysis. PLoS ONE. 2014;9(4):e94381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ‘van t Klooster MA , van Klink NEC, Leiten FSS, Zelmann R, Gebbink TA, Gosselaar PH, et al. Residual fast ripples in the intraoperative corticogram predict epilepsy surgery outcome. Neurology. 2015;85(2):120–8. [DOI] [PubMed] [Google Scholar]
- 24. Hussain SA, Mathern GW, Hung P, Weng J, Sankar R, Wu JY. Intraoperative fast ripples independently predict postsurgical epilepsy outcome: comparison with other electrocorticographic phenomena. Epilepsy Res. 2017;135:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Klink NE, ‘van t Klooster MA , Zelmann R, Leijten FSS, Ferrier CH, Braun KPJ, et al. High frequency oscillations in intra‐operative electrocorticography before and after epilepsy surgery. Clin Neurophysiol. 2014;125(11):2212–9. [DOI] [PubMed] [Google Scholar]
- 26. Frauscher B, von Ellenrieder N, Zelmann R, Rogers C, Nguyen DK, Kahane P, et al. High‐frequency oscillations in the normal human brain. Ann Neurol. 2018;84(3):374–85. [DOI] [PubMed] [Google Scholar]
- 27. Liu S, Parvizi J. Cognitive refractory state caused by spontaneous epileptic high‐frequency oscillations in the human brain. Sci Transl Med. 2019;11(514):1–14. [DOI] [PubMed] [Google Scholar]
- 28. Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High‐frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72(11):979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Klink NEC, ‘van t Klooster MA , Leijten FSS, Jacobs J, Braun KPJ, Zijlmans M. Ripples on rolandic spikes: a marker of epilepsy severity. Epilepsia. 2016;57(7):1179–89. [DOI] [PubMed] [Google Scholar]
- 30. Hamer HM, Hong SB. Is an epilepsy presurgical evaluation necessary for mid‐grade and high‐grade brain tumors presenting with seizures? Epilepsia. 2013;54(Suppl 9):56–60. [DOI] [PubMed] [Google Scholar]
- 31. Berendsen S, Varkila M, Kroonen J, Seute T, Snijders TJ, Kauw F, et al. Prognostic relevance of epilepsy at presentation in glioblastoma patients. Neuro Oncol. 2016;18(5):700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


