Abstract
Autumn senescence in aspen (Populus tremula) is precisely timed every year to relocate nutrients from leaves to storage organs before winter. Here we demonstrate how stem girdling, which leads to the accumulation of photosynthates in the crown, influences senescence. Girdling resulted in an early onset of senescence, but the chlorophyll degradation was slower and nitrogen more efficiently resorbed than during normal autumn senescence. Girdled stems accumulated or retained anthocyanins potentially providing photoprotection in senescing leaves. Girdling of one stem in a clonal stand sharing the same root stock did not affect senescence in the others, showing that the stems were autonomous in this respect. One girdled stem with unusually high chlorophyll and nitrogen contents maintained low carbon‐to‐nitrogen (C/N) ratio and did not show early senescence or depleted chlorophyll level unlike the other girdled stems suggesting that the responses depended on the genotype or its carbon and nitrogen status. Metabolite analysis highlighted that the tricarboxylic acid (TCA) cycle, salicylic acid pathway, and redox homeostasis are involved in the regulation of girdling‐induced senescence. We propose that disrupted sink‐source relation and C/N status can provide cues through the TCA cycle and phytohormone signaling to override the phenological control of autumn senescence in the girdled stems.
1. INTRODUCTION
During the growing season, a considerable amount of the mineral nutrients in a tree is located in the leaves to allow for efficient photosynthesis. Autumn senescence is a strategy deployed by trees to reallocate the mineral nutrients from the leaves in to the bark, stem, twigs, and roots (Black et al., 2001; Cooke & Weih, 2005) for re‐use next season. This is achieved by degradation of macromolecules such as protein, chlorophyll, nucleic acids, and lipids, and by the transfer of metabolites from leaves to storage organs. To allow for nutrient recovery, senescence should begin well before frost occurs but the longer leaves are actively photosynthesizing, the more carbohydrates can be produced. The timing of senescence is therefore a delicate trade‐off; late senescence facilitates more carbon gain and potential biomass production, but may compromise nutrient resorption. Obviously, there should be a strong selection pressure on trees to properly time their autumn senescence.
We have previously demonstrated strong genotypic control on the initiation of autumn senescence and that a given genotype of Populus tremula (European aspen) commences senescence around the same date every year (Fracheboud et al., 2009; Keskitalo et al., 2005; Michelson et al., 2018) regardless of weather conditions, suggesting that light cues provide the trigger. Other autumn phenology traits have been shown to be under photoperiodic control; bud set, for example, is controlled by phytochrome A and the CONSTANS and FLOWERING LOCUS T module (Böhlenius et al., 2006; Olsen et al., 1997). The light cue that triggers autumn senescence in aspen seems to not to be photoperiod per se (Michelson et al., 2018), but as photoperiod triggers bud set, and bud set is a prerequisite of autumn senescence, photoperiodic regulation is also involved in the regulation of senescence, albeit perhaps indirectly.
Leaves of aspen turn yellow in autumn as a consequence of chlorophyll degradation during senescence, but they also have the capacity to accumulate anthocyanins under certain conditions. Anthocyanin production is enhanced in response to environmental stresses such as nutrient deficiency, drought, low temperature, and high light (Hoch et al., 2003; Landi et al., 2015) and their accumulation is often associated with high carbohydrate levels (Murakami et al., 2008; Schaberg et al., 2008; Solfanelli et al., 2006). Anthocyanins absorb light—also in the ultra violet range—and therefore participate in photoprotection against excess light through light shielding, acting as antioxidants and participating in reactive oxygen species (ROS) scavenging, and through their sugar‐buffering role (Hoch et al., 2003; Landi et al., 2015; Lo Piccolo et al., 2020). In senescing leaves, anthocyanins have been proposed to prevent photo‐oxidative stress and thus prolong leaf life span and nutrient resorption (Hoch et al., 2003; Lo Piccolo et al., 2018). Anthocyanins and other leaf pigments are proposed to act as signals to animals interacting with the plants, including pollinators and frugivores (Archetti, 2009). The role of anthocyanins in autumn senescence has been debated in the literature (e.g., Archetti, 2009, Ougham et al., 2008) and it is unclear which of these functions are important in autumn leaves.
Sugar accumulation and an increase in the pool of free amino acids are associated with leaf senescence (Watanabe et al., 2013; Wingler et al., 2009; Wingler & Roitsch, 2008), and there is considerable crosstalk between primary carbon and nitrogen metabolism, ROS, and phytohormone signaling (Leon & Sheen, 2003; Yanagisawa et al., 2003). However, it is not clear which is the key signal that triggers autumn senescence, or if one even exists. In order to study the interaction between internal and external factors regulating senescence, we capitalized on a useful feature of our experimental system, aspen, namely that trees in a clonal stand are genetically identical and share the same root system, but the stems could be subjected to different treatments. In this contribution, we aimed to manipulate photosynthate levels and use a gardening practice known as stem girdling, an experimental strategy where the phloem is excised in a circle around the tree stem while leaving the xylem intact. Phloem transport of photosynthates from leaves to roots is blocked but since the xylem remains undamaged, root uptake and transport of water and nutrients are not affected. Even if phloem transport is disrupted by girdling in one aspen stem, other stems can provide photosynthates for the whole root system. A girdled stem can remain alive for many years and the root system seems to remain perfectly vital. Compared to girdling of a stem of an individual tree, the girdling of one stem of a clonal stand reduces the collateral effects on the root system and soil processes.
Aspen loads sugars into the phloem passively by diffusion (Hubeau et al., 2019), therefore the blocked phloem transport by girdling is expected to reduce phloem loading of sugars and lead to accumulation of photosynthates in the leaves. Regier et al. (2010) performed girdling on poplars (Populus deltoides × nigra cv. Dorskamp) to elucidate the interruption of carbohydrate transport and how this can influence plant winter survival and bud flush. Another study showed that a subset of genes initially identified as modulated by nitrogen availability also responded to girdling, demonstrating the interplay between nitrogen and carbohydrate metabolism (Cooke et al., 2003). Branch girdling of sugar maples (Acer saccarum March.) resulted in increased foliar sugar concentrations and enhanced anthocyanin expression during autumn (Murakami et al., 2008). Despite that girdling has been applied to study carbon dynamics in forest trees (Rademacher et al., 2019), very few studies have investigated how girdling and consequently disrupted sink‐source relationship affects autumn phenology (Kraj, 2017). As far as we know this is the first detailed study on girdling effects on autumn senescence in free‐growing tree stands in natural conditions.
We expected that girdling would lead to the accumulation of photosynthates in the leaves, and that this could affect the onset of leaf senescence. In this study, we provide data from multiple years and in several genotypes. We first performed a pilot study on girdling and secondly on tree on the Umeå University campus, which has been studied extensively (Andersson et al., 2004; Bhalerao et al., 2003; Fracheboud et al., 2009; Keskitalo et al., 2005). Thirdly, we studied the effects of girdling on four other aspen stands. To study metabolic factors underlying the girdling‐induced responses, leaf pigment analysis, dynamic metabolomic data analysis, and differential correlation network analysis were employed and combined with transcript profiling. In addition, we studied how girdling affected the herbivore community in leaves. By combining all these data, we were able to get information of the interaction between (1) the timing of senescence, (2) herbivore preferences, (3) carbon and nitrogen allocation, and (4) the metabolic status of the tree.
2. MATERIALS AND METHODS
2.1. Plant material and girdling treatments
The aspen (Populus tremula L.) stand in the pilot experiment was grown in Obbola (63.42°N, 20.19°E), approximately 15 km north of Umeå, in Sweden. The 12 stems of ca. 30‐year old clonal stand were girdled to various extents in July 2006: either <3 cm (class I), 3–7 cm (class II) or > 10 cm (class III) section of the bark was removed with a sharp knife.
One stem of the tree (genotype 201) growing on the Umeå University campus (60.819°N, 20.311°E) was girdled on 2 July 2008 (the day of the year, DOY 184). Based on the pilot experiment, we chose to apply class II girdling for further experiments and removed a ~ 7 cm wide section of the bark.
On 15 June 2009 (DOY 166), one stem in four aspen stands in Täfteå, approximately 10 km east of Umeå (63.84°N, 20.52°E) was girdled. To minimize variation in light conditions, the chosen stands faced an open area—a field or a road.
The timing of girdling was in all three sub‐experiments such that the trees had fully expanded leaves but had not yet set bud. Girdling was done at approximately 1.5 m height and another stem of comparable size in the same stand was chosen as a control.
Weather conditions (daily mean of air temperature, precipitation, sunshine hours) for the autumns of 2008 and 2009 were obtained from Swedish meteorological and hydrological institute (SMHI, http://smhi.se) (Figure S1).
2.2. Chlorophyll and anthocyanin measurements
Chlorophyll and anthocyanin content indices were measured with CCM‐200 instruments (Opti‐sciences, Hudson, USA). In the pilot experiment, 10 leaves were measured on 16 September 2008 (DOY 260), 2 years after girdling. The girdled stems were divided into three damage classes based on the extent of the girdle: healed (class I), partially healed (class II), and unhealed (complete girdle, class III). The effect of the damage class on chlorophyll and anthocyanin content indices was tested with one‐way anova followed by Tukey's post hoc test, P < 0.05 considered significant (IBM SPSS Statistics, version 25). For the tree 201, 20 leaves were measured every day from 24 August to 24 September 2008 (DOY 237–269). For the four aspen stands, five leaves were measured twice per week from 6 August to 9 October 2009 (DOY 218–282).
2.3. Start and rate of senescence
The initiation of rapid chlorophyll degradation was chosen as a proxy for the start of senescence. The onset date and the rate of senescence were determined based on chlorophyll content index (CCI) by fitting the data with a non‐linear Boltzmann function (OriginPro version 9, 2019, OriginLab Corporation) as described in Figure S2.
2.4. Leaf carbon and nitrogen analysis
As the purpose of autumn senescence is nutrient, especially nitrogen, salvage, we determined carbon (C) and nitrogen (N) concentrations and C/N ratio in the leaves of control and girdled stems in the four aspen stands. Five leaves were sampled twice weekly from 10 August to 28 September 2009 (DOY 222–271) and their areas were determined with LAMINA image analysis software (Bylesjö et al., 2008). Leaves were pooled, freeze‐dried, and ground with a mortar and pestle. C and N concentrations (area and mass based) of the samples (~ 4 mg dry weight) were determined with a Flash EA 1112 Nitrogen and Carbon Analyzer (Thermo Scientific) in three technical replicates. The proportion (%) of remobilized N from the leaves of control and girdled stems was determined based on pre‐senescence and post‐senescence concentrations. The effect of girdling was tested with one‐way anova with Tukey's post hoc test, P < 0.05 considered significant (SPSS).
2.5. Transcriptomics
Two years after girdling, on 3 September 2010 (DOY 246), leaves from girdled and control stems of the tree 201 were sampled and mRNA was extracted and sequenced as described in Sundell et al. (2015). At that date, the girdled stem had initiated senescence, while the control stem had not. The gene expression data were deposited in the Populus tremula expression atlas of the Plant Genome Integrative Explorer platform (https://plantgenie.org/; Sundell et al., 2015). We used the data to identify differentially expressed genes (DEGs, |log2 fold change| ≥ 1.5) in the leaves between control and girdled stems (Table S1). Gene ontology (GO) enrichment analyzes of DEGs were performed with PANTHER (http://pantherdb.org/; Mi et al., 2018) using the best hits in Arabidopsis thaliana (Arabidopsis) (Table S2). The gene expression patterns were studied at one time point during leaf senescence, thus the genetic regulation behind girdling‐induced senescence should be interpreted with caution. However, we believe that a snapshot of gene expression patterns during girdling‐induced senescence complemented with time‐course metabolite data can provide insight into which biological processes are affected in the leaves of girdled stems.
2.6. Metabolomics
Leaf sampling for pigment analysis was performed 11 times from 13 August to 24 September 2008 (DOY 226–268) from the control and girdled stems of the tree 201. Leaf sampling for GC–MS metabolite analysis was performed twice a week throughout the autumn, from 10 August to 28 September 2009 (DOY 222–271) from the four aspen stands. In both experiments, samples were collected between 11 AM and 1 PM. Five visually healthy short shoot leaves were collected from the lower part of the canopy into one scintillation vial and immediately frozen in liquid nitrogen and stored at −80°C. Leaf samples were ground in liquid nitrogen with a mortar and pestle.
Pigments (chlorophyll a, chlorophyll b, lutein, beta‐carotene, antheraxanthin, violaxanthin, neoxanthin, zeaxanthin) were quantified with HPLC‐DAD according to Król et al. (1995). De‐epoxidation state (DEPS) of xanthophyll cycle pigments was determined based on the equation: DEPS = (0.5 × Ax + Zx)/(Ax + Vx + Zx), where Ax, Vx, and Zx stand for antheraxanthin, violaxanthin, and zeaxanthin, respectively.
Soluble metabolites were extracted and analyzed by gas chromatography–mass spectrometry (GC–MS) from the leaf powder (~10 mg fresh weight) with a method described in Gullberg et al. (2004) and Kusano et al. (2011). Peak areas were quantified with in‐house software (Swedish Metabolomics Centre, SMC) and metabolites were annotated based on retention index and mass spectra using reference standards, in‐house libraries (SMC), and mass spectral libraries (NIST, Golm, FiehnLib). Metabolite peak areas were normalized by the peak area of the internal standard, succinic acid‐d5, and the fresh weight of the sample.
Statistics were performed with normalized log10‐transformed and Pareto‐scaled data. The main effects of time (DOY), treatment (girdling), and their interaction (time × treatment) on metabolite levels and ratios were tested with two‐way repeated measures anova, FDR‐adjusted P < 0.05 considered significant (MetaboAnalyst, Chong et al., 2018). Heatmaps were generated with multiple experiment viewer (MeV, version 4.9, http://mev.tm4.org) to visualize the temporal patterns in the metabolite levels in the leaves of control and girdled stems. Metabolite levels were normalized to z‐scores and hierarchical clustering was based on Pearson correlations and average linkage.
Principal component analysis (PCA, Simca P+, version 15, Umetrics, Sweden) was performed to display the general variation in the GC–MS data. anova‐simultaneous component analysis (ASCA) was performed with 100 permutations to test the effects of time, treatment and interaction (time × treatment) on the overall metabolite profile (MetaboAnalyst). Dynamic metabolomic data analysis by multivariate Empirical Bayes analysis (MEBA) for time‐series was performed to identify metabolites that showed the most variable temporal patterns between control and girdled stems (MetaboAnalyst).
Orthogonal projection to latent structures discriminant analysis (OPLS‐DA) was performed to identify metabolites that accounted for the separation of control and girdling samples (Simca P+). OPLS‐DA models were constructed separately for stand 1, which did not show girdling‐induced senescence or depletion of chlorophyll content, and for stands 2, 3, and 4, which did. Both models were of good quality with R2X, R2Y, and Q2 > 0.9 and significant with CV‐anova (analysis of variance of cross‐validated predictive residuals) of P < 0.05 (Table S3). A shared and unique structures (SUS)‐plot was constructed in order to detect which metabolites responded similarly and differently to girdling between stand 1 and the other tree stands.
Differential correlations were determined using the DiffCorr package (version 0.4.1, Fukushima, 2013) in R. Pairwise metabolite‐metabolite correlations were determined based on Pearson correlation coefficients and the differential correlations between control and girdling treatments were determined based on Fisher's z‐test. Differential correlations were visualized in a network using the MetScape plug‐in in Cytoscape (version 3.7.0, Shannon et al., 2003). Metabolite‐metabolite pairs that had significantly different correlations (P < 0.05) and > 0.7 difference in correlation coefficients were included in the network. Edges were colored based on the change in correlation coefficient in response to girdling (orange = positive, blue = negative). The metabolites (nodes) with the highest degrees are regarded as important metabolite “hubs,” which can be involved in the regulation of girdling‐induced responses.
3. RESULTS AND DISCUSSION
3.1. Girdling can override the phenological control of autumn senescence and induce flowering in aspen
Firstly, we wanted to determine the extent to which the functional phloem could be removed without killing the tree. Therefore, in 2006 we started a pilot experiment where several stems of a clonal stand of aspen were girdled to various extents. This stand of aspen had been observed for 10 years before the start of the experiment, and all stems had had a fully coordinated autumn senescence. All girdled stems remained viable irrespective of the extent of the girdle, but distinct senescence behavior among stems developed. Two years after girdling in 2008, stems with only a 3 cm section of the bark removed (class I) had efficiently healed the wound (Figure 1A), and they did not differ visually from non‐girdled trees. Stems that had had a wider section of bark removed (class II) had partially sealed the wound (Figure 1A) and therefore in part restored phloem transport between the crown and the root, while those that had a ~ 10 cm wide section removed (class III) were unable to heal it, and therefore maintained an interrupted phloem transport system (Figure 1A). Leaves from the three classes of stems differed in their content of chlorophyll and anthocyanins in the autumn (Figure 1B). Class I stems that had efficiently healed the girdle had higher chlorophyll and lower anthocyanin levels than class III stems that displayed a complete girdle (Figure 1C). Class II stems had an intermediate phenotype (Figure 1C). This effect progressed from year to year; chlorophyll degradation and anthocyanin accumulation appeared to start earlier in the summer but all stems were still alive in 2011, 5 years after girdling, when the trees were felled.
FIGURE 1.

Girdling of aspen trees in the pilot experiment. Stems were girdled in 2006 to various extents and after 2 years, in 2008, the trees were scored and divided in three classes: healed (class I), partially healed (class II), and unhealed (class III) (A). Classes differed with respect to their senescence phenotypes (B) and chlorophyll and anthocyanin content indices (C). Bars represent mean ± se, n = 4. Different letters indicate significant differences in chlorophyll content index or anthocyanin content index between groups (one‐way anova, Tukey's post hoc test, P < 0.05)
We also observed a strong effect of girdling on flowering. Mature aspens in northern Europe flower very infrequently. However, class III stems flowered every year (2007–2010), class I stems did not flower at all and class II stems had, again, an intermediate phenotype. Stem girdling is commonly used in horticulture to enhance flowering and fruit yield (reviewed in Goren et al., 2004). Enhanced allocation of photosynthates from the leaves to remaining sink tissues above the girdle, such as into developing buds, could explain the induced flowering the following season. From this experiment, we concluded that aspen stems survived extensive girdling but that it resulted in chlorophyll depletion, anthocyanin accumulation and induced flowering.
3.2. Girdling results in early onset of leaf senescence and slow chlorophyll degradation
One of the seven stems of the aspen stand on the Umeå university campus (genotype 201) was girdled in July 2008 and chlorophyll and anthocyanin levels of stems were monitored throughout the autumn (Figure 2). The control stem started rapid chlorophyll degradation around 7th of September (DOY 251) consistent with other study years (Figure 2C, Keskitalo et al., 2005, Fracheboud et al., 2009). The girdled stem started chlorophyll degradation ca. 6 days earlier, around 1st of September (DOY 245, Figure 2C).
FIGURE 2.

Girdling of aspen tree 201 and photosynthetic pigments in the leaves of control and girdled stems in 2008. Senescence phenotype on 19 September (DOY 263, A), the girdle on 2 July (DOY 184, B), chlorophyll (C) and anthocyanin content indices (D) and the onset and rate of senescence during autumn (C). Data are means of 20 leaves (± se for anthocyanin content index). Pigment levels (E), de‐epoxidation state (DEPS) of xanthophyll cycle pigments (F), and chlorophyll‐to‐carotenoid ratio (Chl/Car, G). Details of statistical results are in Table S4. Data are mean of five leaves ± se. Two‐way anova (P < 0.01**, < 0.05*). DOY = the day of the year
Since mineral nutrient levels in the natural ecosystem are highly variable, and different aspen genotypes may have different responses to girdling, to confirm our observations, one stem in each of the four aspen stands was girdled in June 2009 (DOY 166) and its senescence behavior and metabolic status were compared to a control stem (Figures 3, 4). Consistent with the pilot experiment, the girdled stems (except in stand 1) contained less chlorophyll than control stems at the beginning of the measurement period in 2009 (Figure 3). Control stems in stands 1, 2, and 4 started to senesce in mid‐September (DOY 257–260). Stand 3 had lower pre‐senescence chlorophyll content and chlorophyll levels were already decreasing in August (Figure 3A) complicating the determination of senescence onset, which could have occurred earlier than estimated. Girdled stems in stand 2 and 4 started to senesce ca. 19 days earlier than control stems, that is, at the beginning of September, whereas in girdled stems in stands 1 and 3, no major changes in the onset of senescence were observed (Figure 3). However, all girdled stems showed lower rates of chlorophyll degradation compared to control stems.
FIGURE 3.
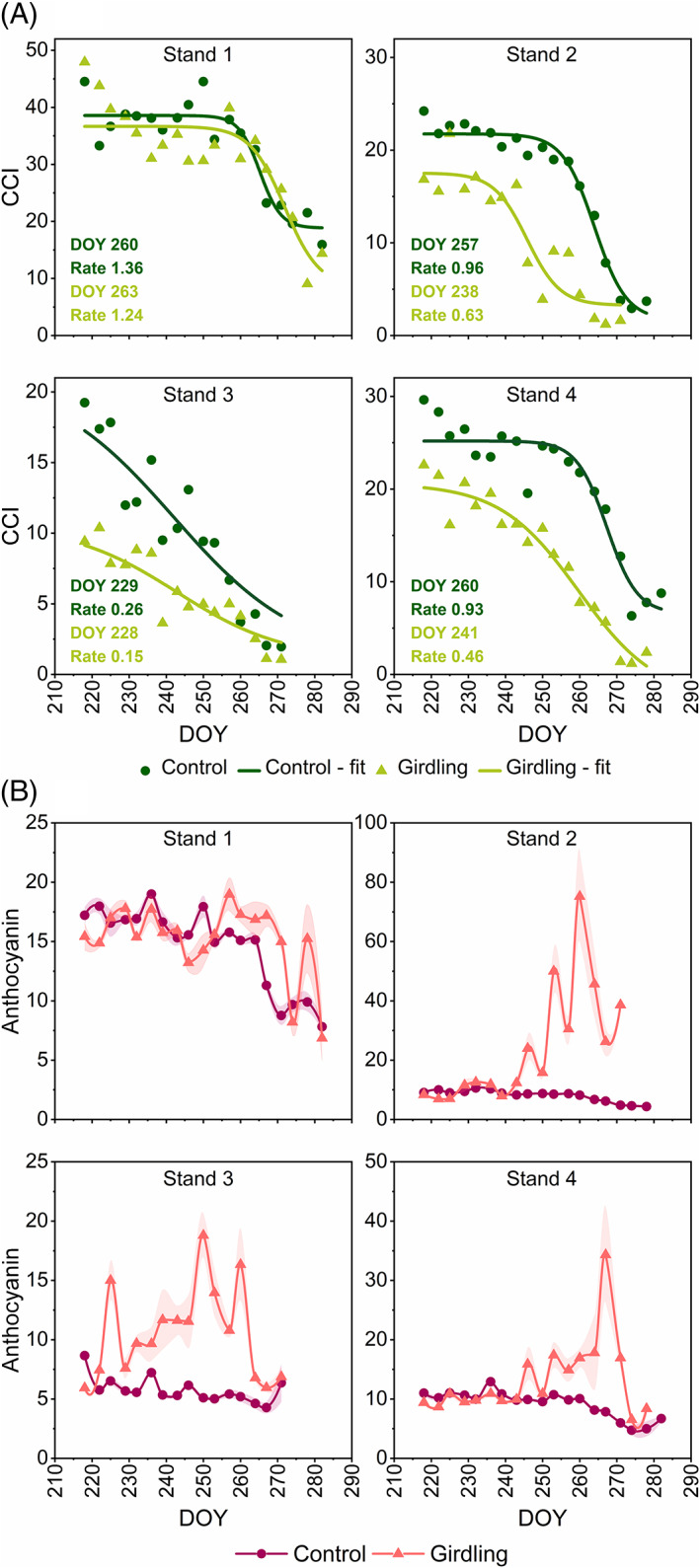
The effect of stem girdling on senescence onset and rate, and anthocyanin content in four aspen stands during autumn 2009. Chlorophyll (A) and anthocyanin (B) content indices in control and girdled stems are means of five leaves (± se for anthocyanin content index), note the different scales of y‐axes. DOY = the day of the year
FIGURE 4.

The effect of girdling on the leaf metabolite profile in four aspen stands during autumn 2009. Principal component analysis (PCA) score plots of metabolite data (133 metabolites) in the leaves of control and girdled stems was affected by time (DOY, the day of the year, A), by the girdling treatment (B) and by tree stand (C). Metabolites that showed significant changes in their levels across time or in response to girdling treatment were included in the heatmap (D) and the pattern for each cluster was visualized (mean z‐score ± standard deviation, E). Details of statistical results are in Table S6. 2‐HG, 2‐hydroxyglutaric acid; 2‐IPM, 2‐isopropylmalate; 3‐HMG, 3‐hydroxy‐3‐methylglutaric acid; AMP, adenosine monophosphate; DHA, dehydroascorbic acid; GABA, γ‐aminobutyric acid; UMP, uridine monophosphate
Stands 1 and 3 displayed abnormal pre‐senescence chlorophyll levels. In stand 1, pre‐senescence chlorophyll levels were much higher (CCI ~ 50) than we ever observed in any natural aspen accession grown in any of our common gardens (unpublished data), and the nitrogen status of this tree stand was higher than of the others (Figure S3). Since this stand, in contrast to all others, was next to an agricultural field, fertilization is one possible reason behind its high N status and senescence behavior. This is in line with our other studies, where we have observed that N fertilization can delay senescence in aspen (Fataftah et al. unpublished). In the case of stand 3, pre‐senescence chlorophyll levels were lower than in the other stands and already depleting at the beginning of August, which may have affected its response to girdling or concealed the effect on the onset. On average, girdled stems started to senesce on DOY 243 and control stems on DOY 252. Based on the evidence from three experiments, we concluded that girdling‐induced senescence was consistently slow, and typically accompanied by declined chlorophyll levels and earlier onset, albeit these responses could vary depending on the genotype or internal nutrient status.
3.3. Girdling induces anthocyanin accumulation
The girdled stems of all aspens we had studied retained or accumulated anthocyanins during the autumn (Figures 1, 2, 3). In tree 201, anthocyanin levels were consistently higher in the girdled stem and did not decrease during the autumn as in the control stem (Figure 2D). As a result, the leaves of the girdled stem appeared red at the time when the leaves of control stem still appeared green (Figure 2A).
To get information about the metabolic status of girdled stems and how it could affect the onset of leaf senescence, we performed pigment analysis and metabolite profiling of leaves during the autumn. Girdling reduced the levels of precursors (shikimic acid and phenylalanine) and induced the production of specific downstream metabolites in the phenylpropanoid pathway (Figure 5). The levels of salicinoids, quinic acid derivatives and flavonols did not change significantly (Figure 5) indicating that girdling enhanced mainly the production of anthocyanins and catechins (flavan‐3‐ols), which form condensed tannins (proanthocyanidins) upon polymerization. Transcriptomic data supported that phenylpropanoid biosynthesis was enhanced by girdling. Genes with enhanced expression in the girdled stem included homologs to Arabidopsis phenylalanine ammonia‐lyase (PAL2), chalchone synthase (CHS), anthocyanin synthase (LDOX/ANS), and MYB111 encoding a transcription factor that upregulate “early” biosynthetic genes in the phenylpropanoid pathway (Stracke et al., 2007) (Table S1, Figure 5). GO term enrichment analysis indicated that the altered phenylpropanoid biosynthesis was reflected also in cell wall biogenesis.
FIGURE 5.
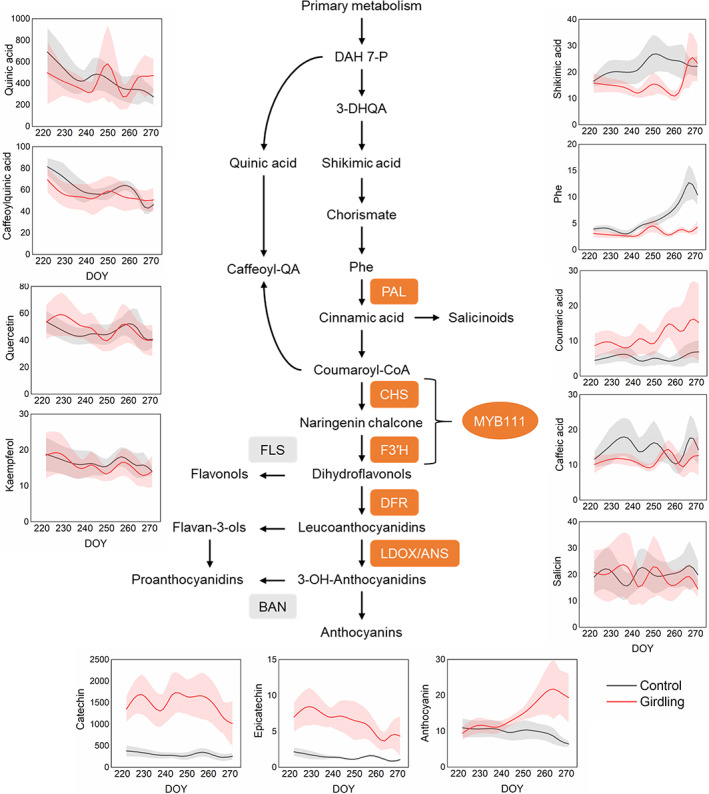
The effect of stem girdling on the phenylpropanoid pathway during autumn. Genes coding the enzymes and transcription factors involved in phenylpropanoid biosynthesis pathway were upregulated (colored in orange) in the leaves of girdled stem. See Table S1 for a summary of differential gene expression and Table S6 for statistical results of metabolite data. Metabolite data are arbitrary units, mean ± se, n = 4. DOY, the day of the year
Photosynthetic pigment analysis showed that in addition to chlorophyll a and b, girdling also reduced the levels of beta‐carotene, lutein, violaxanthin, and neoxanthin (Figure 2E, Table S4). On the other hand, antheraxanthin (Ax) and zeaxanthin (Zx) levels increased around the time of senescence onset in girdled (DOY 243) and control stems (DOY 251) (Figure 2E, Table S4). However, in the control stem the increase was transient (5 days) whereas in the girdled stem it was maintained longer (2 weeks) (Figure 2E). Increase of de‐epoxidation state of xanthophyll cycle pigments (DEPS) coincided with Ax and Zx accumulation (Figure 2F). Furthermore, the chlorophyll to carotenoid ratio remained lower in girdling than in control until late stages of senescence because of increased antheraxanthin and zeaxanthin levels and partly because chlorophyll degradation was faster than carotenoid degradation (Figure 2G). Low chlorophyll‐to‐carotenoid ratios are symptomatic of stress and senescence (Junker & Ensminger, 2016). Carotenoids can act as antioxidants and account for photoprotection by dissipating excess energy from PSII by non‐photochemical quenching (Havaux et al., 2007; Jahns et al., 2009; Tian et al., 2017). At a glance, the results reflect that the changes in the levels of xanthophyll cycle pigments may play a role in senescence, since in the control stem they clearly coincided with senescence onset. However, daily temperature variation was large that day, and at night the temperature dropped to 1°C (Figure S1). In the same tree in 2003, the changes in the epoxidation state of xanthophyll cycle pigments occurred on a cold day (DOY 245), but senescence started about 1 week later (Keskitalo et al., 2005). In line with the previous report, our data (Figure 2E,F) suggest that xanthophyll cycle pigments may have responded to the cold weather conditions, but whether they have a role in the onset of autumn senescence remains to be verified experimentally.
In the leaves of girdled stems, the early activation of violaxanthin de‐epoxidase, low chlorophyll to carotenoid ratio, and anthocyanin accumulation can be mechanisms of sustained photoprotection due to girdling (Urban & Alphonsout, 2007). In addition, anthocyanin accumulation has been proposed to provide photoprotection through light shielding in order to facilitate nutrient resorption during senescence in deciduous tree species (Hoch et al., 2003; Lo Piccolo et al., 2018).
Autumnal leaf color has been suggested to be a signal for insect herbivores of traits related to defense or nutrient status of the host (Hamilton & Brown, 2001, Archetti et al., 2009, Döring et al., 2008). In light of this, we undertook a study to investigate whether the differential levels of pigments between the girdled and control stems influenced the arthropod herbivore community (Note S1). Briefly, our results suggested that herbivores were more attracted to the leaves of the girdled than control stems (Note S1; Table S5). We believe that factors such as sink capacity and phenology that affect the intensity and the timing of nutrient mobilization are such strong drivers for differences in autumn coloration in trees that they are likely to account for the variation in insect herbivory.
3.4. Stems that display girdling‐induced senescence and depleted chlorophyll content show high carbon‐to‐nitrogen ratio
This study also illustrates how nutrient conditions can affect senescence in the natural environment. The four aspen stands were chosen with the intention to be similar, but the nutrient status of the stands was actually different. Unlike the other girdled trees, the girdled stem in stand 1, which was located next to an agricultural field and exhibited exceptionally high chlorophyll content, did not show early onset of senescence or decline in chlorophyll content in pre‐senescent leaves (Figure 3A). Nor did its foliar N content decrease in response to girdling like in the other girdled stems (Figure S3). This suggested that its high N status and C/N ratio that was maintained below 30 (Figures 6; S3) could counteract girdling‐induced leaf senescence. The C/N ratio in the other girdled stems was consistently around 40 or above (Figure 6). The typical repression of photosynthetic genes by accumulating sugars, cytosolic carbohydrates, or starch, is not apparent when nitrogen supply is high (Aoyama et al., 2014; Martin et al., 2002; Pourtau et al., 2006). An interaction between C and N status in the initiation of leaf senescence has been well documented in annuals (Wingler et al., 2006; Wingler & Roitsch, 2008) and our observations support that the cross‐talk between C and N metabolism has an important role in the regulation of chlorophyll content and the onset of autumn senescence in aspen.
FIGURE 6.

Leaf area‐based carbon and nitrogen concentrations (mg cm− 2) in pre‐senescence (pre‐sen) and post‐senescence (post‐sen) leaves, the proportion of remobilized N (%), and carbon‐to‐nitrogen ratio (C/N) in control and girdled stems in four aspen stands during autumn 2009. Different letters indicate significant differences between groups. Bars represent mean ± se, n = 4, except C/N ratio data are mean of three technical replicates. DOY, the day of the year
Nitrogen was more efficiently remobilized in the girdled stems than in the control stems, which could be achieved by the slower senescence process and by the activation of plausible photo‐protective mechanisms (Figures 6, 2 and 3). This indicates that under stressful conditions, early and slow senescence can be beneficial for nutrient resorption.
3.5. Metabolic changes associated with girdling‐induced senescence are mainly similar to developmental senescence
The early senescence phenotype reflected the overall metabolite profile in the leaves of girdled stems—the metabolite shift toward a senescence profile occurred earlier in girdled stems than in control stems (Figure 4). The levels of 22 metabolites increased and nine metabolites decreased during senescence irrespective of the treatment (Table S6), and the results resembled senescence‐associated metabolic changes described in other plant species (Clément et al., 2018; Diaz et al., 2005; Moschen et al., 2016; Watanabe et al., 2013). In line with the early onset of senescence and nutrient remobilization, the levels of free amino acids showed early accumulation (tyrosine, histidine, proline, threonine, glutamine, cluster IV Figure 4) or were consistently elevated (tryptophan, methionine, cluster V Figure 4) in the leaves of girdled stems than in control stems. The levels of branched chain amino acids (valine, leucine, and isoleucine) increased earlier but less in girdled stems than in control stems (cluster III, Figure 4). The accumulation of free amino acids and especially branched chain and aromatic (phenylalanine, tyrosine, tryptophan) amino acids have been associated with developmental and dark‐induced senescence in Arabidopsis (Law et al., 2018; Watanabe et al., 2013).
Girdling already caused major changes in the primary metabolite and secondary metabolite pathways in the pre‐senescent leaves at the beginning of August (DOY 222) (Figure 4B). Treatment affected the levels of 67 metabolites (30 increased, 37 decreased) (Table S6). Time and treatment effects on overall metabolite profile were significant (P < 0.01) in anova‐simultaneous component analysis (ASCA). Time × treatment interaction term was not significant (P = 0.77) indicating that although the timing and intensity varied, the overall metabolic changes during senescence process were mainly similar in the leaves of control and girdled stems.
Reduced photosynthetic rate is a typical response to girdling (De Schepper et al., 2010; De Schepper & Steppe, 2011; Urban et al., 2004) and could be postulated based on the metabolite results. Decreased levels of several C3, C4, and C5 carbohydrates (clusters I and II Figure 4) and glycine‐to‐serine (Gly/Ser) ratio (Figure 7A) already in the pre‐senescence leaves of girdled stems suggested early downregulation of photosynthesis and photorespiration, typical for early stage of leaf senescence (Keskitalo et al., 2005). A decrease in Gly/Ser ratio was observed in control stems when chlorophyll degradation started and thus it appeared to be a good senescence marker in aspen leaves (Figure 7A).
FIGURE 7.

The effect of girdling on the metabolite ratios (A), metabolite relationships (B), and the comparison of metabolite responses (C) in four aspen stands during autumn 2009. (A) Metabolite ratios are expressed as mean ± se, n = 4, two‐way anova P < 0.01**, <0.05*. The average dates of senescence onset in control and girdled stems are marked with arrows. (B) Differential correlation network. Edge width is proportional to the strength of differential correlation and color represents more positive (orange) and negative (blue) correlation in girdling than in control samples. The metabolites with high degrees are shown. (C) A Shared and Unique Structures plot (SUS‐plot) of discriminant analysis (OPLS‐DA). The SUS‐plot was constructed to compare girdling‐induced metabolite responses between tree stand 1 that did not display girdling‐induced senescence or decrease in chlorophyll levels and other tree stands that did. Metabolites that responded similarly to girdling in all four tree stands are located in the upper right (levels increased) and in the lower left (levels decreased) quartiles. Metabolites that showed opposite responses to girdling in stand 1 compared to the other tree stands are located in the upper left (increased in stand 1 and decreased in the other stands) and lower right quartiles (decreased in stand 1 and increased in the other stands). For a clear visualization only the selected metabolites with high p(corr) values in both models are labeled in the plot. Figures with all metabolite labels are in Figure S4. α‐KG, α‐ketoglutaric acid; 2‐IPM, 2‐isopropylmalic acid; AMP, adenosine monophosphate; Asc, ascorbic acid; DHA, dehydroascorbic acid; DOY, the day of the year; UMP, uridine monophosphate
Is girdling‐induced senescence the same as autumn senescence? At the molecular level, all plant senescence programs largely involve the same molecular players, but the triggering factors differ. It has been shown that the signaling pathways can vary between developmental senescence and stress‐induced senescence (Buchanan‐Wollaston et al., 2005; van der Graaff et al., 2006). Not only girdling, but factors such as drought, air pollutants and pathogen infections could cause leaf senescence throughout the season, but we believe that autumn senescence is a process that aspens initiate at a certain day of the year, if leaves have not already started to senescence triggered by other factors. This would be compatible with an idea that senescence is the default pathway in leaf development and that several factors are needed to counteract it, but if one of them disappears, leaves will enter a common senescence program.
3.6. Accumulation of soluble carbohydrates is not the primary trigger of senescence in girdled stems
Carbohydrate accumulation has often been related to the onset of leaf senescence (Wingler et al., 2006). Stem girdling induced accumulation of soluble sugars and starch in the leaves of sugar maple, citrus (Citrus reticulata Blanco), and beech (Fagus sylvatica) (Kraj, 2017; Murakami et al., 2008; Rivas et al., 2008). Carbohydrate accumulation in response to girdling is known to inhibit photosynthesis due to feedback regulation (e.g., Urban & Alphonsout, 2007), but it does not necessarily trigger senescence (Holland et al., 2016). Furhermore, in transgenic hybrid poplar (Populus alba L. × P. grandidentata Michx.) expressing Arabidopsis sucrose phosphate synthase (AtSPS) showed enhanced sucrose levels and delayed onset of leaf senescence (Park et al., 2009) suggesting that the relationship between carbohydrate accumulation and senescence onset is not straightforward. We studied if the changes in carbohydrate levels would account for the premature leaf senescence observed in girdled stems. The level of sucrose, the major product of photosynthesis and primary transported carbohydrate, was unaltered by girdling, consistent with the passive phloem loading in aspen (Hubeau et al., 2019). The increase in glucose (and fructose) levels occurred after DOY 243, which would be too late to account for the onset of senescence in girdled stems (cluster IV, Figure 4).
Nevertheless, carbohydrate metabolism was affected by girdling treatment—in pre‐senescence leaves, glucose levels were slightly lower, but glucose‐6‐phosphate levels were higher (cluster V, Figure 4) that can inhibit hexokinase activity and affect sugar sensing (Jang et al., 1997; Li & Sheen, 2016). Elevated glucose‐6‐phosphate levels can induce starch biosynthesis or glucose breakdown through glycolysis as well as the oxidative pentose phosphate pathway (OPPP), which provides precursors for nucleic acid and phenylpropanoid biosynthesis and NADPH for reductive reactions that are the basis of biosynthetic processes and the antioxidant system.
Other differences in carbohydrate metabolism between girdled and control stems were detected in raffinose family oligosaccharides (RFOs), a group of transportable carbohydrates. Raffinose showed the most different time‐related patterns between girdled and control stems based on dynamic metabolomic analysis by MEBA (Table 1, Table S7); it did not accumulate during senescence in the girdled stems like it did in the control stems (cluster III, Figure 4). Instead, the leaves of girdled stems exhibited constantly high levels of galactinol, which is synthesized from myo‐inositol and UDP‐galactose by galactinol synthase (cluster V, Figure 4). myo‐Inositol level, on the other hand, decreased earlier in girdled stems than in control stems (cluster I, Figure 4). RFOs accumulate in response to several environmental stresses such as cold and drought proposedly to function as osmoprotectants and ROS scavengers (Nishizawa et al., 2008). In autumn leaves, RFOs may account for the mobilization of carbohydrates and also for cold tolerance (Unda et al., 2011). High levels of polyols such as galactinol and erythritol (cluster V, Figure 4) can act as osmolytes in the leaves of girdled stems. Taken together, the observed changes in carbohydrate metabolism could affect the senescence process, but the accumulation of soluble sugars seems not to be the trigger for leaf senescence in aspen.
TABLE 1.
Top 25 metabolites showing the most variable temporal patterns between girdled and control stems in four aspen stands in 2009
| Metabolite | Hotelling‐T2 |
|---|---|
| Raffinose | 53.303 |
| Arabitol | 48.411 |
| Lysine | 39.045 |
| Glucose | 37.76 |
| Lactic acid | 36.067 |
| Erythritol | 34.382 |
| Malic acid | 31.461 |
| Heptulose 1 | 30.257 |
| Orotic acid | 28.053 |
| 2‐Hydroxyglutaric acid (2‐HG) | 27.588 |
| Arabinose | 25.473 |
| Heptulose 2 | 25.298 |
| Glycerol‐3‐phosphate | 25.192 |
| Rhamnose | 24.228 |
| Dehydroascorbic acid (DHA) dimer | 22.876 |
| 2‐Isopropylmalic acid (2‐IPM) | 21.636 |
| Fumaric acid | 21.331 |
| Epigallocatechin | 20.996 |
| Fructose | 20.897 |
| Catechin | 20.582 |
| α‐Ketoglutaric acid (α‐KG) | 20.402 |
| β‐Aminoisobutanoic acid (BAIB) | 19.548 |
| Uridine monophosphate (UMP) | 17.965 |
| Aspartic acid | 17.805 |
| Histidine | 17.158 |
Metabolites were ranked based on Hotelling‐T2 statistics using Multivariate Empirical Bayesian time‐series Analysis (MEBA). A complete list of statistically significant metabolites is in Table S7.
3.7. Salicylic acid metabolism is affected by stem girdling
As the girdled stem in stand 1 did not initiate premature leaf senescence or show decline in chlorophyll content, unlike the other girdled stems (Figure 3), we compared the metabolite responses separating stand 1 from the other three stands to identify candidate metabolic markers for girdling‐induced senescence. A group of metabolites that separated stand 1 from the others was phenolic acids: salicylic acid, benzoic acid, and caffeic acid that increased in response to girdling in stand 1 and decreased in the other stands (Figure 7C) indicating that salicylic acid may be involved in the regulation of senescence in aspen. One possible explanation is that elevated salicylic acid levels may avert girdling‐induced senescence in stand 1 due to its antagonistic effect on the jasmonate signaling pathway (Miao & Zentgraf, 2007; Van der Does et al., 2013; Yin et al., 2020), that could be activated by girdling, based on the enhanced expression of PR3 (PATHOGENESIS RELATED PROTEIN 3, Potra001809g14625, log2fc 3.88) and repressed expression of JAZ8 (JASMONATE‐ZIM‐DOMAIN PROTEIN 8, Potra004547g25056, log2fc − 1.51), a repressor of jasmonic acid signaling (Table S1).
Lower salicylic acid levels in girdled stems can be explained by the enhanced expression of DLO2 (DMR6‐LIKE OXYGENASE 2, Potra001205g10420, log2fc 2.03) that encodes a α‐ketoglutarate‐dependent dioxygenase (2‐ODD) that converts salicylic acid to 2,3‐dihydroxybenzoic acid. Low levels of salicylic acid, as in the leaves of girdled stems (except in stand 1) (Figure 7C), have been associated with slow progression of leaf senescence (Morris et al., 2000). Salicylic acid promotes leaf senescence by inducing the expression of senescence‐associated genes such as SAG12 (Buchanan‐Wollaston et al., 2003; Morris et al., 2000), which is a commonly used marker for leaf senescence. However, SAG12 expression was not upregulated in the senescing leaves of girdled stem (Potra000590g04457, log2fc − 0.60). Salicylic acid, WRKY75 transcription factor (Potra001336g11465, log2fc − 1.12), and ROS have shown to form an amplification loop causing a steady rise in their levels in an age‐dependent manner (Guo et al., 2017).
The tight control of ROS production and elimination is maintained by antioxidant compounds, such as carotenoids, tocopherols, ascorbate and glutathione, and ROS‐scavenging enzymes (Foyer & Noctor, 2011). Girdling has been shown to enhance ROS production and to induce enzymatic antioxidant systems in citrus and beech trees (Kraj, 2017; Rivas et al., 2008). Elevated levels of α‐tocopherol, as were found here in the leaves of girdled stems (cluster V, Figures 4; Figure 7C), have been found in senescing leaves and can prevent lipid peroxidation in thylakoid membranes (Falk & Munné‐Bosch, 2010; Munné‐Bosch, 2005). The ascorbate pool was more reduced in the pre‐senescent leaves of girdled stems than in control stems (Figure 7A). After senescence was initiated, dehydroascorbate levels increased (DHA dimer, cluster IV Figure 4) in parallel with increasing oxidation state of ascorbate pool in girdled stems (Figure 7A). Ascorbic acid and DHA dimer levels were reduced by girdling in stand 1, while the responses were opposite in the other tree stands (Figure 7C) suggesting that cellular redox status can be involved in the initiation of premature senescence in aspen. The elevated levels of antioxidant metabolites and the redox state of ascorbate pool suggested induction of the antioxidant system in pre‐senescent leaves of girdled stems, which is likely to affect ROS signaling network in tight interplay with other signaling pathways.
Salicylic acid pathway was differentially regulated between normal developmental senescence and dark‐ or starvation‐induced senescence in Arabidopsis (Buchanan‐Wollaston et al., 2005; van der Graaff et al., 2006). The results from those studies suggested that the induction of the salicylic acid pathway is characteristic for developmental leaf senescence, and our results support that conclusion since girdling‐induced senescence was accompanied by low salicylic acid levels. We propose that salicylic acid may be involved in the regulation of autumn senescence through its crosstalk with other phytohormone pathways and once initiated, the low levels of salicylic acid may suppress the progression of senescence in girdled stems through its interaction with ROS and senescence associated transcriptional regulatory network.
3.8. Is tricarboxylic acid cycle the key pathway regulating girdling‐induced senescence?
Many of the metabolites that showed either different levels (Figure 4D) or temporal patterns between girdled and control stems (Table 1) or between the tree stands (Figure 7C), or were identified as key nodes in the differential correlation network (Figure 7B), were linked to the tricarboxylic acid cycle (TCA) cycle. Those metabolite included α‐ketoglutaric acid, malic acid fumaric acid, cis‐aconitic acid, 2‐hydroxyglutaric acid, proline, aspartate, and alanine. We propose that TCA cycle intermediates, α‐ketoglutarate and malate in particular, could act as “hubs” affecting metabolic responses to girdling. α‐Ketoglutarate is a precursor for glutamate‐derived amino acids and can be formed from proline catabolism, via alanine and aspartate aminotransferases or via 2‐hydroxyglutarate dehydrogenases. Furthermore, a malate–aspartate shuttle transfers redox equivalents between mitochondria and the cytosol by interconverting malate and aspartate through oxaloacetate. The process also involves interconversion of α‐ketoglutarate and glutamate and metabolites can be transferred between mitochondria and the cytosol by aspartate–glutamate and malate‐α‐ketoglutarate antiporters.
During senescence, the production of α‐ketoglutarate drives the reassimilation of ammonium released from protein catabolism to form glutamine (Law et al., 2018), which was elevated by girdling (Cluster IV Figure 4). Together with asparagine, glutamine contributes to nitrogen translocation from senescing leaves to storage organs in poplar (Populus trichocarpa) (Couturier et al., 2010). The higher glutamine‐to‐glutamate ratio (Gln/Glu) indicated higher ammonium assimilation in the leaves of girdled stems than in control stems (Figure 7A). The glutamate‐to‐α‐ketoglutarate ratio (Glu/α‐KG) and asparagine‐to‐aspartate ratio (Asn/Asp) showed different temporal patterns in the leaves of girdled and control stems and the changes in the ratios corresponded to the timing of senescence in both treatments (Figure 7A). These patterns can be related to the activation of N recycling and transport processes. The TCA cycle is a central energy producing pathway integrating carbon and nitrogen metabolism and as the C/N ratio was disturbed by girdling (Figure 5) due to reduced sink strength for phloem transport to the root, our data suggested that the TCA cycle could regulate tree responses to girdling and that the changes in TCA cycle status could preceed the onset of senescence.
The TCA cycle has also gained attention in previous senescence studies. Changes in the TCA cycle can preceed senescence in Arabidopsis (Watanabe et al., 2013) and the TCA cycle and carbohydrate metabolism were the main altered pathways in senescing sunflower (Helianthus annuus) leaves (Moschen et al., 2016). Antisense inhibition of malate dehydrogenase, fumarase or succinate dehydrogenase, did not affect senescence (Araújo et al., 2011; Nunes‐Nesi et al., 2005; Nunes‐Nesi et al., 2007), whereas antisense inhibition of α‐ketoglutarate dehydrogenase (αKGDH) reduced respiration rate and led to early leaf senescence in tomato (Solanum lycopersicum) (Araújo et al., 2012). αKGDH is regarded as the rate‐limiting factor affecting the flux through TCA cycle and toward amino acid biosynthesis (Bunik & Fernie, 2009). It is also a major mitochondrial redox regulator since it supplies reducing equivalents in the form of NADH (Bunik & Fernie, 2009). α‐Ketoglutarate and ascorbate act as co‐substrates for α‐ketoglutarate‐dependent dioxygenases (2‐ODDs), enzymes that catalyze a number of oxidation reactions in primary and secondary metabolic pathways (Araújo et al., 2014). Perhaps α‐ketoglutarate provides the link among primary carbohydrate and amino acid metabolism, phenylpropanoid biosynthesis, redox metabolism, and phytohormone metabolism, which were all altered in the leaves of girdled stems (Figures 4 and 7).
3.9. Concluding remarks—physiological and metabolic interactions during senescence are complex
In this contribution, we have provided data that could help to understand the effect of girdling in aspen, but hopefully also leaf senescence in general. This has been done by studying many aspects of physiology and metabolism over several years, and in several aspen stands, which not only are different genotypes but also exposed to different conditions. Like several other studies that have aimed to understand leaf physiology and metabolism during senescence, we have illustrated that the physiological and metabolic interactions are highly complex. We also note that it is easy to identify changes that are consequences of leaf senescence, but hard to understand which changes may cause it; the identification of the key factor(s) that lead to leaf senescence is still the holy grail of senescence research.
However, we think that the data presented here lead to several conclusions that we think are of general relevance, namely: (1) girdling can override the environmental control on the timing of leaf senescence in autumn and cause premature leaf senescence (Figures 1, 2, 3); (2) this girdling‐induced senescence is—in terms of chlorophyll degradation—slower than the one induced “by the calendar” (Figures 2 and 3); (3) the early and slow senescence process allows the girdled stems to remobilize nitrogen more efficiently (Figure 6); (4) girdling induces flowering in aspen and (5) alters herbivore community structure (Table S5). (6) At the metabolite level, girdling can induce anthocyanin accumulation that could together with low chlorophyll‐to‐carotenoid ratio and activated xanthophyll cycle provide photoprotection during senescence (Figures 2, 3, 4, 5); (7) the accumulation of soluble sugars does not account for the premature leaf senescence in girdled stems, but high pre‐senescence C/N ratio (and starch accumulation) could potentially do (Figure 6); (8) the effect of girdling on senescence appears to be linked to the status of the TCA cycle (Figure 7), at the crossroads of carbon and nitrogen metabolism, and to (ix) salicylic acid metabolism (Figure 7) in interaction with ROS and other phytohormone signaling pathways.
By taking all these findings into account, we propose a model of girdling effects on tree physiology, foliar pigments, and metabolic status during leaf senescence in aspen (Figure 8). Girdling effects on senescence are consequences of the reduced sink strength for phloem transport, initially increased accumulation of photosynthates that in turn disturbs carbon and nitrogen homeostasis and inhibits photosynthesis due to feedback regulation. It is unlikely that any other effect of girdling will be persistent over the years and disappear if phloem transport is re‐established through healing of the wound, and that the effect would be quantitative. In addition, it seems that if premature senescence has been induced in one stem of a tree stand, no additional increase in chlorophyll degradation rate occurs in that stem at the date when the rapid chlorophyll degradation of the other stems begins. This is consistent with a model where senescence is a program that could be triggered by different factors; only if the program has not already been initiated by other factors will the environmental signal, presumably given by light, lead to the execution of the autumn senescence. In conclusion, girdling can override the phenological control of autumn senescence in aspen, in a complex interaction with sink strength and the metabolic status of the tree.
FIGURE 8.

A schematic model of girdling effects on tree physiology, foliar pigments, and metabolic status during leaf senescence in aspen. Stem girdling blocks phloem transport from leaves to roots and photosynthates accumulate above the girdle. However, in a clonal stand another stem can sustain the common root system with photosynthates. In the control stems, an environmental signal is proposed to trigger normal developmental autumn senescence and the metabolic program that acts through the interaction of TCA cycle, phytohormone, and ROS signals. Stem girdling disrupts sink‐source relations that induce flowering and leads to altered leaf pigment composition, herbivore community structure, and high C/N ratio. High leaf C/N ratio in turn affects the metabolic flux through the TCA cycle, the key pathway integrating carbon and nitrogen metabolism and redox homeostasis. Altered TCA cycle status and redox homeostasis may provide cues to initiate premature leaf senescence in the girdled stems, and once initiated, the low levels of salicylic acid may suppress the progression of senescence. High nitrogen status can overcome girdling‐induced leaf senescence by maintaining low C/N ratio in conjunction with primary carbon and nitrogen metabolism regulated at the level of TCA cycle. Red denotes higher levels and blue denotes lower levels in girdled stems compared to control stems. Anth, anthocyanin content; Car, carotenoid content; Chl, chlorophyll content; DOY, the day of the year
AUTHOR CONTRIBUTIONS
Stefan Jansson, Erik Edlund, Lars Björkén, and Kathryn M. Robinson designed the experiments; Erik Edlund, Lars Björkén, and Kathryn M. Robinson performed the experiments; Stefan Jansson supervised the experiments; Jenna Lihavainen, Pushan Bag, and Kathryn M. Robinson analyzed the data; Jenna Lihavainen wrote the article with contributions of all the authors; all authors discussed the results and commented on the manuscript; Stefan Jansson agrees to serve as the author responsible for contact and ensures communication.
Supporting information
Figure S1 Weather data in autumn 2008 and 2009.
Figure S2. Fitting the chlorophyll content index data with a non‐linear Boltzmann function.
Figure S3. The effect of girdling on leaf carbon (C) and nitrogen (N) concentrations in four aspen stands during autumn 2009.
Figure S4. A differential metabolite‐metabolite correlation network between girdling and control treatments, and Shared and Unique Structures (SUS)‐plot comparing girdling‐induced metabolite responses in four aspen stands during autumn 2009.
Table S1. List of differentially expressed genes (DEGs) in the leaves of the control and girdled stem in tree 201 in autumn 2010.
Table S2. GO enrichment analysis of DEGs in the leaves of the control and girdled stem in tree 201 in autumn 2010.
Table S3. Orthogonal projections to latent structures discriminant analysis (OPLS‐DA) model diagnostics of GC–MS metabolite data from the leaves of control and girdled stems in four aspen stands during autumn 2009.
Table S4. Statistics of pigment data from leaves of the control and girdled stem in tree 201 during autumn 2008.
Table S5. Damage to leaves of tree 201 caused by arthropod herbivores.
Table S6. Statistics of GC–MS metabolite data from the leaves of control and girdled stems in four aspen stands during autumn 2009.
Table S7. Multivariate Empirical Bayes time‐series analysis (MEBA) statistics of metabolite data from the leaves of control and girdled stems in four aspen stands during autumn 2009.
Note S1. Herbivore community differs between girdled and control stems.
ACKNOWLEDGMENTS
This research has been supported by funding from the Swedish Research Council VR, Formas, Kempestiftelserna, Swedish Foundation for Strategic Research (SSF), Trees for the Future (T4F) project and SE2B Horizon 2020 (grant no. 675006, Solar Energy to Biomass). The authors also thank the Swedish Metabolomics Centre (SMC) for their support in metabolite analyzes and UPSC bioinformatics facility for their support in transcriptome data analyzes.
Lihavainen J, Edlund E, Björkén L, Bag P, Robinson KM, Jansson S. Stem girdling affects the onset of autumn senescence in aspen in interaction with metabolic signals. Physiologia Plantarum. 2021;172:201–217. 10.1111/ppl.13319
Edited by I. Ensminger
Funding information H2020 Marie Skłodowska‐Curie Actions, Grant/Award Number: 675006; Kempestiftelserna; Stiftelsen för Strategisk Forskning; Svenska Forskningsrådet Formas; Trees for the Future; Vetenskapsrådet
DATA AVAILABILITY STATEMENT
Transcriptome data are openly available in Plant Genome Integrative Explorer platform (https://plantgenie.org/). Raw metabolite data were generated at Swedish Metabolomics Centre (SMC) and derived data are available on request. Other data supporting the findings of this study are available within the article and/or its supplementary materials.
REFERENCES
- Andersson, A. , Keskitalo, J. , Sjödin, A. , Bhalerao, R. , Sterky, F. , Wissel, K. , et al. (2004) A transcriptional timetable of autumn senescence. Genome Biology, 5, R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama, S. , Huarancca Reyes, T. , Guglielminetti, L. , Lu, Y. , Morita, Y. , Sato, T. , et al. (2014) Ubiquitin ligase ATL31 functions in leaf senescence in response to the balance between atmospheric CO2 and nitrogen availability in Arabidopsis. Plant & Cell Physiology, 55, 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, W.L. , Martins, A.O. , Fernie, A.R. & Tohge, T. (2014) 2‐Oxoglutarate: linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Frontiers in Plant Science, 5, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, W.L. , Nunes‐Nesi, A. , Osorio, S. , Usadel, B. , Fuentes, D. , Nagy, R. , et al. (2011) Antisense inhibition of the iron‐Sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid–mediated effect on stomatal aperture. Plant Cell, 23, 600–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, W.L. , Tohge, T. , Osorio, S. , Lohse, M. , Balbo, I. , Krahnert, I. , et al. (2012) Antisense inhibition of the 2‐oxoglutarate dehydrogenase complex in tomato demonstrates its importance for plant respiration and during leaf senescence and fruit maturation. Plant Cell, 24, 2328–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti, M. (2009) Classification of hypotheses on the evolution of autumn colours. Oikos, 118, 328–333. [Google Scholar]
- Bhalerao, R. , Keskitalo, J. , Sterky, F. , Erlandsson, R. , Bjorkbacka, H. , Birve, S.J. , et al. (2003) Gene expression in autumn leaves. Plant Physiology, 131, 430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, B.L. , Parmentier Line, C. , Fuchigami, L.H. & Coleman, G.D. (2001) Ecotypic and genetic variation in poplar bark storage protein gene expression and accumulation. Tree Physiology, 21, 1289–1297. [DOI] [PubMed] [Google Scholar]
- Böhlenius, H. , Huang, T. , Charbonnel‐Campaa, L. , Brunner, A.M. , Jansson, S. , Strauss, S.H. , et al. (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science, 312, 1040–1043. [DOI] [PubMed] [Google Scholar]
- Buchanan‐Wollaston, V. , Earl, S. , Harrison, E. , Mathas, E. , Navabpour, S. , Page, T. , et al. (2003) The molecular analysis of leaf senescence–a genomics approach. Plant Biotechnology Journal, 1, 3–22. [DOI] [PubMed] [Google Scholar]
- Buchanan‐Wollaston, V. , Page, T. , Harrison, E. , Breeze, E. , Lim, P.O. , Nam, H.G. , et al. (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation‐induced senescence in Arabidopsis. The Plant Journal, 42, 567–585. [DOI] [PubMed] [Google Scholar]
- Bunik, V.I. & Fernie, A.R. (2009) Metabolic control exerted by the 2‐oxoglutarate dehydrogenase reaction: a cross‐kingdom comparison of the crossroad between energy production and nitrogen assimilation. The Biochemical Journal, 422, 405–421. [DOI] [PubMed] [Google Scholar]
- Bylesjö, M. , Segura, V. , Soolanayakanahally, R.Y. , Rae, A.M. , Trygg, J. , Gustafsson, P. , et al. (2008) LAMINA: a tool for rapid quantification of leaf size and shape parameters. BMC Plant Biology, 8, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, J. , Soufan, O. , Li, C. , Caraus, I. , Li, S. , Bourque, G. , et al. (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research, 46, W486–W494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément, G. , Moison, M. , Soulay, F. , Reisdorf‐Cren, M. & Masclaux‐Daubresse, C. (2018) Metabolomics of laminae and midvein during leaf senescence and source–sink metabolite management in Brassica napus L. leaves. Journal of Experimental Botany, 69, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, J.E.K. , Brown, K.A. , Wu, R. & Davis, J.M. (2003) Gene expression associated with N‐induced shifts in resource allocation in poplar. Plant, Cell and Environment, 26, 757–777. [Google Scholar]
- Cooke, J.E.K. & Weih, M. (2005) Nitrogen storage and seasonal nitrogen cycling in Populus: bridging molecular physiology and ecophysiology. The New Phytologist, 167, 19–30. [DOI] [PubMed] [Google Scholar]
- Couturier, J. , Doidy, J. , Guinet, F. , Wipf, D. , Blaudez, D. & Chalot, M. (2010) Glutamine, arginine and the amino acid transporter Pt‐CAT11 play important roles during senescence in poplar. Annals of Botany, 105, 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper, V. & Steppe, K. (2011) Tree girdling responses simulated by a water and carbon transport model. Annals of Botany, 108, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper, V. , Steppe, K. , Van Labeke, M.C. & Lemeur, R. (2010) Detailed analysis of double girdling effects on stem diameter variations and sap flow in young oak trees. Environmental and Experimental Botany, 68, 149–156. [Google Scholar]
- Diaz, C. , Purdy, S. , Christ, A. , Morot‐Gaudry, J.F. , Wingler, A. & Masclaux‐Daubresse, C. (2005) Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis. A metabolic profiling approach. Plant Physiology, 138, 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring, T.F. , Archetti, M. & Hardie, J. (2008) Autumn leaves seen through herbivore eyes. Proceedings of the Royal Society B: Biological Sciences, 276, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, J. & Munné‐Bosch, S. (2010) Tocochromanol functions in plants: antioxidation and beyond. Journal of Experimental Botany, 61, 1549–1566. [DOI] [PubMed] [Google Scholar]
- Foyer, C.H. & Noctor, G. (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiology, 155, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracheboud, Y. , Luquez, V. , Björkén, L. , Sjödin, A. , Tuominen, H. & Jansson, S. (2009) The control of autumn senescence in European aspen. Plant Physiology, 149, 1982–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima, A. (2013) DiffCorr: an R package to analyze and visualize differential correlations in biological networks. Gene, 518, 209–214. [DOI] [PubMed] [Google Scholar]
- Goren, R. , Huberman, M. & Goldschmidt, E.E. (2004) Girdling: physiological and horticultural aspects. American Society for Horticultural Science, 30, 1–36. [Google Scholar]
- Gullberg, J. , Jonsson, P. , Nordström, A. , Sjöström, M. & Moritz, T. (2004) Design of experiments: an efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Analytical Biochemistry, 331, 283–295. [DOI] [PubMed] [Google Scholar]
- Guo, P. , Li, Z. , Huang, P. , Li, B. , Fang, S. , Chu, J. , et al. (2017) A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell, 29, 2854–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, W.D. & Brown, S.P. (2001) Autumn tree colours as a handicap signal. Proceedings of the Royal Society of London. Series B: Biological Sciences, 268, 1489–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux, M. , Dall'Osto, L. & Bassi, R. (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiology, 145, 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch, W.A. , Singsaas, E.L. & McCown, H.B. (2003) Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiology, 133, 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, V. , Fragner, L. , Jungcurt, T. , Weckwerth, W. & Brüggemann, W. (2016) Girdling interruption between source and sink in Quercus pubescens does not trigger leaf senescence. Photosynthetica, 54, 589–597. [Google Scholar]
- Hubeau, M. , Mincke, J. , Vanhove, C. , Fayolle, A. , Epila, J. , Leroux, O. , et al. (2019) 11C‐autoradiographs to image phloem loading. Frontiers in Forests and Global Change, 2, 20. [Google Scholar]
- Jahns, P. , Latowski, D. & Strzalka, K. (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochimica et Biophysica Acta ‐ Bioenergetics, 1787, 3–14. [DOI] [PubMed] [Google Scholar]
- Jang, J.C. , León, P. , Zhou, L. & Sheen, J. (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell, 9, 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker, L.V. & Ensminger, I. (2016) Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiology, 36, 694–711. [DOI] [PubMed] [Google Scholar]
- Keskitalo, J. , Bergquist, G. , Gardström, P. & Jansson, S. (2005) A cellular timetable of autumn senescence. Plant Physiology, 139, 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraj, W. (2017) Stem girdling affects the carbon/nitrogen imbalance and oxidative stress, and induces leaf senescence in phenological forms of beech (Fagus sylvatica). Acta Biologica Cracoviensia Series Botanica, 59, 67–79. [Google Scholar]
- Król, M. , Spangfort, M.D. , Huner, N.P. , Oquist, G. , Gustafsson, P. & Jansson, S. (1995) Chlorophyll a/b‐binding proteins, pigment conversions, and early light‐induced proteins in a chlorophyll b‐less barley mutant. Plant Physiology, 107, 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano, M. , Fukushima, A. , Redestig, H. & Saito, K. (2011) Metabolomic approaches toward understanding nitrogen metabolism in plants. Journal of Experimental Botany, 62, 1439–1453. [DOI] [PubMed] [Google Scholar]
- Landi, M. , Tattini, M. & Gould, K.S. (2015) Multiple functional roles of anthocyanins in plant‐environment interactions. Environmental and Experimental Botany, 119, 4–17. [Google Scholar]
- Law, S.R. , Chrobok, D. , Juvany, M. , Delhomme, N. , Lindén, P. , Brouwer, B. , et al. (2018) Darkened leaves use different metabolic strategies for senescence and survival. Plant Physiology, 177, 132–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon, P. & Sheen, J. (2003) Sugar and hormone connections. Trends in Plant Science, 8, 110–116. [DOI] [PubMed] [Google Scholar]
- Li, L. & Sheen, J. (2016) Dynamic and diverse sugar signaling. Current Opinion in Plant Biology, 33, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Piccolo, E. , Landi, M. , Massai, R. , Remorini, D. & Guidi, L. (2020) Girled‐induced anthocyanin accumulation in red‐leafed Prunus cerasifera: effect on photosynthesis, photoprotection and sugar metabolism. Plant Science, 294, 110456. [DOI] [PubMed] [Google Scholar]
- Lo Piccolo, E. , Landi, M. , Pellegrini, E. , Agati, G. , Giordano, C. , Giordani, T. , et al. (2018) Multiple consequences induced by epidermally‐located anthocyanins in young, mature and senescent leaves of Prunus. Frontiers in Plant Science, 9, 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, T. , Oswald, O. & Graham, I.A. (2002) Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: nitrogen availability. Plant Physiology, 128, 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi, H. , Muruganujan, A. , Ebert, D. , Huang, X. & Thomas, P.D. (2018) PANTHER version 14: more genomes, a new PANTHER GO‐slim and improvements in enrichment analysis tools. Nucleic Acids Research, 47, D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, Y. & Zentgraf, U. (2007) The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell, 19, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson, I.H. , Ingvarsson, P.K. , Robinson, K.M. , Edlund, E. , Eriksson, M.E. , Nilsson, O. , et al. (2018) Autumn senescence in aspen is not triggered by day length. Physiologia Plantarum, 162, 123–134. [DOI] [PubMed] [Google Scholar]
- Morris, K. , Mackerness, S.A.H. , Page, T. , John, C.F. , Murphy, A.M. , Carr, J.P. , et al. (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. The Plant Journal, 23, 677–685. [DOI] [PubMed] [Google Scholar]
- Moschen, S. , Higgins, J. , Di Rienzo, J.A. , Heinz, R.A. , Paniego, N. & Fernandez, P. (2016) Network and biosignature analysis for the integration of transcriptomic and metabolomic data to characterize leaf senescence process in sunflower. BMC Bioinformatics, 17, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné‐Bosch, S. (2005) Linking tocopherols with cellular signaling in plants. The New Phytologist, 166, 363–366. [DOI] [PubMed] [Google Scholar]
- Murakami, P.F. , Schaberg, P.G. & Shane, J.B. (2008) Stem girdling manipulates leaf sugar concentrations and anthocyanin expression in sugar maple trees during autumn. Tree Physiology, 28, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Nishizawa, A. , Yabuta, Y. & Shigeoka, S. (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiology, 147, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes‐Nesi, A. , Carrari, F. , Gibon, Y. , Sulpice, R. , Lytovchenko, A. , Fisahn, J. , et al. (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. The Plant Journal, 50, 1093–1106. [DOI] [PubMed] [Google Scholar]
- Nunes‐Nesi, A. , Carrari, F. , Lytovchenko, A. , Smith, A.M. , Loureiro, M.E. , Ratcliffe, R.G. , et al. (2005) Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiology, 137, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, J.E. , Junttila, O. , Nilsen, J. , Eriksson, M.E. , Martinussen, I. , Olsson, O. , et al. (1997) Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimation. The Plant Journal, 12, 339–1350. [Google Scholar]
- Ougham, H. , Hörtensteiner, S. , Armstead, I. , Donnison, I. , King, I. , Thomas, H. , et al. (2008) The control of chlorophyll catabolism and the status of yellowing as a biomarker of leaf senescence. Plant Biology, 10, 4–14. [DOI] [PubMed] [Google Scholar]
- Park, J.Y. , Canam, T. , Kang, K.Y. , Unda, F. & Mansfield, S.D. (2009) Sucrose phosphate synthase expression influences poplar phenology. Tree Physiology, 29, 937–946. [DOI] [PubMed] [Google Scholar]
- Pourtau, N. , Jennings, R. , Pelzer, E. , Pallas, J. & Wingler, A. (2006) Effect of sugar‐induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta, 224, 556–568. [DOI] [PubMed] [Google Scholar]
- Rademacher, T.T. , Basler, D. , Eckes‐Shephard, A.H. , Fonti, P. , Friend, A.D. , Le Moine, J. , et al. (2019) Using direct phloem transport manipulation to advance understanding of carbon dynamics in Forest trees. Frontiers in Forests and Global Change, 2, 11. [Google Scholar]
- Regier, N. , Streb, S. , Zeeman, S.C. & Frey, B. (2010) Seasonal changes in starch and sugar content of poplar (Populus deltoides × nigra cv. Dorskamp) and the impact of stem girdling on carbohydrate allocation to roots. Tree Physiology, 30, 979–987. [DOI] [PubMed] [Google Scholar]
- Rivas, F. , Fornes, F. & Agustí, M. (2008) Girdling induces oxidative damage and triggers enzymatic and non‐enzymatic antioxidative defences in citrus leaves. Environmental and Experimental Botany, 64, 256–263. [Google Scholar]
- Schaberg, P.G. , Murakami, P.F. , Turner, M.R. , Heitz, H.K. & Hawley, G.J. (2008) Association of red coloration with senescence of sugar maple leaves in autumn. Trees, 22, 573. [Google Scholar]
- Shannon, P. , Markiel, A. , Ozier, O. , Baliga, N.S. , Wang, J.T. , Ramage, D. , et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research, 13, 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfanelli, C. , Poggi, A. , Loreti, E. , Alpi, A. & Perata, P. (2006) Sucrose‐specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiology, 140, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke, R. , Ishihara, H. , Huep, G. , Barsch, A. , Mehrtens, F. , Niehaus, K. , et al. (2007) Differential regulation of closely related R2R3‐MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. The Plant Journal, 50, 660–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell, D. , Mannapperuma, C. , Netotea, S. , Delhomme, N. , Lin, Y.C. , Sjödin, A. , et al. (2015) The plant genome integrative explorer resource: PlantGen IE. org. New Phytologist, 208, 1149–1156. [DOI] [PubMed] [Google Scholar]
- Tian, L. , Xu, P. , Chukhutsina, V.U. , Holzwarth, A.R. & Croce, R. (2017) Zeaxanthin‐dependent nonphotochemical quenching does not occur in photosystem I in the higher plant Arabidopsis thaliana . Proceedings of the National Academy of Sciences, 114, 4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unda, F. , Canam, T. , Preston, L. & Mansfield, S.D. (2011) Isolation and characterization of galactinol synthases from hybrid poplar. Journal of Experimental Botany, 63, 2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, L. & Alphonsout, L. (2007) Girdling decreases photosynthetic electron fluxes and induces sustained photoprotection in mango leaves. Tree Physiology, 27, 345–352. [DOI] [PubMed] [Google Scholar]
- Urban, L. , Léchaudel, M. & Lu, P. (2004) Effect of fruit load and girdling on leaf photosynthesis in Mangifera indica L. Journal of Experimental Botany, 55, 2075–2085. [DOI] [PubMed] [Google Scholar]
- Van der Does, D. , Leon‐Reyes, A. , Koornneef, A. , Van Verk, M.C. , Rodenburg, N. , Pauwels, L. , et al. (2013) Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1‐JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell, 25, 744–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff, E. , Schwacke, R. , Schneider, A. , Desimone, M. , Flügge, U.I. & Kunze, R. (2006) Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiology, 141, 776–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, M. , Balazadeh, S. , Tohge, T. , Erban, A. , Giavalisco, P. , Kopka, J. , et al. (2013) Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiology, 162, 1290–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler, A. , Masclaux‐Daubresse, C. & Fischer, A.M. (2009) Sugars, senescence, and ageing in plants and heterotrophic organisms. Journal of Experimental Botany, 60, 1063–1066. [DOI] [PubMed] [Google Scholar]
- Wingler, A. , Purdy, S. , MacLean, J.A. & Pourtau, N. (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. Journal of Experimental Botany, 57, 391–399. [DOI] [PubMed] [Google Scholar]
- Wingler, A. & Roitsch, T. (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biology, 10, 50–62. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, S. , Yoo, S.D. & Sheen, J. (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature, 425, 521–525. [DOI] [PubMed] [Google Scholar]
- Yin, R. , Liu, X. , Yu, J. , Ji, Y. , Liu, J. , Cheng, L. , et al. (2020) Up‐regulation of autophagy by low concentration of salicylic acid delays methyl jasmonate‐induced leaf senescence. Scientific Reports, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Weather data in autumn 2008 and 2009.
Figure S2. Fitting the chlorophyll content index data with a non‐linear Boltzmann function.
Figure S3. The effect of girdling on leaf carbon (C) and nitrogen (N) concentrations in four aspen stands during autumn 2009.
Figure S4. A differential metabolite‐metabolite correlation network between girdling and control treatments, and Shared and Unique Structures (SUS)‐plot comparing girdling‐induced metabolite responses in four aspen stands during autumn 2009.
Table S1. List of differentially expressed genes (DEGs) in the leaves of the control and girdled stem in tree 201 in autumn 2010.
Table S2. GO enrichment analysis of DEGs in the leaves of the control and girdled stem in tree 201 in autumn 2010.
Table S3. Orthogonal projections to latent structures discriminant analysis (OPLS‐DA) model diagnostics of GC–MS metabolite data from the leaves of control and girdled stems in four aspen stands during autumn 2009.
Table S4. Statistics of pigment data from leaves of the control and girdled stem in tree 201 during autumn 2008.
Table S5. Damage to leaves of tree 201 caused by arthropod herbivores.
Table S6. Statistics of GC–MS metabolite data from the leaves of control and girdled stems in four aspen stands during autumn 2009.
Table S7. Multivariate Empirical Bayes time‐series analysis (MEBA) statistics of metabolite data from the leaves of control and girdled stems in four aspen stands during autumn 2009.
Note S1. Herbivore community differs between girdled and control stems.
Data Availability Statement
Transcriptome data are openly available in Plant Genome Integrative Explorer platform (https://plantgenie.org/). Raw metabolite data were generated at Swedish Metabolomics Centre (SMC) and derived data are available on request. Other data supporting the findings of this study are available within the article and/or its supplementary materials.


